Abstract
Apc, a negative regulator of the canonical Wnt signaling pathway, is a bona-fide tumor suppressor whose loss of function results in intestinal polyposis. APC is located in a commonly deleted region on human chromosome 5q, associated with myelodysplastic syndrome (MDS), suggesting that haploinsufficiency of APC contributes to the MDS phenotype. Analysis of the hematopoietic system of mice with the Apcmin allele that results in a premature stop codon and loss of function showed no abnormality in steady state hematopoiesis. Bone marrow derived from Apcmin mice showed enhanced repopulation potential, indicating a cell intrinsic gain of function in the long-term hematopoietic stem cell (HSC) population. However, Apcmin bone marrow was unable to repopulate secondary recipients because of loss of the quiescent HSC population. Apcmin mice developed a MDS/myeloproliferative phenotype. Our data indicate that Wnt activation through haploinsufficiency of Apc causes insidious loss of HSC function that is only evident in serial transplantation strategies. These data provide a cautionary note for HSC-expansion strategies through Wnt pathway activation, provide evidence that cell extrinsic factors can contribute to the development of myeloid disease, and indicate that loss of function of APC may contribute to the phenotype observed in patients with MDS and del(5q).
Introduction
The canonical Wnt-β-catenin pathway is an evolutionarily conserved and tightly regulated pathway in development. Apc (adenomatosis polyposis coli) is a critical negative regulator of this pathway and functions as a bona-fide tumor suppressor gene. In the absence of Wnt ligands, a master complex comprising Apc, GSK-3β, Axin, and CK1 phosphorylates cytoplasmic β-catenin, marking it for ubiquitination and subsequent proteasomal degradation. Wnt ligand binding to the membrane coreceptors (LRP5/6 and Frizzled) inhibits this complex, allowing nuclear translocation of dephosphorylated β-catenin, where it activates a large number of context-dependent target genes.1
Deletion of both alleles of Apc in the murine hematopoietic compartment (by the use of an Mx1-Cre–inducible Apcfl/fl model) results in marked hematopoietic defects, including bone marrow necrosis, loss of hematopoietic stem and progenitor cells (HSPCs) through apoptosis and exit from cell cycle, and failure of cell-intrinsic HSPC function.2 The Apcmin allele is a point mutation in the Apc gene (g.2549T>A, p.850L>X) that leads to a premature stop codon and formation of a truncated nonfunctional Apc protein.3,4 Homozygotes have an early embryonic lethal phenotype, whereas heterozygous Apcmin mice are viable and fertile, although with reduced life expectancy. This model has been extensively studied in the context of its analogous human disease, familial adenomatous polyposis, a hereditary condition predisposing one to multiple intestinal neoplasia. Within the hematopoietic system, Apcmin mice develop progressive thymic atrophy and loss of T cells with age.5
Furthermore, the authors of a recent report6 indicate that Apc regulates HSPC function, with increased engraftment frequency in bone marrow derived from Apcmin in competitive transplantation assays. APC is on human chromosome 5q22, a region that is frequently deleted in de novo and therapy-related myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) associated with del (5q). This observation suggests the possibility that haploinsufficiency of APC might contribute to the MDS/AML phenotype. In support of this hypothesis, haploinsufficiency of RPS147 or EGR18,9 recently has been shown to contribute to the phenotype of the “5q minus syndrome,” which is characterized by small deletions on 5q, macrocytic anemia, micromegakaryocytes, and normal-to-elevated platelets. In addition, haploinsufficiency of a number of non-5q–associated genes, such as p27Kip110 and RUNX1,11-13 have been shown to contribute to the pathogenesis of hematologic malignancies.
There is graded regulation of the hematopoietic stem cell (HSC) compartment by the canonical Wnt/β-catenin pathway. For example, constitutive activation of β-catenin through mutation of GSK-3β phosphorylation sites results in increased HSC numbers and consequent apoptosis, HSC depletion, and myeloid failure.14,15 Less-profound activation of the pathway by a selective small molecule inhibitor of GSK-3β is associated with HSC expansion with enhanced repopulating potential, sparking an interest in ex vivo HSC-expansion strategies to improve the outcome of HSPC transplantation.16 In consonance with these observations, pathway inhibition through ectopic expression of inhibitors reduces the repopulating potential of HSC.17 Somewhat paradoxically, however, there is a minimal hematopoietic phenotype observed after deletion of β-catenin (and its orthologue γ-catenin) in hematopoietic cells.18,19 This apparent contradiction may be explained by an incompletely characterized functional redundancy in that β/γ-catenin double-knockout HSCs retain the ability to transduce canonical Wnt signals.19 The noncanonical Wnt pathways also are important in the maintenance of HSC function, and exogenous Wnt4 can expand HSPC independently of β-catenin.20 Wnt5a can also enhance the repopulating potential of HSPCs.21
Wnt β-catenin signaling also regulates the cell cycle in HSCs. Either activation14,15 or inhibition of Wnt signaling22 is associated with the loss of HSC quiescence. This in turn may affect the long-term repopulating ability of HSCs.23,24 Wnt signaling is also critical in the interaction of HSCs with the bone marrow hematopoietic microenvironment. Cell extrinsic inhibition of Wnt, either through niche-specified expression of Dickkopf-122 or deletion of Secreted Frizzled-related Protein 1,25 causes loss of HSC function and HSC exit from quiescence. Finally, Wnt activation is observed in AML stem cells26 and candidate stem cells in blast crisis chronic myeloid leukemia.27
On the basis of these cumulative data, we hypothesized that HSPC function would be modulated by haploinsufficiency of APC in patients with myeloid malignancy associated with del(5q) involving the 5q22 locus. To test this hypothesis, we analyzed the HSPC compartment of Apcmin mice. We report that the Apcmin allele causes altered HSPC function in vivo and that these mice develop an age-dependent, cell-extrinsic MDS/myeloproliferative disorder (MDS/MPD). These findings have direct clinical relevance because ex vivo HSC-expansion strategies incorporating Wnt modulation are being actively pursued. In addition, we provide further evidence for a role of the hematopoietic niche in the pathogenesis of myeloid malignancies.
