Abstract
Loss of a whole chromosome 5 or a deletion of the long arm of chromosome 5, −5/del(5q), is a recurring abnormality in myeloid neoplasms. The APC gene is located at chromosome band 5q23, and is deleted in more than 95% of patients with a −5/del(5q), raising the question of whether haploinsufficiency of APC contributes to the development of myeloid neoplasms with loss of 5q. We show that conditional inactivation of a single allele of Apc in mice leads to the development of severe anemia with macrocytosis and monocytosis. Further characterization of the erythroid lineage revealed that erythropoiesis is blocked at the early stages of differentiation. The long-term hematopoietic stem cell (LT-HSC) and short-term HSC (ST-HSC) populations are expanded in Apc-heterozygous mice compared with the control littermates; however, the HSCs have a reduced capacity to regenerate hematopoiesis in vivo in the absence of a single allele of Apc. Apc heterozygous myeloid progenitor cells display an increased frequency of apoptosis, and decreased in vitro colony-forming capacity, recapitulating several characteristic features of myeloid neoplasms with a −5/del(5q). Our results indicate that haploinsufficiency of Apc impairs hematopoiesis, and raise the possibility that loss of function of APC contributes to the development of myelodysplasia.
Introduction
Myelodysplastic syndromes (MDSs) are a group of heterogeneous disorders of hematopoietic stem cells (HSCs) characterized by blood cytopenias due to ineffective hematopoiesis.1 Approximately 30% to 40% of primary MDS transforms to acute myeloid leukemia (AML).1 MDS can arise de novo or as a result of previous cytotoxic therapy (t-MDS), including chemotherapy, radiation therapy, and immunosuppressive therapy. Recurring karyotypic abnormalities, including −5/del(5q), −7/del(7q), +8, and del(20q), have been identified in approximately 50% of patients with a primary MDS.2,3 Loss of a whole chromosome 5 or a del(5q) are among the most common recurring cytogenetic abnormalities, and are noted in approximately 10% to 15% of patients with primary MDS or AML de novo, and in more than 40% of patients with t-MDS/t-AML.2,4 MDS with an isolated del(5q) (formerly termed the 5q− syndrome) is a distinct subtype of MDS, characterized by macrocytosis, anemia, and a low rate of leukemic transformation.5 In contrast, the advanced stages of MDS, AML de novo, or t-MDS/t-AML with −5/del(5q) are characterized by complex karyotypes, a poor prognosis, and relative resistance to conventional therapies.4,6,7 These features suggest that additional acquired genetic alterations occur in the advanced stages of MDS or t-MDS/t-AML, accelerating the progression of the disease.
Two commonly deleted segments (CDSs) within 5q33.1 and 5q31 have been identified by cytogenetic analysis of MDS with an isolated del(5q) (5q33.1), or MDS, AML de novo, and t-MDS/t-AML with a del(5q) (5q31).8,9 Thus far, biallelic deletions or inactivating mutations have been not reported in genes located in the 5q CDSs8-10 The existing data support a haploinsufficiency model, in which loss of a single allele of 1 or more genes on 5q contributes to the pathogenesis of MDS or t-MDS/t-AML with a −5/del(5q).10,11 A number of genes located on 5q, including RPS14,12 EGR1,13 NPM1,14 and CTNNA1,15 have been implicated in the development of myeloid disorders due to a gene dosage effect.
The APC tumor suppressor gene is located at chromosome band 5q23, and loss of a single allele of APC occurs in more than 95% of patients with myeloid neoplasms and −5/del(5q). Loss of function of APC is responsible for the initiation and progression of colorectal cancer.16 APC, a multifunctional protein, is involved in the regulation of the Wnt signaling pathway via its ability to control the degradation of β-catenin.16 Other cellular processes in which APC plays a role include cell migration, cell adhesion, spindle assembly, and chromosome segregation.17 Recently, down-regulation of Apc has been implicated in activation of the mTOR pathway, independent of β-catenin–dependent transcription.18 Using a conditional knockout mouse model, we demonstrated that Apc is a critical regulator of hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) survival. Loss of Apc results in rapid exhaustion of HSCs and HPCs, leading to bone marrow failure in mice.19 These mice displayed defective erythroid and myeloid differentiation. In this study, we used this conditional model to determine whether loss of a single allele of Apc contributes to the development of myeloid disorders. Our data reveal that Apc haploinsufficient mice develop a hematologic disorder that recapitulates several characteristic features of human MDS.
Methods
Apc-mutant mice
Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice were generated by crossing Apc fl/fl mice20 with Mx1-Cre transgenic mice.21 Genotyping was performed by polymerase chain reaction (PCR) analysis of tail DNA using primers P3 (5′-GTTCTGTATCATGGAAAGATAGGTGGTC-3′), P4 (5′-CACTCAAAACGCTTTTGAGGGTTGATTC-3′), and P5 (5′-GAGTACGGGGTCTCTGTCTCAGTGAA-3′).20 To induce deletion of exon 14 of Apc, mice were injected intraperitoneally with pI-pC 2 or 3 times (6-10 μg per gram of body weight, every 2 days) at 1 to 2 months of age. The efficiency of deletion in hematopoietic cells was verified by PCR using primers P3 and P4. The expression of Apc in HSCs and subsets of myeloid progenitors, including common myeloid progenitors (CMPs), as well as more restricted granulocyte-monocyte progenitors and megakaryocyte-erythroid progenitors (GMPs and MEPs) after induction of Apc deletion, was analyzed by quantitative reverse transcriptase (qRT)–PCR using primers A (5′-ACAAGACGGCAGCTGGAGTATGAA-3′) and B (5′-TGGATCCTGGCTATTCTTCGCTGT-3′). Bone marrow cells were collected from mice 2 months of age or older, and HSCs and HPCs were isolated by flow sorting. All animal protocols were approved by the animal care and use committee of the University of Chicago.