Methods
Apcmin mice
Apcmin mice were obtained from The Jackson Laboratory (stock number 002020) and maintained in a pathogen-free animal facility according to Children's Hospital Boston protocols. Apcmin mice are maintained in the heterozygous state and have been backcrossed against C57Bl6 inbred mice for more than 10 generations.
Peripheral blood analysis
Peripheral blood was collected by retro-orbital venous blood sampling. Peripheral blood counts were analyzed on a Hemavet analyzer (Drew Scientific). Peripheral blood films were fixed in methanol and stained with the May-Grünwald-Giemsa (Sigma-Aldrich) Romanowsky stain. Peripheral blood images were taken with a Nikon Eclipse E400 microscope and digital camera (SPOT Diagnostics, model 2.2.1).
Immunophenotype analysis
For immunophenotype analysis of bone marrow and spleen cells, mice were killed according to institutional guidelines. Bone marrow was flushed with phosphate-buffered saline containing 2% fetal bovine serum and filtered through 100-μm filters before undergoing red cell lysis (ACK lysis buffer; Lonza). All antibodies were obtained from BD Biosciences unless otherwise stated, and concentrations used are per the manufacturer's recommendation. For examination of HSPC by flow cytometry, bone marrow cells were stained with a lineage cocktail of rat anti–mouse antibodies against antigens expressed on all mature blood cells, including Ter119, CD3, CD4, CD8, CD19, B220, Mac-1, Gr-1, and IL7-R (Caltag, Invitrogen). Lineage-positive cells were detected by the use of a goat-anti–rat Pe-Cy5.5–conjugated antibody. HSPCs were then stained with combinations of cKit (clone 2B8), Sca-1 (D7), FcGRII/III (93; eBioscience), CD34 (RAM34; eBioscience), Flk2 (A2F10.1), CD150 (TC15-12F12.2), and CD48 (HM48-1). For determination of chimerism in transplantation assays, CD45.1 (A20; eBioscience) and CD45.2 (104) were additionally used. For phenotyping of lineage-positive cells within peripheral blood, bone marrow and spleen CD3 (145-2c11), Gr1 (RB6-8C5), Mac1 (M1/70), B220 (RA3-6B2), CD71 (C2), and Ter-119 (Ter-119; eBioscience) were used.
To determine HSPC populations and for flow-sorting purification, the following gating strategies were used: LKS+ (lineagelow, cKithigh, Sca-1+), long-term HSC (LKS+CD34−Flk2− and LKS+CD150+CD48−),28-30 granulocyte-macrophage progenitors (GMP; lineagelowcKithighSca-1−CD34+FcGRII/III+), common myeloid progenitors (CMP; lineagelowcKithighSca-1−CD34+FcGRII/III−), and megakaryocyte-erythroid progenitors (MEP; lineagelowcKithighSca-1−CD34−FcGRII/III−). Hoechst 33258 (H21491; Invitrogen) was used to label nonviable cells.
For intracellular flow cytometry, LKS+ cells were isolated as described previously. The cells were fixed in 1.4% paraformaldehyde and permeabilized in 100% ice-cold methanol. The cells stained with anti-β catenin (1:100; Cell Signaling; 9562) or anti–phospho-β catenin (1:100; Cell Signaling; 9561) and counterstained with Cy2 anti–rabbit secondary antibody (1:50; Jackson ImmunoResearch Laboratories). Identically prepared samples without the primary antibody were used as a negative control to determine background staining. The active fraction of β catenin (dephosphorylated) was determined by expressing a ratio of total:phosphorylated β catenin.
Cell-cycle analysis
Cell-cycle status was determined by the use of Hoechst 33342 staining (final concentration 20 μg/mL) of DNA and pyronin Y RNA staining (final concentration, 10 μg/mL) of LKS+ populations in the presence of verapamil (final concentration, 50μM) to inhibit Hoechst efflux. For analysis of cell cycle after transplantation experiments, CD45.2-positive LKS+ cells were sorted (purity > 90%) and stained with Hoechst 33342/pyronin Y.
Apoptosis analysis
Apoptosis was assessed by flow cytometry by the use of the Annexin V:PE Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer's instructions.
Colony-formation assays
Colony-formation assays were performed in cytokine-enriched methylcellulose (M3434; StemCell Technologies) according to the manufacturer's instructions. Colonies were scored according to number and morphology. All biologic replicates were performed in duplicate.
Competitive repopulation and transplantation assays
In all experiments described, the bone marrow derived from 6- to 8-week-old Apcmin or wild-type (WT) control mice expressed the CD45.2 antigen. Cells were treated with red cell lysis (ACK buffer; Lonza) and counted. For competitive transplantation experiments, 5 × 105Apcmin or WT bone marrow cells were injected together with equal numbers of CD45.1/45.2 congenic competitor bone marrow into the lateral tail vein of lethally irradiated (11 Gy in 2 separate fractions at least 3 hours apart) CD45.1 congenic recipient mice (The Jackson Laboratory). For noncompetitive transplantation experiments, 106Apcmin or WT bone marrow cells were injected into the lateral tail vein of lethally irradiated CD45.1 congenic recipient mice. For reverse transplantation, 106 CD45.1 bone marrow cells were injected into sublethally irradiated Apcmin or WT mice (a single fraction of 5.5 Gy). For MDS/MPD transplantability experiments, 106Apcmin or WT bone marrow cells were injected into sublethally irradiated CD45.1 mice.
Gene expression microarray experiments
LKS+ cells were isolated from Apcmin or WT mice by the use of high-speed multiparameter flow cytometry as described previously. At least 2 × 104 cells per mouse were isolated with confirmed purity in excess of 90%. The cells were treated with RLT lysis buffer (QIAGEN) containing beta-mercaptoethanol to stabilize RNA. RNA was extracted with the use of the QIAGEN RNeasy Micro Kit according to manufacturer's instruction. The RNA was amplified with a linear amplification protocol (Nugen Ovation V2 amplification system). cDNA was fragmented and biotinylated before hybridization onto Affymetrix mouse genome 430 2.0 Array chips. Gene set-enrichment analysis (GSEA) was performed by the use of a database of molecular signature datasets (http://www.broadinstitute.org/gsea/msigdb/index.jsp). The microarray dataset reported in this article has been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE20352 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20352).
Analysis for Apc DNA copy number
Genomic DNA was extracted from spleens of Apcmin or WT mice (Puregene; Gentra Systems Inc). The following primers were used to amplify genomic DNA and determine copy number (in comparison with WT controls). Apc1, forward, 5′-CCT CTC ACC GGA GTA AGC AG-3′, Apc1, reverse, 5′-AGG AAG AAG AGC TGG GCA AT-3′; Apc2, forward, 5′-CCA GCT GAC CTA GCC CAT AA-3′, Apc2, reverse, 5′-TCT TGC CCA CCT TTC ATT CT-3′; and Apc3, forward, 5′-AGC ACC TCT CAA TGC TTG GT-3′, Apc3, reverse, 5′-ACG GGG TAA GAC AGT TGC AC-3′.
Real-time quantitative polymerase chain reaction analysis
RNA was isolated from the bone marrow of Apcmin or WT mice (RNeasy Mini Kit; QIAGEN). Complementary DNA was synthesized (Taqman reagents), and relative transcript quantification was performed with SYBR Green reagent with the use of Applied Biosystems 7300 Real-Time PCR System and SDS software (all from Applied Biosystems). Gene expression was compared with the β-actin housekeeping gene.
Statistical analysis
Graphpad Prism, version 5.0a, was used to analyze results and create graphs. All comparisons represent 2-tailed t tests unless otherwise specified. All flow cytometry data were analyzed with FlowJo software (TreeStar).
Results
Young Apcmin mice have normal steady-state hematopoiesis
To characterize baseline hematopoietic function in young Apcmin mice, we performed retro-orbital venous blood sampling at 4, 8, and 12 weeks of age. There was no statistically significant difference in baseline blood parameters, including hematocrit (HCT), white blood cell count (WCC), neutrophil, lymphocyte, or platelet count at any of the time points (Figure 1A). Romanowsky staining of blood smears revealed no abnormalities of red blood cell, white blood cell, or platelet morphology compared with age-matched WT controls (Figure 1B). Bone marrow immunophenotype analysis showed no differences between Apcmin and WT mice in myeloid, erythroid, B lymphocyte, or T lymphocyte proportions (data not shown).
Apcmin mice have normal hematopoiesis at steady state. (A) Full blood count examination at 4, 8, and 12 weeks. (B) Peripheral blood morphology demonstrating lymphocytes (top) and neutrophils (bottom). (C) LKS+ (lineagelowcKithighSca-1+ enriched for hematopoietic stem cells) numbers as a percentage of total bone marrow cells. (D) GMP numbers. (E) CMP numbers. (F) MEP numbers. (G) Hematopoietic stem and progenitor cell numbers expressed as a percentage of LKS+ cells. Multipotent progenitors (MPP) LKS+CD34+Flk2+, short-term hematopoietic stem cells (ST-HSC) LKS+CD34+Flk2−, and long-term HSC (LT-HSC) LKS+CD34−Flk2−. (H) Long-term HSC, CD150+CD48−LKS+ expressed as a percentage of LKS+ cells. (I) Colony-forming ability of 104 bone marrow cells in cytokine-enriched methylcellulose (M3434; StemCell Technologies) and scored for colony (> 50 cells) number and morphology after 7 days. Megakaryocyte colonies (CFU-Mk), megakaryocyte-erythroid colonies (CFU-EMk), mixed multilineage colonies (CFU-GEMM), granulocyte-monocyte colonies (CFU-GM), and blast forming units-erythroid colonies (BFU-E). A total of n = 3 is shown for each condition.
Apcmin mice have normal hematopoiesis at steady state. (A) Full blood count examination at 4, 8, and 12 weeks. (B) Peripheral blood morphology demonstrating lymphocytes (top) and neutrophils (bottom). (C) LKS+ (lineagelowcKithighSca-1+ enriched for hematopoietic stem cells) numbers as a percentage of total bone marrow cells. (D) GMP numbers. (E) CMP numbers. (F) MEP numbers. (G) Hematopoietic stem and progenitor cell numbers expressed as a percentage of LKS+ cells. Multipotent progenitors (MPP) LKS+CD34+Flk2+, short-term hematopoietic stem cells (ST-HSC) LKS+CD34+Flk2−, and long-term HSC (LT-HSC) LKS+CD34−Flk2−. (H) Long-term HSC, CD150+CD48−LKS+ expressed as a percentage of LKS+ cells. (I) Colony-forming ability of 104 bone marrow cells in cytokine-enriched methylcellulose (M3434; StemCell Technologies) and scored for colony (> 50 cells) number and morphology after 7 days. Megakaryocyte colonies (CFU-Mk), megakaryocyte-erythroid colonies (CFU-EMk), mixed multilineage colonies (CFU-GEMM), granulocyte-monocyte colonies (CFU-GM), and blast forming units-erythroid colonies (BFU-E). A total of n = 3 is shown for each condition.
We next assessed the numbers of immunophenotypically defined HSPC hematopoietic numbers in Apcmin or WT mice. There was no difference in 4 bone (bilateral tibia and femur) nucleated cell count (42.2 × 106 WT vs 40.7 × 106Apcmin; P = .81). In addition, there were no differences in the number of LKS+ cells (lineagenegative, cKithigh, Sca-1+) that are enriched for HSCs (Figure 1C). There were no differences observed in more mature myeloid progenitors, including GMPs, CMPs, or MEPs (Figure 1D-F, respectively). We further fractioned the HSC compartment and observed no difference in the frequency of LKS+ CD34−Flk2− (enriched for long-term HSC), LKS+CD34+Flk2− (containing short-term HSC), LKS+Flk2+ (multipotent progenitor) cells (Figure 1G) or LKS+CD150+CD48− (Figure 1H).28,29 In addition, there was no difference in the ability of Apcmin bone marrow to form colonies in cytokine-enriched methylcellulose (M3434; StemCell Technologies), and colonies had normal lineage commitment as defined by colony morphology (Figure 1I). There was no significant difference in cell-cycle status of LKS+ cells between Apcmin and WT mice at baseline (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Taken together, these data demonstrate that young Apcmin mice have normal hematopoiesis.