Histology and peripheral blood analyses
Bone marrow (BM), spleen (SP), and peripheral blood (PB) smears were stained with May-Gruenwald-Giemsa. Peripheral blood was collected by tail-bleeding. The white blood cell (WBC), red blood cell (RBC), and platelet counts; hemoglobin level; and WBC differentials were determined with a Hemavet counter (CDC Technologies).
Colony-forming unit assays
For colony-forming unit (CFU) assays, total BM cells, isolated from Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice 2 months after induction, were plated in duplicate in methylcellulose medium (Methocult M3434) supplemented with IL-3, IL-6, Epo, and SCF, and scored after 10 days. Burst-forming unit-erythroid (BFU-E) and CFU-erythroid (CFU-E) assays were performed using the protocols provided by StemCell Technologies. The CFU-E colonies were scored in 2 to 3 days, whereas BFU-E colonies were scored after 10 days.
Flow cytometric analysis
Single-cell suspensions from BM, SP, and PB were stained with the indicated fluorochrome-conjugated antibodies. Flow cytometric analysis of HSCs and subsets of HPCs has been described previously.22,23 Dead cells were excluded by DAPI or propidium iodide staining. Flow cytometry was performed using BD FACSCanto or LSRII flow cytometers. Cell-cycle analysis with DAPI staining was performed as described previously.24 For the detection of apoptosis, BM or SP cells were stained with antibody conjugates, Annexin V and 7-ADD, or DAPI. All data were analyzed by FlowJo software (TreeStar).
Competitive repopulation assay
BM cells (2 × 106, CD45.2+) from Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+ (control) mice 4 weeks after 3 injections of pI-pC were mixed 1:1 with the competitor BM cells (CD45.1+CD45.2+) from wild-type mice, and transplanted into lethally irradiated (9.6 Gy [960 rad]) B6.SJL (CD45.1+) mice by retro-orbital injection.
Results
Loss of a single allele of Apc leads to a lethal anemia with monocytosis and macrocytosis
Loss of a single allele of APC occurs in more than 95% of patients with a myeloid neoplasm characterized by a −5/del(5q), raising the question of whether haploinsufficiency of APC contributes to the pathogenesis of myeloid diseases. To address this question, we generated a cohort of Mx1-Cre+Apcfl/+ and control Mx1-Cre−Apcfl/+ mice. At 2 months of age, we induced the Apc deletion by pI-pC injection (3 doses; Figure 1A), and monitored the hematologic parameters of these cohorts of mice over time. All of the Mx1-Cre+Apcfl/+mice died within 8 months after induction of Apc loss, whereas none of the control Mx1-Cre−Apcfl/+mice died (Figure 1B). None of the mice showed abnormal hematologic parameters in the PB within 2 to 3 months after induction. However, all mice with loss of a single allele of Apc (referred to herein as Apc-heterozygous mice) became moribund due to severe anemia within 3 to 8 months after induction. At this time, the Apc-heterozygous mice showed normal WBCs, and normal differential neutrophil and lymphocyte counts (data not shown), but had a significantly increased monocyte count (1.5 ± 0.6 for Apc-heterozygous mice vs 0.7 ± 0.2 for control mice; P < .001) compared with control mice, consistent with a monocytosis (Figure 1C). In addition, the Apc-heterozygous mice displayed significantly lower red blood cell (RBC) counts (2.8 ± 0.6 vs 9.4 ± 0.6; P < .001), and hemoglobin levels (5.1 ± 1.9 vs 14 ± 0.9; P < .001) compared with control mice. The mean red cell volume (MCV; 63 ± 9 vs 44.8 ± 1.5; P < .001) and red cell distribution width (RDW; 31 ± 8.6 vs 20 ± 1.1; P < .001) were elevated dramatically in Apc-heterozygous mice, reflecting a macrocytosis that is consistent with dyserythropoiesis (Figure 1C). Because an alteration in maturation of myeloid cells may not be detected by complete blood count (CBC) analysis, we performed flow cytometric analysis to determine the proportion of neutrophils, monocytes, and megakarocytes, defined as Gr-1+ Mac-1+, Mac-1+Gr-1low, and CD41+ cell populations, respectively, in BM, and SP from Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+ mice. The Gr-1+ Mac-1+ cells are comparable in BM and SP from Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice, whereas Mac-1+Gr-1low cells were increased in BM, but not SP, from Apc-heterozygous mice (Figure 2A). However, we found that the CD41+ megakarocytes were significantly decreased in BM and SP from Mx1-Cre+Apcfl/+ mice, when they become moribund (several months after induction; Figure 2B).
Haploinsufficiency of Apc leads to lethality. (A) Analysis of the deletion of Apc in primary mice as determined by semiquantitative polymerase chain reaction of genomic DNA from bone marrow (BM) cells 4 days after induction. (B) Kaplan-Meier survival curve of primary Mx1-Cre+Apcfl/+ (n = 13) and Mx1-Cre−Apcfl/+ (n = 13) mice. (C) Peripheral blood counts of primary mice after induction. The deletion of a single allele of Apc was induced in Mx1-Cre+Apcfl/+ mice treated with 3 doses of pI-pC, referred to as Δ/+ mice. Control Mx1-Cre−Apcfl/+ mice are referred as fl/+ mice. The data were collected when Δ/+ mice became moribund within 3 to 8 months after induction. n = 13; ***P < .001.
Haploinsufficiency of Apc leads to lethality. (A) Analysis of the deletion of Apc in primary mice as determined by semiquantitative polymerase chain reaction of genomic DNA from bone marrow (BM) cells 4 days after induction. (B) Kaplan-Meier survival curve of primary Mx1-Cre+Apcfl/+ (n = 13) and Mx1-Cre−Apcfl/+ (n = 13) mice. (C) Peripheral blood counts of primary mice after induction. The deletion of a single allele of Apc was induced in Mx1-Cre+Apcfl/+ mice treated with 3 doses of pI-pC, referred to as Δ/+ mice. Control Mx1-Cre−Apcfl/+ mice are referred as fl/+ mice. The data were collected when Δ/+ mice became moribund within 3 to 8 months after induction. n = 13; ***P < .001.