Apcmin bone marrow has enhanced repopulating potential in competitive bone marrow transplantation assays
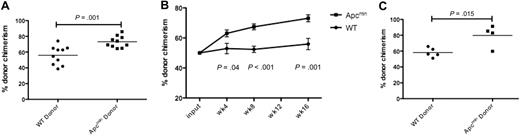
We next performed competitive bone marrow transplantation to assess HSC function. A total of 5 × 105 bone marrow cells from Apcmin or WT donors (expressing CD45.2) were mixed with equal numbers of cells derived from CD45.1/CD45.2 double-positive congenic competitor marrow and injected into lethally irradiated congenic recipients expressing CD45.1. We observed a statistically significant enhanced chimerism of Apcmin bone marrow evident by 4 weeks that persisted at 8 and 16 weeks after transplantation (Figure 2A-B). To confirm that the peripheral blood chimerism reflected the activity of primitive stem and progenitor cell populations, we assessed the chimerism of LKS+ cells in the bone marrow 16 weeks after transplantation. Consistent with the peripheral blood findings, there was a statistically significant increase in CD45.2 chimerism in bone marrow LKS+ cells (Figure 2C). The increased repopulating potential in the context of normal HSPC numbers indicates that Apcmin mice have intrinsic enhancement of short-term and long-term HSC function.
Apcmin bone marrow has enhanced repopulating potential. (A) Whole-blood chimerism (expressed as the fraction of CD45.2-positive donor cells divided by CD45.2 and CD45.1/2 competitor cells) 16 weeks after transplantation into lethally irradiated 45.1 recipients. Representative data of 2 independent experiments. Apcmin 73.1% versus WT 55.9% chimerism, P = .001 (B) Whole-blood chimerism of transplant recipients at 4, 8, and 16 weeks after transplantation. Mean ± SEM (C) Bone marrow chimerism, gated on LKS+ cells 16 weeks after transplantation into lethally irradiated recipients. Apcmin 79.8% versus 58.2% WT, P = .015
Apcmin bone marrow has enhanced repopulating potential. (A) Whole-blood chimerism (expressed as the fraction of CD45.2-positive donor cells divided by CD45.2 and CD45.1/2 competitor cells) 16 weeks after transplantation into lethally irradiated 45.1 recipients. Representative data of 2 independent experiments. Apcmin 73.1% versus WT 55.9% chimerism, P = .001 (B) Whole-blood chimerism of transplant recipients at 4, 8, and 16 weeks after transplantation. Mean ± SEM (C) Bone marrow chimerism, gated on LKS+ cells 16 weeks after transplantation into lethally irradiated recipients. Apcmin 79.8% versus 58.2% WT, P = .015
To determine whether this gain of function was intrinsic to HSC and to exclude a contribution by supporting cells that may have facilitated engraftment, we transplanted highly purified HSC subsets (150 cells, long-term HSC, lineagelowcKit+Sca-1+CD48−CD150+) in competition with whole bone marrow (3 × 105 cells) into lethally irradiated CD45.1 recipients. At 16 weeks after transplantation, there was a strong trend toward increased chimerism in the recipients of Apcmin long-term HSC, although there was no difference found at earlier time points. These data indicate that Apcmin mice have enhanced HSC function that is intrinsic to immunophenotypically defined LT-HSC (supplemental Figure 2).
To confirm a primary role for activation of the Wnt signaling pathway in this phenotype, we isolated LKS+ cells from Apcmin or WT hosts and performed genome-wide transcriptional profiling followed by GSEA.31 We assessed candidate pathways that might contribute to altered self-renewal in this compartment, including the canonical Wnt, Notch, Bmi-1, and Hedgehog pathways. Apcmin LKS+ cells showed significant enrichment for Wnt pathway members (P = .02, false-discovery rate = .03) by GSEA; however, there was no enrichment for Notch, Hedgehog, or Bmi-1 (supplemental Figure 3). To confirm that canonical Wnt signaling was activated, we performed intracytoplasmic staining for βcatenin and phosphorylated β-catenin in LKS+ cells. There was an increase in the active (dephosphorylated) β-catenin fraction in Apcmin LKS+ cells (supplemental Figure 4). Finally, there was increased expression of Lef1 in bone marrow from Apcmin mice, determined by real-time polymerase chain reaction analysis (supplemental Figure 5). These data are consistent with a role for Wnt pathway activation in enhancing HSC function in Apcmin mice. Collectively, these data would support a potential role for pathway activation ex vivo as a strategy for therapeutic expansion of the HSC compartment.
Apcmin bone marrow has impaired repopulating potential in secondary recipients
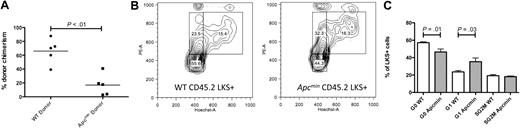
To assess the more subtle effects of haploid loss of Apc in the murine hematopoietic compartment, we performed 2 rounds of noncompetitive serial transplantation of Apcmin or WT bone marrow into congenic recipient mice. Lethally irradiated primary recipients (expressing CD45.1) that had received 106Apcmin or WT bone marrow cells (expressing CD45.2) without competitor bone marrow were killed after 16 weeks, and the bone marrow was harvested. At 16 weeks after transplantation, there was no difference in peripheral blood counts, recipient CD45.2 chimerism, or LKS+ chimerism between recipients of Apcmin or WT marrow (LKS+ chimerism 84.9% WT vs 87.4% Apcmin; P = .66). To determine the long-term repopulating potential of Apcmin bone marrow in secondary recipients, bone marrow from the primary recipients was injected into lethally irradiated secondary recipients. However, of considerable interest, the secondary recipients of Apcmin bone marrow had significantly reduced donor chimerism at 16 weeks after secondary transplantation (Figure 3A). This reduced chimerism was sustained after 26 weeks (CD45.2 chimerism 8.5% Apcmin vs 54.9% WT; P < .01).
Apcmin bone marrow has impaired repopulating potential in secondary recipients. (A) Whole-blood chimerism 16 weeks after transplantation of Apcmin or WT bone marrow into lethally irradiated secondary CD45.1 recipients. CD45.2 chimerism 16.9% Apcmin versus 66% WT P < .01, representative data from 2 experiments. (B) Cell-cycle analysis on CD45.2+LKS+ cells 16 weeks after transplantation. Hoechst 33342 (HoechstA, x-axis) and pyronin Y (PyY, y-axis) were used to resolve G0 (quiescent, HoelowPyYlow), G1 (HoelowPyYhigh), and SG2M (cycling HoehighPyYhigh) populations. (C) Histogram representation of cell-cycle data, expressed as the percentage of CD45.2LKS+ cells in each gate. Quiescent fraction 42.8% Apcmin versus 53.4% WT, P = .01, G1 phase 32.6% Apcmin versus 22.1% WT, P = .03.