Apc-heterozygous mice displayed an alteration in the distribution of CD41+ megakaryocytes and monocytes. Flow cytometric analysis of mature myeloid cells from BM and spleen (SP) from Mx1-Cre+Apcfl/+ mice and the control Mx1-Cre−Apcfl/+ mice was performed 3 to 4 months after induction. The percentage of (A) Gr-1+Mac-1+ and GrlowMac-1+ or (B) CD41+cells is indicated (average ± SD of 4 animals). *P < .05; **P < .01.
Apc-heterozygous mice displayed an alteration in the distribution of CD41+ megakaryocytes and monocytes. Flow cytometric analysis of mature myeloid cells from BM and spleen (SP) from Mx1-Cre+Apcfl/+ mice and the control Mx1-Cre−Apcfl/+ mice was performed 3 to 4 months after induction. The percentage of (A) Gr-1+Mac-1+ and GrlowMac-1+ or (B) CD41+cells is indicated (average ± SD of 4 animals). *P < .05; **P < .01.
Although morbidity in Apc-heterozygous mice appeared to be the result of severe anemia, we performed gross anatomic analysis of moribund mice. There were no tumors of the liver, spleen, or kidney. Mx1-Cre+Apcfl/fl mice displayed liver abnormalities after ablation of Apc due to a significantly increased frequency of apoptosis19 ; however, morphologic analysis of the liver from the moribund Apc-heterozygous mice revealed that these mice had normal liver morphology (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The acquired anemia in Apc-heterozygous mice is not due to loss of blood, or a deficiency of vitamin B12 or folic acid.
Mx1-Cre–induced excision is not absolutely limited to hematopoietic cells.21 To determine whether Mx1-Cre+Apcfl/+ mice develop intestinal polyps as a result of the deletion of 1 Apc allele from expression of Mx1-Cre in intestinal epithelial cells after pI-pC induction, we have examined the entire length of the intestinal tract of Mx1-Cre+Apcfl/+ mice when they became anemic. Although most anemic mice developed polyps (4 of 5 mice examined), these mice had relatively few polyps,3-14 which were located in the proximal colon. In contrast, ApcMin/+ mice develop more than 50 polyps, on average, throughout the intestinal tract (the ApcMin mutation results in truncation of the Apc gene at position 850).25 Chronic blood loss can result in iron deficiency, and RBCs often appear microcytic; however, we did not observe intestinal bleeding. Moreover, we found that the anemic mice had normal serum iron levels (103 ± 76 vs 128 ± 27 μg/dL in controls; n = 4), and developed macrocytic anemia, suggesting that the acquired anemia in Mx1-Cre+Apcfl/+ mice after pI-pC induction is not the result of blood loss. Vitamin B12 or folic acid deficiency is the major cause of macrocytic anemia. However, treatment of the mice with vitamin B12 or folic acid (500 μg/kg weight) for 7 to 10 days did not rescue the anemia phenotype (data not shown), suggesting that acquired anemia in Mx1-Cre+Apcfl/+ mice is not due to defective intestinal function.
Haploinsufficiency of Apc leads to a block in differentiation in the early stages of erythroid development
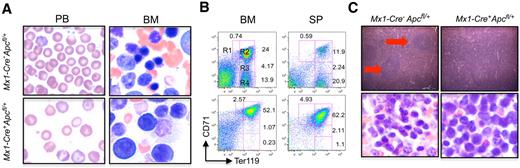
To characterize the anemia occurring in Apc-heterozygous mice, we performed morphologic analysis of mature erythroid cells. Examination of the PB smears from the Apc-heterozygous mice showed anisocytosis, poikilocytosis, and macrocytosis (Figure 3A left panel). Erythroid blasts were increased significantly in BM from Apc heterozygous mice (Figure 3A right panel). Erythroblasts can be recognized morphologically, and are defined by expression of the Ter119 and CD71 cell surface markers.26 From least to most differentiated, there are 4 cell populations with specific staining characteristics: R1 (Ter119lowCD71hi), R2 (Ter119hiCD71hi), R3 (Ter119hiCD71med), and R4 (Ter119hiCD71low), corresponding to proerythroblasts, basophilic erythroblasts, late basophilic and polychromatophilic erythroblasts, and orthochromatophilic erythroblasts, respectively. To determine whether the acquired anemia in Apc-heterozygous mice is due to the arrest of differentiation of erythroid progenitors at the early stages, we performed flow cytometric analysis of single-cell suspensions from bone marrow by immunostaining with anti-Ter119 and anti-CD71 antibodies. Anemic Apc-heterozygous mice had a significantly increased proportion of the proerythroblast (R1, 3.2 ± 0.9 vs 0.8 ± 0.3; n = 4, P < .01) and the basophilic erythroblast (R2, 29.7 ± 2.2 vs 17.4 ± 8.4; n = 4, P < .03) populations, and a significantly decreased proportion of the orthochromatophilic erythroblasts (R4, 2.1 ± 1.5 vs 13.9 ± 7.6; n = 4, P < .03), compared with Apc-control mice, whereas the frequency of late basophilic and polychromatophilic erythroblasts (R3) was comparable (2.2 ± 0.9 vs 2.8 ± 1.4; n = 4; Figure 3B; left panel).
Apc-heterozygous mice developed ineffective erythropoiesis with erythroid dysplasia. (A) PB smears (left panels) and representative Giemsa-stained BM smears (right panels). (B) Flow cytometric analysis of BM and SP cells from representative Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice after pI-pC induction. The single-cell suspensions were simultaneously stained with anti-CD71 and anti-Ter119 antibodies. The numbers indicate the percentages of cells in each population. (C). Hematoxylin and eosin (H&E)–stained sections of adult SP (top panels) and Giemsa-stained spleen touch-preparations (bottom panels) from either control or anemic Apc heterozygous mice. The red arrows identify white pulp. Samples were obtained from both Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice 4 to 8 months after induction of the Apc deletion by pI-pC. Images were obtained using an Olympus microscope BX45 (Model U-DO3) equipped with an Olympus DP12 digital camera (A panels: 100× oil objective/1.25 NA, C top panels: 10× Plan air objective/0.3 NA, C bottom panels: 50× oil objective/0.9 NA), and processed using Microsoft PowerPoint.
Apc-heterozygous mice developed ineffective erythropoiesis with erythroid dysplasia. (A) PB smears (left panels) and representative Giemsa-stained BM smears (right panels). (B) Flow cytometric analysis of BM and SP cells from representative Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice after pI-pC induction. The single-cell suspensions were simultaneously stained with anti-CD71 and anti-Ter119 antibodies. The numbers indicate the percentages of cells in each population. (C). Hematoxylin and eosin (H&E)–stained sections of adult SP (top panels) and Giemsa-stained spleen touch-preparations (bottom panels) from either control or anemic Apc heterozygous mice. The red arrows identify white pulp. Samples were obtained from both Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice 4 to 8 months after induction of the Apc deletion by pI-pC. Images were obtained using an Olympus microscope BX45 (Model U-DO3) equipped with an Olympus DP12 digital camera (A panels: 100× oil objective/1.25 NA, C top panels: 10× Plan air objective/0.3 NA, C bottom panels: 50× oil objective/0.9 NA), and processed using Microsoft PowerPoint.
In addition, the anemic Apc-heterozygous mice developed splenomegaly, with spleens that were enlarged up to 7 to 10 times the normal size (supplemental Figure 2A). These mice showed a marked expansion of the red pulp with predominantly extramedullary erythropoiesis, and the absence of white pulp (Figure 3C). Flow cytometric analysis revealed that the R1 (3.8 ± 1.4 vs 0.7 ± 0.5; n = 4, P < .01) and R2 (65.8 ± 14.4 vs 12.3 ± 4.6; n = 4, P < .001) cell populations were increased significantly, and the R4 cell population (4.1 ± 3.9 vs 27.6 ± 9; n = 4, P < .01) was significantly decreased in spleens from Apc-heterozygous mice compared with control mice (Figure 3B; right panel). Two broad types of erythroid progenitors, the BFU-E and the more mature CFU-E, have been identified by classic colony-forming assays.27 We determined the frequency of BFU-E and CFU-E in BM and SP from Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice 3 months after pI-pC induction. Our data showed that the frequency of BFU-E was slightly increased in BM and SP, whereas CFU-E was dramatically increased in SP, but not in BM (supplemental Figure 2B). Together, these data indicate that the differentiation of erythroid progenitors from Apc-heterozygous mice was blocked at the CFU-E, early proerythroblast, and basophilic erythroblast stages, leading to ineffective erythropoiesis, and suggest that Apc plays a critical role in erythropoiesis.
Apc haploinsufficiency results in increased proliferation of splenic CD71+Ter119high erythroblasts
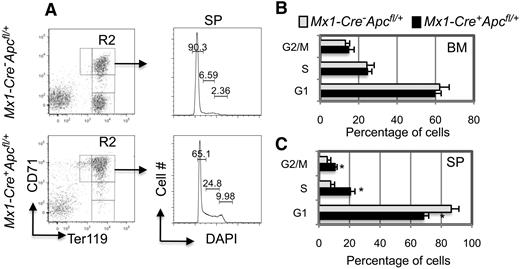
We performed cell-cycle analysis to determine whether the CD71+Ter119high erythroblasts that accumulate in the BM and SP of Mx1-Cre+Apcfl/+ mice 3 to 6 months after pI-pC induction displayed an increased proliferative rate. Interestingly, we found that the frequency of proliferation of BM blasts is comparable in Mx1-Cre+Apcfl/+ and control mice (Figure 4B). Although the proportions of splenic erythroblasts in the S and G2/M phases were significantly increased in the Apc-heterozygous mice compared with control mice, it is likely that this is the consequence of the extensive extramedullary erythropoiesis in the spleen as a result of Apc haploinsufficiency (Figure 4A,C). There was no significant alteration in the frequency of apoptosis in the CD71+Ter119high erythroblasts from BM and SP in Mx1-Cre+Apcfl/+ and control mice after pI-pC induction (supplemental Figure 3).
Analysis of the proliferation of erythroblasts (CD71+Ter119+) in BM and SP from Apc-heterozygous mice. (A) Representative histograms of flow cytometric analysis of the cell cycle in splenic erythroblasts. (B-C) Histograms depicting the cell-cycle status of erythroblasts from (B) BM and (C) SP. Samples were obtained from both Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice with anemia 4 to 6 months after induction of the Apc deletion (average ± SD of 4-6 animals).*P < .01.
Analysis of the proliferation of erythroblasts (CD71+Ter119+) in BM and SP from Apc-heterozygous mice. (A) Representative histograms of flow cytometric analysis of the cell cycle in splenic erythroblasts. (B-C) Histograms depicting the cell-cycle status of erythroblasts from (B) BM and (C) SP. Samples were obtained from both Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice with anemia 4 to 6 months after induction of the Apc deletion (average ± SD of 4-6 animals).*P < .01.