Apcmin bone marrow has impaired repopulating potential in secondary recipients. (A) Whole-blood chimerism 16 weeks after transplantation of Apcmin or WT bone marrow into lethally irradiated secondary CD45.1 recipients. CD45.2 chimerism 16.9% Apcmin versus 66% WT P < .01, representative data from 2 experiments. (B) Cell-cycle analysis on CD45.2+LKS+ cells 16 weeks after transplantation. Hoechst 33342 (HoechstA, x-axis) and pyronin Y (PyY, y-axis) were used to resolve G0 (quiescent, HoelowPyYlow), G1 (HoelowPyYhigh), and SG2M (cycling HoehighPyYhigh) populations. (C) Histogram representation of cell-cycle data, expressed as the percentage of CD45.2LKS+ cells in each gate. Quiescent fraction 42.8% Apcmin versus 53.4% WT, P = .01, G1 phase 32.6% Apcmin versus 22.1% WT, P = .03.
Wnt signaling is known to regulate the cell cycle of HSC,14,15,22,24,25 and the preservation of a quiescent HSC population is essential for efficient transplantation.22,23 Therefore, we hypothesized that the reduced contribution of Apcmin bone marrow in secondary repopulation assays might be caused by a loss of quiescence in Apcmin HSC that is only seen after the stress of transplantation. To test this hypothesis, we examined the cell-cycle status of LKS+ cells 16 weeks after primary transplantation into lethally irradiated recipient mice by the use of Hoechst 33342 and pyronin Y staining. In contrast to baseline hematopoiesis, where no difference was observed (supplemental Figure 1), Apcmin LKS+ cells showed exit from quiescence after 16 weeks of transplantation (Figure 3B-C). To determine whether Apcmin marrow also demonstrates impaired stress response in other contexts, we treated Apcmin or WT mice with the 5-fluorouracil (5-FU, 150 mg/kg) and monitored leukocytopenia. There was no difference in the depth or duration of the leukocyte nadir after 5-FU treatment. Furthermore, there was no difference in apoptosis determined by annexin V flow cytometry staining of peripheral blood leukocytes 5 days after 5-FU (supplemental Figure 6). These observations indicate that the Apcmin mutation alters HSC quiescence and results in impaired function of the long-term HSC compartment.
Aged Apcmin mice develop MDS/MPD
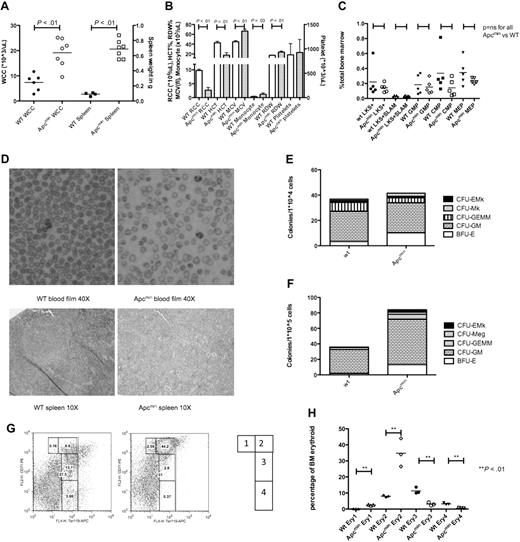
Long-term analysis revealed that aged Apcmin mice developed leukocytosis and splenomegaly (median age, 182 days; range, 136-210 days; Figure 4A). These animals demonstrated neutrophilia and monocytosis with macrocytic anemia and anisopoikilocytosis. Platelet numbers were not significantly different from control mice (Figure 4B). To determine whether these phenotypic differences were a consequence of changes in defined primitive populations or lineage skewing within the bone marrow HSPC compartment, we performed immunophenotypic characterization. There were no differences in LKS+, LT-HSC (LKS+CD150+CD48−), GMP, CMP, or MEP populations (Figure 4C).
Aged Apcmin mice develop MDS/MPD. (A) White cell count (WCC × 103/μL) and spleen weight (mg) from Apcmin mice with MDS/MPD. WCC 19.2 × 103/μL Apcmin versus 7.4 × 103/μL WT, P < .01; neutrophil count 5.7 × 103/μL Apcmin versus 1.6 × 103/μL WT, P < .01; and spleen weight 0.69 g Apcmin versus 0.09 g WT, P < .01. (B) Peripheral blood parameters, including red cell count (RCC × 106/μL), hematocrit (HCT%), mean corpuscular volume (MCV fl), monocyte count (×103/μL), red cell distribution width (RDW%), and platelet count (×103/μL). Red cell count: 2.7 × 106/μL Apcmin versus 9.7 × 106/μL WT, P < .01; HCT: 17.4% Apcmin versus 42.8% WT, P < .01; MCV: 66.5 fl Apcmin versus 45 fl WT P < .01; monocyte count: 1.2 × 103/μL Apcmin versus 0.3 × 103/μL WT, P = .03; RDW: 23.7% Apcmin versus 17.3% WT, P < .01. (C) Hematopoietic stem and progenitor cell numbers expressed as a percentage of total bone marrow. LKS+ (lineagelowcKithighSca-1+ enriched for HSCs), LKS+SLAM (LKS+CD150+CD48− highly enriched for long-term HSC), GMPs, CMPs, and MEPs. (D) Peripheral blood films (magnification ×40) and splenic histology (magnification ×10) demonstrating macrocytic anemia with anisopoikilocytosis in Apcmin mice and effacement of normal splenic architecture by extramedullary hematopoiesis (images taken with Nikon Eclipse E400 microscope and digital camera; SPOT Diagnostic Instruments, model 2.2.1). (E) Colony-formation assays in cytokine-enriched methylcellulose from 104 bone marrow cells and (F) 105 spleen cells. Megakaryocyte colonies (CFU-Mk), megakaryocyte-erythroid colonies (CFU-EMk), mixed multilineage colonies (CFU-GEMM), granulocyte-monocyte colonies (CFU-GM), and blast forming units-erythroid colonies (BFU-E). Bone marrow: 41.5 Apcmin versus 36.8 colonies/104 cells WT, P = .35; BFU-E: 10.2 Apcmin versus 3.3 colonies/104 cells WT, P < .01; spleen: 84.2 Apcmin versus 35.8 colonies/105 cells WT, P < .01; CFU-GM: 58.8 Apcmin versus 30.8 colonies/105 cells WT, P < .01; BFU-E: 13.2 Apcmin versus 1.8 colonies/105 cells WT, P < .01). (G) Erythroid maturation flow cytometric analysis. Ery1 (CD71high, Ter119mid), Ery2 (CD71high, Ter119high), Ery3 (CD71mid, Ter119high), and Ery4 (CD71low, Ter119high). (H) Graphical representation of erythroid maturation demonstrating shift toward immature (Ery1/2) development. 2.5% and 34.8% Apcmin versus 0.2% and 8.0% WT for Ery1 and Ery2, respectively, P < .01; 3.1% and 0.6% Apcmin versus 11.4% and 3.2% WT for Ery3 and Ery4, respectively, P < .01.
Aged Apcmin mice develop MDS/MPD. (A) White cell count (WCC × 103/μL) and spleen weight (mg) from Apcmin mice with MDS/MPD. WCC 19.2 × 103/μL Apcmin versus 7.4 × 103/μL WT, P < .01; neutrophil count 5.7 × 103/μL Apcmin versus 1.6 × 103/μL WT, P < .01; and spleen weight 0.69 g Apcmin versus 0.09 g WT, P < .01. (B) Peripheral blood parameters, including red cell count (RCC × 106/μL), hematocrit (HCT%), mean corpuscular volume (MCV fl), monocyte count (×103/μL), red cell distribution width (RDW%), and platelet count (×103/μL). Red cell count: 2.7 × 106/μL Apcmin versus 9.7 × 106/μL WT, P < .01; HCT: 17.4% Apcmin versus 42.8% WT, P < .01; MCV: 66.5 fl Apcmin versus 45 fl WT P < .01; monocyte count: 1.2 × 103/μL Apcmin versus 0.3 × 103/μL WT, P = .03; RDW: 23.7% Apcmin versus 17.3% WT, P < .01. (C) Hematopoietic stem and progenitor cell numbers expressed as a percentage of total bone marrow. LKS+ (lineagelowcKithighSca-1+ enriched for HSCs), LKS+SLAM (LKS+CD150+CD48− highly enriched for long-term HSC), GMPs, CMPs, and MEPs. (D) Peripheral blood films (magnification ×40) and splenic histology (magnification ×10) demonstrating macrocytic anemia with anisopoikilocytosis in Apcmin mice and effacement of normal splenic architecture by extramedullary hematopoiesis (images taken with Nikon Eclipse E400 microscope and digital camera; SPOT Diagnostic Instruments, model 2.2.1). (E) Colony-formation assays in cytokine-enriched methylcellulose from 104 bone marrow cells and (F) 105 spleen cells. Megakaryocyte colonies (CFU-Mk), megakaryocyte-erythroid colonies (CFU-EMk), mixed multilineage colonies (CFU-GEMM), granulocyte-monocyte colonies (CFU-GM), and blast forming units-erythroid colonies (BFU-E). Bone marrow: 41.5 Apcmin versus 36.8 colonies/104 cells WT, P = .35; BFU-E: 10.2 Apcmin versus 3.3 colonies/104 cells WT, P < .01; spleen: 84.2 Apcmin versus 35.8 colonies/105 cells WT, P < .01; CFU-GM: 58.8 Apcmin versus 30.8 colonies/105 cells WT, P < .01; BFU-E: 13.2 Apcmin versus 1.8 colonies/105 cells WT, P < .01). (G) Erythroid maturation flow cytometric analysis. Ery1 (CD71high, Ter119mid), Ery2 (CD71high, Ter119high), Ery3 (CD71mid, Ter119high), and Ery4 (CD71low, Ter119high). (H) Graphical representation of erythroid maturation demonstrating shift toward immature (Ery1/2) development. 2.5% and 34.8% Apcmin versus 0.2% and 8.0% WT for Ery1 and Ery2, respectively, P < .01; 3.1% and 0.6% Apcmin versus 11.4% and 3.2% WT for Ery3 and Ery4, respectively, P < .01.
These observations had phenotypic similarity to certain aspects of human MDS/MPD. In addition, splenic histology showed effacement of the normal white-red pulp architecture caused by extramedullary hematopoiesis (Figure 4D). To determine the relative lineage contributions to bone marrow hematopoiesis and splenic infiltration, we performed colony assays in cytokine-enriched methylcellulose media (M3434; StemCell Technologies). There were no differences in the absolute numbers of bone marrow colonies; however, there was evidence of erythroid lineage skewing present (Figure 4E). In contrast, there were increased total myeloid colonies in the spleen of Apcmin mice as well as increased granulocytic and erythroid colonies (Figure 4F).
Finally, because there was preservation of erythroid colony formation with macrocytic anemia and abnormal red cell morphology, we used flow cytometry to more carefully examine the bone marrow erythroid maturation in Apcmin mice with MDS/MPD. The stages of maturation were defined as Ery1 (CD71high, Ter119mid), Ery2 (CD71high, Ter119high), Ery3 (CD71mid, Ter119high), and Ery4 (CD71low, Ter119high). There was a marked shift toward immature erythropoiesis in Apcmin mice with increased Ery1 and Ery2 and a block in later stages Ery3 and Ery4 maturation (Figures 4G-H). Taken with the aforementioned granulocytic and erythroid findings, the increased bone marrow erythropoiesis with abnormal maturation, macrocytic anemia and abnormal red cell morphology shows features of both MDS and MPD.
To assess the possibility that this phenotype was associated with loss of function of the second Apc allele, we assayed for evidence of genomic deletion of the Apc locus by quantitative polymerase chain reaction on genomic DNA derived from mice with evidence of MDS/MPD. There was no evidence for genomic deletions involving the residual WT Apc gene in any of the Apcmin MDS/MPD mice examined. Furthermore, there was no evidence of loss of heterozygosity in the remaining WT Apc allele, arguing against a secondary event leading to WT Apc inactivation (n = 4; supplemental Figure 7).