Loss of a single allele of Apc results in expansion of HSCs
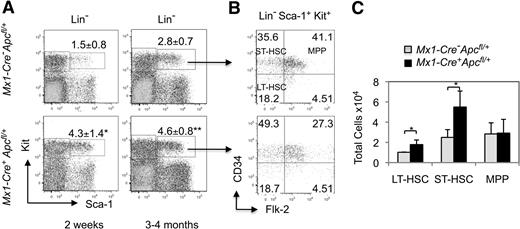
To determine whether loss of a single allele of Apc affects the HSC population, we analyzed the LSK (Lin− Sca-1+ Kit+), an HSC-enriched population, from Mx1-Cre+Apcfl/+ and control mice 2 weeks or 3 to 4 months after induction. The total number of BM cells from Apc-heterozygous mice and control mice was comparable (supplemental Figure 4A). The number of LSKs is increased in Mx1-Cre+Apcfl/+ mice at both 2 weeks and 3 to 4 months after induction compared with control mice (Figure 5A). LSK cells contain both long-term (LT)–HSCs (Lin− Sca-1+ Kit+ Flt3− CD34− cells) and short-term (ST)–HSCs (Lin− Sca-1+ Kit+ Flt3− CD34+ cells), which are capable of maintaining hematopoiesis for a lifetime, or for a few weeks (8-12 weeks), respectively. The multipotential progenitor (MPP; Lin− Sca-1+ Kit+ Flt3+ CD34+) population is immediately downstream of HSCs.28 We demonstrated that the proportion of ST-HSCs is increased significantly, whereas the proportion of MPPs is decreased in LSKs from Apc-heterozygous mice compared with control mice (Figure 5B). However, the total number of LT-HSCs and ST-HSCs are significantly expanded in Apc-heterozygous mice, whereas the total number of MPPs are comparable in both Apc-heterozygous and control mice (Figure 5C).
LT-HSCs and ST-HSCs are expanded in the absence of a single allele of Apc. (A) Flow cytometric analysis of LSKs (Lin− Sca-1+ Kit+) in primary mice. Comparison of the frequency of LSKs in Mx1-Cre+Apcfl/+ mice and the control Mx1-Cre−Apcfl/+ mice 2 weeks after induction (left panels) and 3 to 4 months after induction (right panels). The percentage of LSK cells is indicated (average ± SD of 3-5 animals).*P < .05; **P < .01. (B) Flow cytometric analysis of the proportion of long-term hematopoietic stem cells (LT-HSC)s, short-term hematopoietic stem cells (ST-HSCs), and multipotential progenitors (MPPs) in the LSK population from representative mice. (C) Total number of LT-HSCs, ST-HSCs and MPPs in BM from Mx1-Cre+Apcfl/+ mice and the control Mx1-Cre−Apcfl/+ mice 3 to 5 months after induction (average ± SD of 3 animals). *P < .05.
LT-HSCs and ST-HSCs are expanded in the absence of a single allele of Apc. (A) Flow cytometric analysis of LSKs (Lin− Sca-1+ Kit+) in primary mice. Comparison of the frequency of LSKs in Mx1-Cre+Apcfl/+ mice and the control Mx1-Cre−Apcfl/+ mice 2 weeks after induction (left panels) and 3 to 4 months after induction (right panels). The percentage of LSK cells is indicated (average ± SD of 3-5 animals).*P < .05; **P < .01. (B) Flow cytometric analysis of the proportion of long-term hematopoietic stem cells (LT-HSC)s, short-term hematopoietic stem cells (ST-HSCs), and multipotential progenitors (MPPs) in the LSK population from representative mice. (C) Total number of LT-HSCs, ST-HSCs and MPPs in BM from Mx1-Cre+Apcfl/+ mice and the control Mx1-Cre−Apcfl/+ mice 3 to 5 months after induction (average ± SD of 3 animals). *P < .05.
Next, we determined whether the distribution of subsets of myeloid progenitor cells is altered in Apc-heterozygous mice by flow cytometric analysis. We found that all subsets of myeloid progenitor cells, including common myeloid progenitors (CMPs), as well as more restricted granulocyte-monocyte progenitors and megakaryocyte-erythroid progenitors (GMPs and MEPs), are comparable in Apc-heterozygous and control mice (supplemental Figure 4B). To examine the efficiency of Cre-mediated deletion of Apc in subsets of hematopoietic stem/progenitor cells after pI-pC induction, we isolated BM cells from 3 Mx1-Cre+Apcfl/+ mice and 1 control Mx1-Cre−Apcfl/+ mouse after pI-pC induction, and plated these cells in methylcellulose-based medium containing SCF, IL-3, IL-6, and Epo. After 10 days, genomic DNA was isolated from 15 to 18 colonies from each mouse, and analyzed by PCR (supplemental Figure 5). In each colony, cells grow from a single HSC/HPC cell; thus, the number of colonies with the deleted allele of Apc reflects the efficiency of Apc deletion in HSCs and myeloid progenitor cells. Of the total 54 colonies analyzed from 3 Mx1-Cre+Apcfl/+ mice, only 1 colony retained the floxed Apc allele, indicating that the deletion of Apc is nearly complete in HSCs and subsets of myeloid progenitor cells. In addition, our data showed that Apc is down-regulated in HSCs and subsets of myeloid progenitors from Apc-heterozygous mice by qRT-PCR analysis (data not shown).
Haploinsufficiency of Apc reduces the capacity of HSCs to reconstitute hematopoiesis in vivo
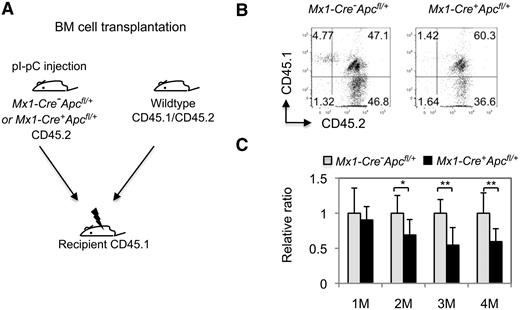
To determine whether loss of a single allele of Apc affects the function of HSCs in vivo, we performed a competitive repopulation assay. We transplanted total CD45.2+-nucleated BM cells from control Mx1-Cre−Apcfl/+ or Mx1-Cre+Apcfl/+ mice 4 weeks after pI-pC treatment, along with an equal number of wild-type CD45.1+CD45.2+ (heterozygous) cells, into lethally irradiated CD45.1+ congenic recipient mice (Figure 6A). Engraftment of donor cells was verified by flow cytometric analysis of CD45 cell-surface marker expression in PB cells 4 weeks after transplantation (Figure 6B). At 1 month after transplantation, recipient mice had an equivalent proportion of Apc-heterozygous or control CD45.2 donor cells versus wild-type cells. However, the Apc-heterozygous donor cells gradually decreased in PB compared with control donor cells (Figure 6C), indicating that Apc-heterozygous HSCs have a reduced capacity to repopulate hematopoiesis in vivo.
Apc haploinsufficiency affects the repopulating capacity of HSCs in vivo. (A) Mice with chimeric BM were generated by transplanting an equal number of wild-type CD45.1+CD45.2+ (CD45 heterozygous) BM cells and CD45.2+ (homozygous) BM cells from Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+ mice 4 weeks after pI-pC induction of the Apc deletion. (B) Flow cytometric analysis of CD45.1- and CD45.2-stained PB cells from representative chimeric Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+ mice 1 month after transplantation. The numbers indicate the percentage of cells in each population. (C) Histogram showing the relative ratio of CD45.2+ versus CD45.1+/CD45.2+-stained PB cells in Mx1-Cre−Apcfl/+ versus Mx1-Cre+Apcfl/+ chimeric mice examined at 1 to 4 months after transplantation (mean ± SD of 8-10 animals). *P < .05; **P < .01.
Apc haploinsufficiency affects the repopulating capacity of HSCs in vivo. (A) Mice with chimeric BM were generated by transplanting an equal number of wild-type CD45.1+CD45.2+ (CD45 heterozygous) BM cells and CD45.2+ (homozygous) BM cells from Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+ mice 4 weeks after pI-pC induction of the Apc deletion. (B) Flow cytometric analysis of CD45.1- and CD45.2-stained PB cells from representative chimeric Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+ mice 1 month after transplantation. The numbers indicate the percentage of cells in each population. (C) Histogram showing the relative ratio of CD45.2+ versus CD45.1+/CD45.2+-stained PB cells in Mx1-Cre−Apcfl/+ versus Mx1-Cre+Apcfl/+ chimeric mice examined at 1 to 4 months after transplantation (mean ± SD of 8-10 animals). *P < .05; **P < .01.
The chimeric mice that received transplants of wild-type competitor BM cells and Apc-heterozygous or control BM cells (n = 10) displayed comparable monocyte counts (0.74 ± 0.2 vs 0.7 ± 0.3 K/μL), RBC counts (8.3 ± 1.1 vs 8.2 ± 1.2 M/μL), hemoglobin levels (11.8 ± 1.2 vs 11.2 ± 0.5 g/dL), MCV (42.7 ± 1.9 vs 41.7 ± 0.5 fL), and RDW (20 ± 2 vs 22.4 ± 3.1%) at 4 months after transplantation. In addition, the frequency of erythroblast subsets was comparable in BM and SP from the chimeric mice with Apc-heterozygous or control BM cells (data not shown). To determine whether the anemia was transplantable, we transplanted bone marrow cells from anemic Apc-heterozygous mice (CD45.2) to lethally irradiated CD45.1 congenic recipient mice to generate chimeric mice. All mice that received cells recovered fully from lethal irradiation. We have not observed the onset of anemia in chimeric mice up to 3 months after transplantation (data not shown); whether these chimeric mice would eventually develop anemia after a longer latency period is unknown.
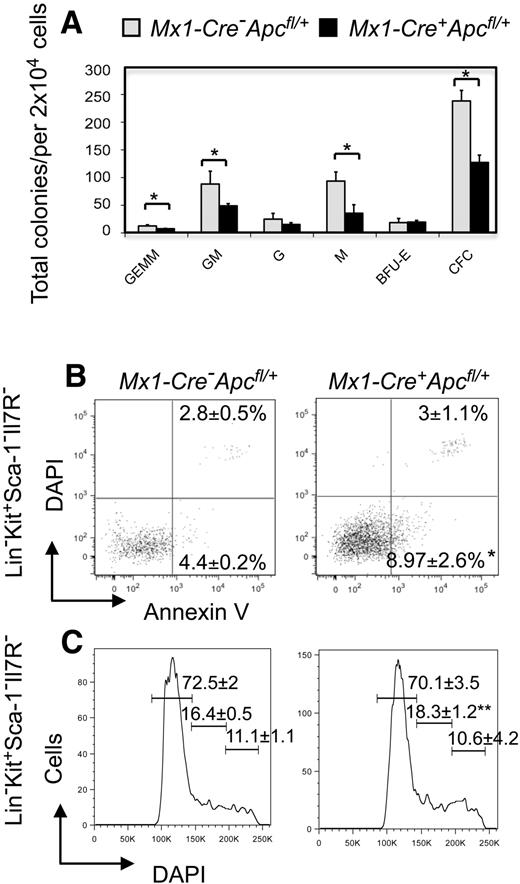
Apc heterozygous myeloid progenitor cells have decreased in vitro colony-forming capacity, and an increased frequency of apoptosis
Previous studies have revealed that there is a high frequency of apoptosis of HPCs in patients with MDS, as well as decreased in vitro colony-forming ability.29-32 To examine the colony-forming capacity of myeloid progenitor cells in BM from Apc-heterozygous mice, we performed in vitro CFU assays. BM cells harvested from Mx1-Cre+Apcfl/+ and control Mx1-Cre−Apcfl/+ mice 2 months after pI-pC induction were plated in methylcellulose medium containing SCF, IL-3, IL-6, and Epo. Apc-heterozygous BM cells gave rise to fewer CFUs, as well as fewer CFU-GEMM, CFU-GM, and CFU-M colonies than did the control BM cells. However, the number of CFU-G and BFU-E colonies was comparable (Figure 7A). These data suggest that the colony-forming capacity is decreased in hematopoietic progenitors from Apc-heterozygous mice. In HPCs (Lin−Sca-1−Kit+Il-7R−), the frequency of early apoptotic cells (Annexin V+DAPI−) was increased 2-fold in Apc-heterozygous mice compared with control mice 3 to 4 months after pI-pC induction (Figure 7B); however, the proportion of myeloid progenitor cells in S and G2/M phases was comparable (Figure 7C). In contrast, the frequency of apoptosis is comparable in mature hematopoietic cells (Lin+; supplemental Figure 6). Together, these data indicate that haploinsufficiency of Apc leads to decreased colony-forming ability of myeloid progenitor cells, which may be due to the increased frequency of apoptosis in these cells.
Analysis of in vitro colony-forming capacity, apoptosis, and proliferation of HPCs from Apc-heterozygous mice. (A) In vitro colony-forming assays. The number of BM CFU-GEMM, CFU-GM, CFU-G, CFU-M, and BFU-E was examined in Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+mice 2 months after induction (mean ± SD of 3 animals). *P < .05. (B-C) Frequency of (B) apoptosis and (C) proliferation in gated HPCs (Lin− Kit+ Sca-1− Il-7R−) stained with Annexin V and DAPI, or DAPI, respectively, from Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice 3 to 4 months after pI-pC induction (mean ± SD of 3 animals). *P < .05; **P = .057.
Analysis of in vitro colony-forming capacity, apoptosis, and proliferation of HPCs from Apc-heterozygous mice. (A) In vitro colony-forming assays. The number of BM CFU-GEMM, CFU-GM, CFU-G, CFU-M, and BFU-E was examined in Mx1-Cre+Apcfl/+ or Mx1-Cre−Apcfl/+mice 2 months after induction (mean ± SD of 3 animals). *P < .05. (B-C) Frequency of (B) apoptosis and (C) proliferation in gated HPCs (Lin− Kit+ Sca-1− Il-7R−) stained with Annexin V and DAPI, or DAPI, respectively, from Mx1-Cre+Apcfl/+ and Mx1-Cre−Apcfl/+ mice 3 to 4 months after pI-pC induction (mean ± SD of 3 animals). *P < .05; **P = .057.
APC is down-regulated in MDS characterized by −5/del(5q)
To determine whether loss of a single allele of APC leads to down-regulation of the expression of APC in patients with MDS, we analyzed the level of expression of APC in CD34+ cells from 55 patients with MDS published previously.33 This analysis revealed that the expression of APC is decreased significantly in CD34+ cells from patients with MDS characterized by a −5/del(5q), but not in CD34+ cells from other patients with MDS (supplemental Figure 7). These results indicate that expression of APC in immature hematopoietic cells is subject to a gene dosage effect, and raise the possibility that haploinsufficiency of APC may play a role in the pathogenesis of myeloid neoplasms with a −5/del(5q).
Discussion
Loss of function of APC leads to colorectal cancer.16 However, whether APC plays a role in the pathogenesis of myeloid neoplasms is unknown. Previously, we showed that Apc is required for the function of HSCs and HPCs.19 In this study, we examined an Apc conditional knockout mouse model, and demonstrate that haploinsufficiency of Apc results in ineffective hematopoiesis. Specifically, induction of ablation of a single allele of Apc results in lethality in mice, due to the development of a severe macrocytic anemia with monocytosis. The Apc-heterozygous mice developed splenomegaly with a marked expansion of red pulp, consistent with extensive extramedullary erythropoiesis in the spleen. Of note, the Apc-heterozygous mice had normal serum iron levels, suggesting that the acquired anemia is unlikely to be due to the loss of blood (eg, as a result of the formation of intestinal polyps and colonic bleeding). In addition, we demonstrate that the anemia resulted from ineffective erythropoiesis, and that the differentiation of Apc-heterozygous erythroid progenitors is blocked at the CFU-E, early proerythroblast, and basophilic erythroblast stages.
Of note, we found that Apc haploinsufficiency affects the functions of HSCs and HPCs. Emerging evidence suggest that MDS, AML de novo, or t-MDS/t-AML with a −5/del(5q) originates in HSCs rather than committed progenitor cells.34,35 In patients with MDS with an isolated del(5q) (the 5q− syndrome), the majority of highly purified CD34+CD38−Thy1+ stem cells contain the del(5q).34,35 In our studies, we demonstrate that loss of a single allele of Apc results in expansion of both LT-HSCs and ST-HSCs, but reduces the ability of HSCs to reconstitute hematopoiesis in vivo. In addition, MDS is associated with increased proliferation and excessive apoptosis of CD34+ HSCs/HPCs in the early stages of the disease. However, the frequency of apoptosis is reduced during the progression to AML.30-32,36 In this study, we show that the frequency of apoptosis is increased significantly in Apc-heterozygous myeloid progenitor cells, but not in mature hematopoietic cells, although the proliferative rate of myeloid progenitor cells is marginally increased in Apc-heterozygous mice compared with control mice. These data suggest that excessive apoptosis of HPCs may be due in part to loss of a single allele of APC in MDS with a del(5q). Although the number and subsets of myeloid progenitor cells are comparable in Apc-heterozygous and control mice, we found that the myeloid progenitor cells from Apc-heterozygous mice have significantly reduced in vitro colony-forming capacity, consistent with the reports that myeloid progenitor cells from most patients with MDS form fewer in vitro hematopoietic colonies compared with those from healthy people.29,34,37 Nonetheless, Apc-heterozygous mice did not develop myeloid leukemia, indicating that additional mutations are required to complete the transformation of normal HSCs to leukemia-initiating cells.
Several genes located on 5q, including RPS14,12 EGR1,13 NPM1,14 and CTNNA1,15 have been implicated in the development of myeloid disorders, due to a gene dosage effect. Of these, only EGR1 and RPS14 are located within the CDSs of 5q. RPS14, which is required for the processing of 18S pre-rRNA,38 was identified as a candidate disease gene in MDS with an isolated del(5q) by Ebert et al.12 Down-regulation of RPS14 in CD34+ BM cells blocks the differentiation of erythroid cells and increases apoptosis in differentiating erythroid cells in vitro.12 However, haploinsufficiency of RPS14 does not account for several features of the disease, including megakaryocytic dysplasia, neutropenia, and clonal dominance. The early growth response 1 protein (EGR1), a positive modulator of macrophage differentiation,39 is a member of the EGR family of zinc-finger transcription factors. We demonstrated previously that loss of a single allele of Egr1 cooperates with mutations induced by the DNA-alkylating agent, N-ethyl-nitrosourea, resulting in a myeloproliferative disorder with ineffective erythropoiesis in mice, suggesting that haploinsufficiency of Egr1 plays a role in leukemogenesis. Nevertheless, the loss of Egr1 alone in vivo does not result in expansion of the HSCs, proliferation of HPCs, or abnormalities in adult hematopoiesis.13 NPM1 is involved in centrosome duplication, and modulates the activity of the TP53 and CDKN2A tumor suppressors.40,41 Absence of Npm1 results in embryonic lethality; however, Npm1 heterozygous mice developed erythroid dysplasia with elevated MCV and RDW, normal RBC counts and hemoglobin levels, and dysplastic megakaryocytes.14 Nevertheless, the role of NPM1 in the pathogenesis of MDS/AML is unclear, because NPM1 is neither deleted in most patients with a del(5q), nor have mutations been identified in the remaining allele.42 Liu et al15 showed that the gene encoding α-catenin (CTNNA1) is down-regulated in leukemia-initiating cells from patients with MDS and AML with a del(5q) compared with patients lacking the del(5q), or normal HSCs. Expression of CTNNA1 is suppressed due to epigenetic silencing in HL-60 cells, a myeloid leukemia cell line used as a model for del(5q) leukemia. Reinduction of α-catenin expression led to reduced proliferation, and an increased frequency of apoptosis, suggesting that down-regulation of α-catenin in HSCs may contribute to transformation of myeloid cells in patients with MDS and AML with a del(5q).15 Analysis of the features of animal models that are null or heterozygous for a null allele of the genes described here shows that each model recapitulates some, but not all, of the features of MDS/AML with a −5/del(5q). Together, these data suggest that haploinsufficiency of more than one gene on 5q is involved in the development of MDS, AML de novo, or t-MDS/t-AML with a −5/del(5q).
Our studies suggest that Apc-heterozygous mice share some of the features of human MDS characterized by a −5/del(5q), raising the possibility that haploinsufficiency of APC cooperates with loss of one or more genes on 5q in the pathogenesis of myeloid neoplasms. Whether the expression of APC is deregulated by other molecular mechanisms in myeloid neoplasms without −5/del(5q) is unknown. Patients with familial adenomatous polyposis coli (FAP) have germline mutations of APC, but have not been reported to be predisposed to developing MDS or AML. It is possible that the effects of germline APC mutations in patients with FAP may differ from that of somatic loss of a single allele of APC acquired in patients with MDS, AML, or t-MDS/t-AML, because the cell context and microenvironment differ when the mutation is induced in adult tissues, compared with germline mutations. Of note is that previous studies have revealed that some patients with FAP develop anemia.43,44 Croner et al undertook a retrospective study of 143 patients with FAP treated between 1971 and 2000.43 They showed that colonic bleeding was present in 42% of patients younger than 20 years, 68% of patients between 20 and 40 years, and 35% of patients older than 40 years, whereas anemia was present in 10% of patients younger than 20 years, 20% of patients between 20 and 40 years, and 32% of patients older than 40 years, indicating that the frequency of anemia is associated with age, rather than the frequency of colonic bleeding in patients with FAP. This observation raises the possibility that the acquired anemia in patients with FAP may result from ineffective erythropoiesis and/or colonic bleeding. Further analysis of the clinical and pathologic features of the anemia in patients with FAP may result in additional insights into the cause of anemia in these patients. Similarly, whether there are defects in the function of HSCs and HPCs in patients with FAP is unknown.
The Wnt pathway represents one of the most significantly deregulated pathways in MDS with an isolated del(5q).8 We showed that loss of Apc increases the stability of β-catenin in HSCs and HPCs, suggesting that loss of Apc activates the Wnt pathway in HSCs and HPCs.19 In addition, activation of the Wnt pathway by conditional expression of a stable form of β-catenin in hematopoietic cells leads to disruption of function of HSCs and a multilineage differentiation block in vivo.45,46 Together, these data suggest that loss of a single allele of Apc results in ineffective hematopoiesis due at least in part to deregulation of the Wnt pathway. In addition to its role in regulating the Wnt pathway, Apc plays a role in chromosome segregation and spindle assembly as well as cell migration and adhesion.17 Apc has been implicated in the regulation of the mTOR pathway, which is critical for regulating cell proliferation and growth.18 Whether the effect of Apc haploinsufficiency on hematopoiesis results from the deregulation of the Wnt pathway, from disruption of other cellular functions of Apc, or both, remains to be determined.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Animal Resource Center at the University of Chicago for the care of the mice used in this study, and the Flow Cytometry Shared Research Facility of the University of Chicago Cancer Research Center for technical assistance. We also thank members of the Le Beau laboratory for helpful discussions.
This work was supported by Public Health Service (PHS) grant CA40046, the Cancer Research Foundation (M.M.L), and the American Cancer Society Illinois Division (09-40; Z.Q.).
Authorship
Contribution: J.W., A.A.F., and J.A. performed research and analyzed data; M.M.L.B. designed research, analyzed data, and wrote the paper; and Z.Q. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhijian Qian, Section of Hematology/Oncology, University of Chicago, 5841 S Maryland Ave, MC2115, Chicago, IL 60637; e-mail: zqian@bsd.uchicago.edu.