APC is located on chromosome 5q22, immediately proximal to the common deleted region in 5q minus syndrome; however, its role in MDS pathogenesis has not been characterized.8 To determine whether findings described in Apcmin mice have implications for human 5q− patients, we examined the expression of APC within a published dataset using CD34+-selected primary bone marrow cells from patients with 5q− syndrome compared with healthy control patients and patients with a low-risk MDS subtype (refractory anemia).32,33 In total, samples from 55 patients with MDS and 11 healthy control patients were analyzed, including 20 patients with 5q− syndrome. Expression level was according to Affymetrix GeneChip Human Genome U133 Plus 2.0 expression arrays. There was reduced expression of APC in cases of MDS with del(5q) (median APC expression decreased by 45%, P < .001), which is consistent with haploinsufficient expression of APC without deletion or epigenetic silencing of the intact allele (supplemental Figure 8). These data suggest that deletion of APC contributes to hematologic abnormalities observed in patients with 5q minus syndrome.
The MDS/MPD that develops in aged Apcmin mice is predominantly cell extrinsic
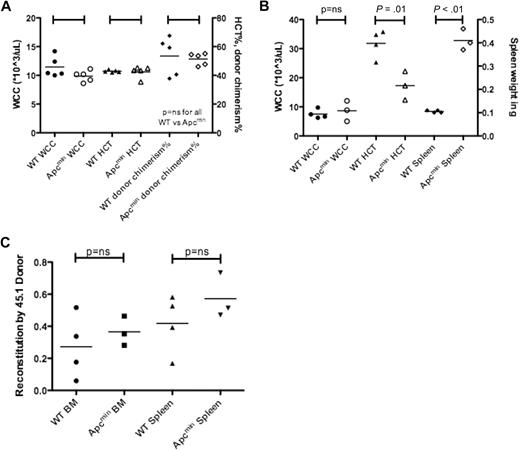
We next performed transplantation experiments to determine whether the MDS/MPD observed in Apcmin mice was a result of cell-intrinsic or cell-extrinsic factors. A total of 1 × 106 bone marrow cells from aged Apcmin mice with MDS/MPD or age-matched WT controls expressing CD45.2 were transplanted into sublethally irradiated WT congenic recipients expressing CD45.1. There were no significant differences after 16 weeks in WCC, HCT, or chimerism of donor-derived cells (Figure 5A).5 Further analysis of these mice after 26 weeks did not reveal any differences in WCC, HCT, platelet count, or chimerism (data not shown). This observation indicated that the MDS/MPD phenotype observed in aged Apcmin mice was not transplantable.
Apcmin MDS/MPD is predominantly cell extrinsic. (A) Transplantation of 106 bone marrow cells from Apcmin MDS/MPD into sublethally irradiated CD45.1 recipients. White cell count (WCC × 103/μL), hematocrit (HCT%), and donor cell chimerism (%)16 weeks after transplantation. WCC: 9.9 × 103/μL Apcmin versus 11.5 × 103/μL WT, P = .12; HCT: 42.5% Apcmin versus 42.9% WT, P = .81; 51.4% Apcmin versus 53.4% WT, P = .75. (B) Bone marrow transplantation of 1 × 106 CD45.1 congenic bone marrow cells into sublethally irradiated Apcmin or WT recipients. Sublethal irradiation was used because of previous reports describing intolerance of lethal irradiation in Apcmin mice.5 WCC × 103/μL, HCT%, spleen weight (g) WCC: 8.64 × 103/μL Apcmin versus 7.53 × 103/μL WT, P = .59; HCT: 17.3% Apcmin versus 31.8% WT, P = .01; spleen: 0.41 g Apcmin versus 0.11 g WT, P < .01. (C) Peripheral blood parameters, including red cell count (RCC × 106/μL), hematocrit (HCT%), mean corpuscular volume (MCV fl), mean corpuscular hemoglobin (pg), red cell distribution width (RDW%), and platelet count (×103/μL).
Apcmin MDS/MPD is predominantly cell extrinsic. (A) Transplantation of 106 bone marrow cells from Apcmin MDS/MPD into sublethally irradiated CD45.1 recipients. White cell count (WCC × 103/μL), hematocrit (HCT%), and donor cell chimerism (%)16 weeks after transplantation. WCC: 9.9 × 103/μL Apcmin versus 11.5 × 103/μL WT, P = .12; HCT: 42.5% Apcmin versus 42.9% WT, P = .81; 51.4% Apcmin versus 53.4% WT, P = .75. (B) Bone marrow transplantation of 1 × 106 CD45.1 congenic bone marrow cells into sublethally irradiated Apcmin or WT recipients. Sublethal irradiation was used because of previous reports describing intolerance of lethal irradiation in Apcmin mice.5 WCC × 103/μL, HCT%, spleen weight (g) WCC: 8.64 × 103/μL Apcmin versus 7.53 × 103/μL WT, P = .59; HCT: 17.3% Apcmin versus 31.8% WT, P = .01; spleen: 0.41 g Apcmin versus 0.11 g WT, P < .01. (C) Peripheral blood parameters, including red cell count (RCC × 106/μL), hematocrit (HCT%), mean corpuscular volume (MCV fl), mean corpuscular hemoglobin (pg), red cell distribution width (RDW%), and platelet count (×103/μL).
Transplantation of WT (CD45.1) bone marrow into sublethally irradiated Apcmin or WT recipients showed no difference in WCC after 16 weeks; however, there was significantly reduced hematocrit and splenomegaly (Figure 5B). These data indicate that the MDS/MPD phenotype was predominantly a consequence of cell-extrinsic factors. However, it is important to note that there was residual host hematopoiesis present, and therefore an effect modulated by residual host HSC cannot be excluded. For example, the residual Apcmin hematopoietic cells may have had paracrine effects on neighboring WT HSC or progenitors.
To further ascertain whether the effects were cell extrinsic, we measured the contribution of host and donor cells to the complement of spleen hematopoiesis. There were no differences in the contribution to hematopoiesis by CD45.1 donor cells, in either bone marrow or splenic hematopoietic cells (supplemental Figure 9). Finally, we observed the same red cell phenotypic abnormalities in the recipients of reverse transplanted bone marrow (reduced red cell count, macrocytosis with anisopoikilocytosis; Figure 5C). Taken together, these data indicate that the MDS/MPD observed in Apcmin mice is predominantly cell extrinsic in origin.
The endosteal surface of trabecular bone represents an important niche for HSC,34 and trabecular bone loss has been associated with cell-extrinsic myeloid dyscrasias.35 To further explore potential mechanisms of the cell-extrinsic changes, we examined the HSC niche of Apcmin mice. Apcmin mice with MDS/MPD had similar trabecular bone and osteoblast appearance on histologic sections of bone marrow, arguing against disruption of the niche as a primary mechanism of the MDS/MPD (supplemental Figure 10). These data suggest that an alteration in circulating factors, rather than architectural modifications in the bone marrow microenvironment, may cause the cell-extrinsic MDS/MPD.
Discussion
The canonical Wnt-β-catenin signaling pathway has diverse and important roles in hematopoiesis and leukemia, ranging from regulation of self-renewal and cell cycle to HSC-niche interactions and pathologic self-renewal in candidate leukemia stem cells. We have observed a cell-intrinsic gain-of-function in Apcmin HSC in primary transplantation assays, evidenced by increased chimerism in competitive transplant recipients using whole bone marrow and highly purified LT-HSC populations. Associated with this, we have observed strong enrichment for Wnt pathway members in gene set-enrichment analysis. These findings are consistent with a recent report6 in which the authors described increased long-term engraftment in Apcmin mice through activation of the Wnt pathway and would at face value provide support for pharmacologic augmentation of the Wnt signaling pathway as a potential therapeutic modality in transplantation medicine.
However, more detailed analysis raises concerns about such strategies. In particular, we observed reduced repopulation in secondary bone marrow transplant recipients as the result of an exit from quiescence of enriched HSC-containing populations (LKS+), although no cell-cycle abnormalities were present at baseline. These data would suggest that transplantation of Apcmin bone marrow and enhanced repopulation of the recipient mice may result in additional HSC cell cycling and exit from quiescence. These HSC that have a modest augmentation of Wnt signaling may have a slow burnout phenotype, akin to premature aging caused by augmented Wnt signaling in other model systems.36 These data would suggest that ex vivo HSC-expansion strategies on the basis of enhanced Wnt signaling should be carefully evaluated for effects on long-term HSC function.
Our findings provide a new model for MDS/MPD because Apcmin mice display abnormal erythroid maturation and extramedullary hematopoiesis but no expansion of HSPC numbers. Cell extrinsic MPD has been described with deficiency of the retinoic acid receptor γ37 or the retinoblastoma protein.35 In the former example, the MPD is mediated by increased expression of the proinflammatory cytokine tumor necrosis factor α and was associated with marked changes in the architecture of trabecular bone. Unlike these previous models,35 we did not see overt changes within the bone marrow microenvironment architecture. This may be because there are abnormalities in the systemic environment that are having their effects directly on hematopoietic cells, rather than primary disruption of the bone marrow microarchitecture. Recently, a similar mechanism leading to alterations in hematopoiesis has been noted in a telomerase deficient mouse model.38 Alternatively, there may be abnormal proliferative signals arising from morphologically normal osteoblasts, other niche components, or paracrine contributions from the residual Apcmin hematopoietic cells. WT Apc allelic loss is common in intestinal adenomas39 ; however, we did not find evidence of genomic deletions in the hematopoietic system of Apcmin mice developing MDS/MPD.
The APC gene is located on chromosome 5q22. Large deletions of 5q are found in patients with MDS and are associated with an adverse prognosis. Although APC lies proximal to the region at band 5q31 that is commonly deleted in 5q− MDS,40-42 neighboring genes are invariably codeleted and may have important disease-modifying functions. Our data demonstrate that APC haploinsufficiency is frequently observed in patients with MDS and provide evidence that codeletion of APC may contribute to the dysplastic phenotype or may be an important disease-modifying allele. Although it is not immediately clear how cell-intrinsic deletion of Apc may lead to a predominantly cell-extrinsic phenotype, recently published data support the tenet that bone marrow niche cells from patients with MDS may have chromosomal instability.43 The direct relevance of the cell-extrinsic phenotype contributions to human disease would be aided by additional correlative studies. For example, a detailed systematic analysis of the hematologic system of patients with familial adenomatous polyposis has not been reported to our knowledge. Interestingly, however, a recent report has documented that treatment with lenalidomide (an effective agent in 5q− syndrome) was beneficial to the ability of patient-derived stromal cells to support hematopoiesis.44
Our data support a role for Wnt signaling, mediated by Apc, in cell-intrinsic HSC function and have implications for the use of Wnt modulation in ex vivo HSC manipulation and transplantation strategies. Furthermore, the development of a MDS/MPD in Apcmin mice provides new evidence that cell extrinsic control of HSC function can contribute to the development of myeloid disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the technical advice and insightful comments from Drs Claudia Scholl, Stefan Fröhling, Ann Mullally, Demetrios Kalaitzidis, Michael Kharas, Peter Miller, and members of the Gilliland and Williams laboratories.
S.W.L. has received funding support from the National Health and Medical Research Council Australia, Australia/US Fulbright Commission, Hematology Society of Australia and New Zealand, and Royal Brisbane and Women's Hospital Foundation. D.A.W. has received funding support from the US National Institutes of Health (DK62757). D.G.G. received funding support from the US National Institutes of Health, the Howard Hughes Medical Institute, the Leukemia & Lymphoma Society, and the Myeloproliferative Disorders Foundation.
Authorship
Contribution: S.W.L., S.M.S., B.L.E., D.A.W., and D.G.G. designed experiments; S.W.L., S.M.S., F.A.-S., S.S., M.P., C.L.C., and J.L.J. performed experiments and analyzed data; S.W.L. wrote the manuscript; and all authors provided critical review of the manuscript.
Conflict-of-interest disclosure: D.G.G is now an employee of Merck. The remaining authors declare no competing financial interests.
Correspondence: Steven W. Lane or D. Gary Gilliland, Department of Hematology, Brigham and Women's Hospital, Harvard Medical School, Karp 8, 1 Blackfan Cir, Boston, MA 02115; e-mail: Steven.lane@childrens.harvard.edu or ggilliland@rics.bwh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal