Abstract
ABT-737 is a small-molecule antagonist of BCL-2 currently under evaluation in clinical trials in the oral form of ABT-263. We anticipate that acquired resistance to this promising drug will inevitably arise. To study potential mechanisms of resistance to ABT-737, we derived resistant lines from initially sensitive OCI-Ly1 and SU-DHL-4 lymphoma cell lines via long-term exposure. Resistance was based in the mitochondria and not due to an inability of the drug to bind BCL-2. Resistant cells had increased levels of BFL-1 and/or MCL-1 proteins, which are not targeted by ABT-737. Proapoptotic BIM was displaced from BCL-2 by ABT-737 in both parental and resistant cells, but in resistant cells, BIM was sequestered by the additional BFL-1 and/or MCL-1. Decreasing MCL-1 levels with flavopiridol, PHA 767491, or shRNA restored sensitivity to ABT-737 resistant cells. MCL-1 was up-regulated not by protein stabilization but rather by increased transcript levels. Surprisingly, in addition to stable increases in MCL-1 transcript and protein in resistant cells, there was a dynamic increase within hours after ABT-737 treatment. BFL-1 protein and transcript levels in resistant cells were similarly dynamically up-regulated. This dynamic increase suggests a novel mechanism whereby modulation of antiapoptotic protein function communicates with nuclear transcriptional machinery.
Introduction
BCL-2 was initially cloned from the breakpoint of the t(14;18) translocation that is found in nearly all cases of follicular lymphoma and in a minority of cases of diffuse large B-cell lymphoma.1-3 BCL-2 was subsequently validated as an oncogene, but an oncogene with a function distinct from prior oncogenes.4,5 Instead of increasing proliferation, it promoted cancer cell accumulation by opposing cell death.6,7 Since that time, nearly 20 years ago, BCL-2 has been an attractive target for therapeutic intervention in cancer. In the past few years, several strategies directed toward antagonizing BCL-2 function have entered clinical trials.
The BCL-2 family proteins control the key step in the intrinsic apoptotic pathway, permeabilization of the mitochondrial outer membrane.8,9 BAX and BAK are proapoptotic proteins that oligomerize to form pores in the mitochondrial outer membrane. Apoptosis via the mitochondrial pathway cannot occur in their absence. To oligomerize, they must be activated. BID and BIM are members of the activator BH3-only subclass of BCL-2 family proteins that can activate BAX and BAK.10,11 It is likely that other proteins, perhaps some as yet undiscovered, share this activator activity.12,13
Antiapoptotic proteins, including BCL-2, MCL-1, BCL-XL, BCL-w, and BFL-1, inhibit cell death primarily by binding and sequestering activator proteins and preventing the activation of BAX and BAK, though they may also sequester certain forms of monomeric BAX and BAK as well.12,14-17 Cells expressing significant amounts of activator proteins such as BIM must sequester the activator proteins with antiapoptotic proteins to stay alive. We describe this condition as being “primed for death.”14 In a prior study, we have found that sensitivity of lymphoma cell lines to BCL-2 antagonism is directly related to the amount of BCL-2 primed with BIM present.18
Perhaps the best characterized strategy for antagonizing BCL-2 function is the small-molecule strategy of Abbott Laboratories.19 Through clever use of a combination of chemical library screening and iterations directed by high-throughput nuclear magnetic resonance (NMR) nicknamed “SAR (structure activity relationship) by NMR,” they produced small molecules that bound with subnanomolar affinity to BCL-2, BCL-XL, and BCL-w. ABT-737 notably does not bind MCL-1 or BFL-1 with high affinity. ABT-737 has been investigated in numerous preclinical studies, and the orally available derivative, ABT-263, is now being tested in clinical trials of non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and small-cell lung cancer.
Like most effective drugs, ABT-737 kills some cells but not others. Studies of de novo sensitivity to the drug have produced 2 main principles: (1) cells with BCL-2 primed with large amounts of activators like BIM tend to be sensitive to ABT-737; and (2) high levels of expression of MCL-1 or BFL-1 can result in decreased sensitivity to ABT-737.14,18,20-25 However, there are no available studies of mechanisms of acquired resistance to ABT-737 or ABT-263. Because acquired resistance is a problem with every drug ever used in oncology, we have investigated whether sensitive lymphoma cell lines can spontaneously select for resistance upon prolonged exposure to ABT-737. We have found that acquired resistance does arise, and that it depends on transcriptional up-regulation of MCL-1 alone or in conjunction with up-regulation of BFL-1. Surprisingly, this novel up-regulation has both a stable component and a dynamic component that responds only after ABT-737 treatment.
Methods
Cell lines
OCI-LY1, OCI-LY1 R7, and OCI-LY1 R10 cell lines were cultured in suspension in Iscove modified Dulbecco medium (IMDM; Invitrogen). SU-DHL-4 and SU-DHL-4 R2 cell lines were cultured in suspension in RPMI-1640 media. All media was supplemented with 10% fetal bovine serum (FBS; BioWhittaker, lot no. 01111939), l-glutamine, penicillin/streptomycin (Invitrogen), and 5.0 μg/mL verapamil (Sigma). MCL-1 ShRNA (pLKO.1puro-MCL-1) or Luc ShRNA (pLKO.1puro-luciferase) was obtained from the RNAi Consortium (Broad Institute of the Massachusetts Institute of Technology and Harvard). Knockdown cells were prepared by infecting lymphoma cells with retroviral supernatants produced by cotransfection of 293T cells with pCMVΔR8.91, pMD.G, and either pLKO.1puro-MCL-1 or pLKO.1puro-luciferase.20,26 Stable clones were selected with puromycin (Sigma; 250 μg/mL).
Development of resistance
ABT-737 resistance was established by short-term in vitro exposures beginning with 5nM and continuing up to 1μM ABT-737. Duration of exposure began at 1 hour and was increased to continuous exposure with a 48-hour passage time between exposures. After cells displayed a viability of approximately 90% and were able to grow at a rate equivalent to the parental lines, drug dose was doubled until they reached 1μM ABT-737. When cells were able to maintain a dose of 1μM for 1 hour, the time was doubled until they reached 8 hours. After 8 hours of exposure was maintainable, cells were moved to continuous culture with 1μM (OCI-LY1 R10 and SU-DHL-4 R2) or 500nM (OCI-LY1 R7) ABT-737. Cell lines OCI-Ly1 R7 and OCI-LY1 R10 originated from 2 independent selection experiments.
Cell viability assay
Cells were treated with ABT-737 as indicated in the figure legends. All cell viability experiments performed on the SU-DHL-4 and SU-DHL-4 R2 cell lines were done in low-FBS (1%) conditions. DMSO was used as a solvent-only negative control. Cells were stained with fluorescent conjugates of annexin-V (BioVision) and/or propidium iodide (PI) and analyzed on a FACSCalibur or FACSCanto machine (BD). Viable cells are annexin-V− and PI−. Cycloheximide and ZVAD.fmk were obtained from Calbiochem. PHA 767491 was obtained from Tocris.
Immunoprecipitation and Immunoblotting
Protein lysates were obtained by lysis in CHAPS buffer (150mM NaCl, 5mM MgCl2, 1mM EGTA, 10mM HEPES, 1% CHAPS [Calbiochem]) containing protease inhibitor cocktail, 100nM NaF, and 1mM NA3VO4. Immunoprecipitation was preformed in 50 μL of lysates containing 100 μg of protein. A total of 0.1 g of protein A beads (Sigma) were prepared in 1 mL of lysis buffer and 1% bovine serum albumin (BSA; Sigma). Lysates were incubated with 5 μL of antibody for 1 hour at 4°C. Extracts were incubated with 30 μL of protein A beads for 1 hour at 4°C. Immunoprecipitates were washed 3 times with CHAPS buffer and boiled in loading buffer (Invitrogen). Samples were separated electrophoretically on NuPage 10% Bis-Tris polyacrylamide gels (Invitrogen). Antibodies used included MCL-1 (Santa Cruz Biotechnology; S-19), BIM (Calbiochem; 22-40), BCL-2 (Epitomics; 1017-1), BCL-2 (BD; 6C8), BCL-2 (BD; /100), BCL-xL (kind gift from Larry Boise, Emory University); BAX (Santa Cruz Biotechnology; N20), BAK (Upstate; NT), BID (Santa Cruz Biotechnology; FL-195), actin (Chemicon; MAB1501), NOXA (Calbiochem), and BCL-w (Calbiochem; 16H12). Polyclonal rabbit antibody directed to human BFL-1 was a kind gift from Dr Jannie Borst (Division of Immunology, The Cancer Institute, Amsterdam).
BH3 profiling
Mitochondria were purified as previously described.18 A total of 0.1 mg of protein/mL mitochondria were suspended in experimental buffer and exposed to BH3 domain peptides for 40 minutes at room temperature. Peptides used are previously described.14 Cytochrome c release was determined by comparison of cytochrome c in the pellet and supernatant and quantified by enzyme-linked immunosorbent assay (ELISA; R&D Systems). OCI-LY1 R10 cells and SU-DHL-4 R2 cells were removed from ABT-737 for 1 and 3 weeks, respectively, before mitochondrial isolation. Before isolation, cells were washed twice in PBS.
RNA isolation and real-time RT-PCR
Total RNA was isolated using the Trizol method (Invitrogen). A total of 4 μg of RNA were reverse-transcribed using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems; N8080234) and amplified using Power SYBR Green Master Mix (Applied Biosystems; 4367659) on the 7300 Real-Time PCR system (Applied Biosystems). MCL-1–specific primers (5′-ATGCTTCGGAAACTGGACAT-3′ [forward] and 5′-TCCTGATGCCACCTTCTAGG-3′ [reverse]), BFL-1–specific primers (5′-TTACAGGCTGGCTCAGGACT-3′ [forward] and 5′- AGCACTCTGGACGTTTTGCT-3′ [reverse]), and actin-specific primers (5′-AGAAAATCTGGCACCACACC-3′ [forward] and 5′-AGAGGCG-TACAGGGATAGCA-3′ [reverse]) amplified fragments of the full-length transcripts. Results were normalized to actin.
Results
B-cell lymphoma cells acquire resistance to ABT-737 after long-term exposure
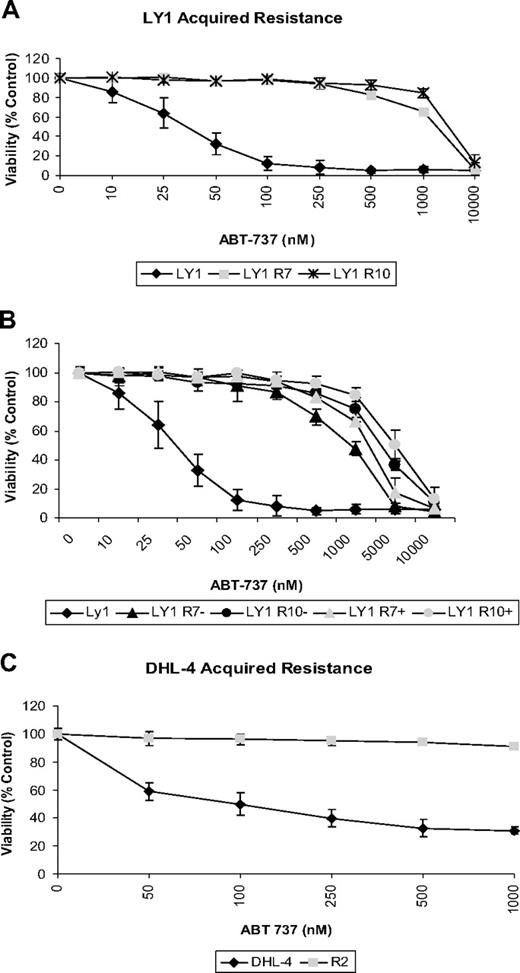
We initiated our study in the diffuse large B-cell lymphoma (DLBCL) lines OCI-Ly1 and SU-DHL-4. These cell lines were chosen based on previous studies in which they demonstrated high sensitivity to the BCL-2 antagonist ABT-737 with EC50 values of 21nM and 140nM, respectively.18 To test whether cancer cells sensitive to ABT-737 would acquire resistance with long-term exposure, we exposed OCI-Ly1 and SU-DHL-4 cells to low doses of ABT-737 for short periods of time over several months. To inhibit resistance based on increased expression of drug efflux pumps, cells treated with ABT-737 and untreated controls were cultured in media containing verapamil.27 Once cell viability was maintained, the dose and time were increased. After several months of treatment, cells were capable of maintaining viability with continuous exposure to ABT-737 at 1μM for the SU-DHL-4 R2 and OCI-LY1 R10 cell lines and 500nM for the OCI-LY1 R7 cell line. OCI-LY1 and SU-DHL-4 cells treated with ABT-737 over an extended period of time acquired resistance to the drug, reaching EC50 values of approximately 2.5μM (Figure 1A) and greater than 1μM (Figure 1C), respectively. The 3 resistant lines, SU-DHL-4 R2, OCI-Ly1 R7, and OCI-Ly1 R10, were independently derived.
B-cell lymphoma cells acquire resistance to ABT-737 after long-term exposure. (A) OCI-Ly1 cells were treated with increasing doses of ABT-737 for 48 hours and stained with annexin-V–FITC for flow cytometry analysis. Before treatments, OCI-Ly1 R7 cells were cultured in media containing 320nM ABT-737 and OCI-Ly1 R10 cells in media with 1μM ABT-737. ABT-737 was withdrawn 24 hours before dose response treatment. Viability is shown as a percentage of DMSO-treated control cells. SD is of quadruplicates and is indicated by error bars. (B) OCI-Ly1 R7 and OCI-Ly1 R10 cells were withdrawn (−) from or continued treatment (+) with ABT-737 for 3 weeks. Cells were then washed and treated with increasing doses of ABT-737 for 48 hours, stained with annexin-V–FITC, and assayed by flow cytometry analysis. Viability is shown as a percentage of DMSO-treated control cells. SD is of quadruplicates and is indicated by error bars. (C) SU-DHL-4 and SU-DHL-4 R2 cells were treated with increasing doses of ABT-737 for 12 hours. After treatment, cells were stained with annexin-V–FITC and PI for flow cytometry analysis. Before treatment, SU-DHL-4 R2 cells were cultured in media containing 1μM ABT-737. ABT-737 was withdrawn 3 weeks before dose response treatment. Viability is shown as a percentage of DMSO-treated control cells. SE is of triplicates and is indicated by error bars.
B-cell lymphoma cells acquire resistance to ABT-737 after long-term exposure. (A) OCI-Ly1 cells were treated with increasing doses of ABT-737 for 48 hours and stained with annexin-V–FITC for flow cytometry analysis. Before treatments, OCI-Ly1 R7 cells were cultured in media containing 320nM ABT-737 and OCI-Ly1 R10 cells in media with 1μM ABT-737. ABT-737 was withdrawn 24 hours before dose response treatment. Viability is shown as a percentage of DMSO-treated control cells. SD is of quadruplicates and is indicated by error bars. (B) OCI-Ly1 R7 and OCI-Ly1 R10 cells were withdrawn (−) from or continued treatment (+) with ABT-737 for 3 weeks. Cells were then washed and treated with increasing doses of ABT-737 for 48 hours, stained with annexin-V–FITC, and assayed by flow cytometry analysis. Viability is shown as a percentage of DMSO-treated control cells. SD is of quadruplicates and is indicated by error bars. (C) SU-DHL-4 and SU-DHL-4 R2 cells were treated with increasing doses of ABT-737 for 12 hours. After treatment, cells were stained with annexin-V–FITC and PI for flow cytometry analysis. Before treatment, SU-DHL-4 R2 cells were cultured in media containing 1μM ABT-737. ABT-737 was withdrawn 3 weeks before dose response treatment. Viability is shown as a percentage of DMSO-treated control cells. SE is of triplicates and is indicated by error bars.
We next tested whether the resistant cells maintained resistance in the absence of continuous exposure to ABT-737. OCI-LY1–derived resistant cells removed from continuous culture with ABT-737 for 3 weeks and cells continuously cultured with the drug were treated with increasing doses of ABT-737. Apoptosis was analyzed by flow cytometry. No changes in viability between continuously treated cells and those previously withdrawn from the drug were observed (Figure 1B). SU-DHL-4 R2 cells also displayed resistance to ABT-737 after a 3-week withdrawal from continuous culture with the drug (Figure 1C).
A priori, this acquired resistance could stem from several different factors. Potential factors include changes affecting the intracellular concentration of the drug, loss of function of proapoptotic proteins, and/or increased levels of antiapoptotic proteins, particularly those not targeted by ABT-737 such as MCL-1 or BFL-1.
BFL-1 and/or MCL-1 proteins are up-regulated in resistant cell lines and are inducible upon treatment with ABT-737
We first investigated whether ABT-737 resistance was mediated by changes in the expression pattern of BCL-2 family proteins. Western blot analysis of BCL-2 family members in cell lines cultured in the absence of ABT-737 for 3 weeks revealed an increase in MCL-1 protein expression in OCI-LY1–derived resistant cells and increases in both MCL-1 and BFL-1 in SU-DHL-4 R2 cells compared with parental cells (Figure 2A,C). Furthermore, expression of BFL-1 and/or MCL-1 in the resistant cell lines appeared to increase further upon either continuous or acute treatment with ABT-737 (Figure 2A-C). In OCI-LY1 R7 cells withdrawn from ABT-737 for 3 weeks, MCL-1 increased within 16 hours after treatment with ABT-737 (Figure 2B). SU-DHL-4 R2 cells withdrawn from treatment for 3 weeks displayed increases in MCL-1 and BFL-1 12 hours after treatment with ABT-737 (Figure 2C). BCL-XL was difficult to detect, but a stable basal increase in BCL-XL was observed in SU-DHL-4 R2 cells, though no increase after ABT-737 treatment was detected. Some contribution by the increase in BCL-XL to acquired resistance cannot be excluded. However, because BCL-XL is also targeted by ABT-737, this modest increase seemed unlikely to contribute to resistance, and so we focused our studies on MCL-1 and BFL-1.
BFL-1 and/or MCL-1 proteins are up-regulated in resistant cell lines and are inducible upon treatment with ABT-737. (A) Immunoblot analysis of OCI-LY1, OCI-LY1 R7, and OCI-LY1 R10 cell line lysates with the indicated antibodies. − indicates that ABT-737 was withdrawn for 3 weeks; +, continuous culture with ABT-737. Unpictured antibody controls confirmed the efficacy of the BCL-XL and BFL-1 antibodies used in this assay. These results are representative of 3 independent experiments. (B) OCI-Ly1 R7 cells were removed from ABT-737–containing media for 3 weeks. ABT-737 was added back to culture for the indicated time frame at 320nM. Whole-cell lysates were made after treatment and analyzed by immunoblot. DMSO was used as a solvent-only negative control. These results are representative of 3 independent experiments. (C) SU-DHL-4 R2 cells were removed from ABT-737–containing media for 3 weeks. SU-DHL-4 and SU-DHL-4 R2 cells were treated with 1μM ABT-737 or DMSO for 12 hours after pretreatment with 10μM ZVAD.fmk or DMSO for 1 hour as indicated. Whole-cell lysates were made after treatment and analyzed by immunoblot. These results are representative of 3 independent experiments. ND indicates that these samples were not analyzed.
BFL-1 and/or MCL-1 proteins are up-regulated in resistant cell lines and are inducible upon treatment with ABT-737. (A) Immunoblot analysis of OCI-LY1, OCI-LY1 R7, and OCI-LY1 R10 cell line lysates with the indicated antibodies. − indicates that ABT-737 was withdrawn for 3 weeks; +, continuous culture with ABT-737. Unpictured antibody controls confirmed the efficacy of the BCL-XL and BFL-1 antibodies used in this assay. These results are representative of 3 independent experiments. (B) OCI-Ly1 R7 cells were removed from ABT-737–containing media for 3 weeks. ABT-737 was added back to culture for the indicated time frame at 320nM. Whole-cell lysates were made after treatment and analyzed by immunoblot. DMSO was used as a solvent-only negative control. These results are representative of 3 independent experiments. (C) SU-DHL-4 R2 cells were removed from ABT-737–containing media for 3 weeks. SU-DHL-4 and SU-DHL-4 R2 cells were treated with 1μM ABT-737 or DMSO for 12 hours after pretreatment with 10μM ZVAD.fmk or DMSO for 1 hour as indicated. Whole-cell lysates were made after treatment and analyzed by immunoblot. These results are representative of 3 independent experiments. ND indicates that these samples were not analyzed.
To test whether or not BFL-1 and/or MCL-1 increase after ABT-737 treatment in the sensitive parental lines, parental cells were pretreated with the caspase inhibitor ZVAD.fmk and then treated with ABT-737. No ABT-737–induced changes in MCL-1 levels were seen in the parental SU-DHL-4 line, though a modest induction of BFL-1 was observed in the SU-DHL-4 cells (Figure 2C). We could not confidently test whether a similar increase in MCL-1 was caused in parental OCI-LY1 cells due to difficulties in obtaining reliable lysates from the dead and dying cells treated with ABT-737, even when using a caspase inhibitor. Expression of other BCL-2 family members, including BIM, BID, BAX, BAK, BFL-1, and NOXA, remained constant across all OCI-LY1–derived cell lines in the presence or absence of ABT-737 (Figure 2A). BCL-2 levels in OCI-LY1 R10 cells do appear to be slightly elevated compared with parental cells, but the magnitude of change is significantly less than for MCL-1. SU-DHL-4 R2 cells displayed slight increases in PUMA and BIM levels and a slight decrease in BAX expression compared with parental SU-DHL-4 cells. These changes were not acutely affected by ABT-737 treatment. It should be noted that while levels of BCL-2 appear to decline in SU-DHL-4 R2 cells compared with the parental SU-DHL-4 cell line (Figure 2C), relative BCL-2 immunoblot signals varied greatly depending on antibody used, rendering accurate comparisons impossible. Our experience suggests that there are differences in posttranslational modification, or even in amino acid sequence via mutation, that are modulating epitope recognition by several BCL-2 antibodies. Binding of BIM by BCL-2 and displacement of Bim by ABT-737 are unaffected, suggesting that function of BCL-2 is nonetheless not detectably altered.
From these data, we conclude that ABT-737–resistant cell lines display a stable up-regulation of BFL-1 and/or MCL-1 compared with sensitive parental cell lines. Upon the addition of ABT-737 to culture, there is an additional dynamic increase in expression of MCL-1 that is not observed in parental cell lines. The SU-DHL-4 R2 cells also display a dynamic increase in BFL-1 expression after treatment with ABT-737 that is recapitulated to a lesser degree in the parental line. However, the level of BFL-1 expression in the SU-DHL-4 parental line remains exceedingly low after treatment with ABT-737 and is barely detectable by immunoblot.
BH3 profiling reveals a mitochondrial basis for acquired resistance to ABT-737
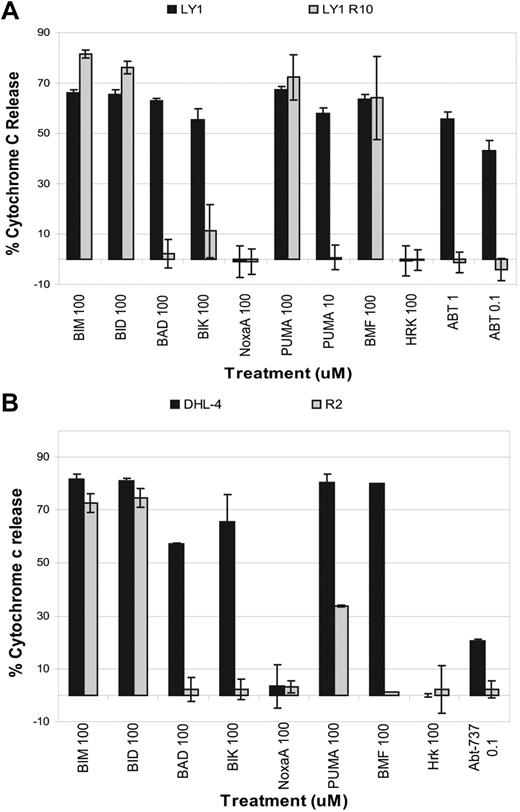
MCL-1 and BFL-1 are antiapoptotic proteins that sequester prodeath BCL-2 family proteins and are largely localized to the mitochondrion.28,29 If changes in the intrinsic apoptotic pathway, such as up-regulation of MCL-1 and BFL-1, were indeed essential for the resistant phenotype, we should be able to observe a difference in the susceptibility of mitochondria to antagonism of BCL-2. Such a difference can be determined using BH3 profiling, a method developed in our laboratory that is used to detect blocks in apoptosis.14,18,20 This tool works by measuring cytochrome c release from isolated mitochondria after treatment with proapoptotic peptides such as PUMA, BAD, NOXA, BIM, and BID. We found that mitochondria from the resistant cells are significantly less sensitive to treatment with BCL-2 antagonists such as the BAD BH3 or PUMA BH3 peptides compared with those obtained from parental cells (Figure 3A-B). In a direct test of mitochondrial sensitivity to ABT-737, mitochondria from the parental cells were more sensitive to ABT-737 treatment than those from the resistant cells. The change in priming status between the mitochondria isolated from the parental SU-DHL-4 cells and those isolated from SU-DHL-4 R2 cells is particularly striking (Figure 3B). Note that observing signal only from PUMA, but not from BAD, NOXA, or BMF, among the sensitizer BH3 domains for SU-DHL-4 R2 indicates an important role for BFL-1 in survival,14,18 consistent with the up-regulation of BFL-1 observed in Figure 2C.
BH3 profiling reveals a mitochondrial basis for acquired resistance to ABT-737. (A) Mitochondria were isolated from OCI-Ly1 and OCI-Ly1 R10 cells and treated with different concentrations of peptides. Cytochrome c release was measured by ELISA. Shown is the average and SE of 3 independent experiments. (B) Mitochondria were isolated from SU-DHL-4 and SU-DHL-4 R2 cells and treated with different peptides. Cytochrome c release was measured by ELISA. Shown is the average and SE of 3 independent experiments. In both cases, ABT-737 was removed from culture medium for at least 1 week.
BH3 profiling reveals a mitochondrial basis for acquired resistance to ABT-737. (A) Mitochondria were isolated from OCI-Ly1 and OCI-Ly1 R10 cells and treated with different concentrations of peptides. Cytochrome c release was measured by ELISA. Shown is the average and SE of 3 independent experiments. (B) Mitochondria were isolated from SU-DHL-4 and SU-DHL-4 R2 cells and treated with different peptides. Cytochrome c release was measured by ELISA. Shown is the average and SE of 3 independent experiments. In both cases, ABT-737 was removed from culture medium for at least 1 week.
These data establish that a cause of resistance is based at the mitochondrion. This finding is consistent with the previously observed alterations in BCL-2 family proteins (Figure 2A,C) and suggests that the observed up-regulations in MCL-1 and BFL-1 play a key role in determining resistance.
ABT-737 reaches its target, BCL-2, in sensitive and resistant cells, and increased MCL-1 and BFL-1 sequester BIM displaced from BCL-2 by ABT-737
These results show a mitochondrial basis to the observed resistance, which argues against upstream effects, such as reduced drug access to mitochondria, being important mechanisms of the resistance. To directly test this, we performed assays directed at comparing the drug reaching its target in parental and resistant cells. We have previously shown that ABT-737's competitive displacement of BIM from BCL-2 appears to play a decisive role in committing the cell to death in many ABT-737–sensitive cells.18,20,27 We next examined how this key event might differ between the parental and resistant cell lines. We immunoprecipitated BCL-2 from parental and resistant whole-cell lysates made using CHAPS detergent in untreated and treated cells and immunoblotted for BIM. In addition, we immunoprecipitated BIM from treated and untreated SU-DHL-4 and SU-DHL-4 R2 CHAPS lysates and immunoblotted for BCL-2. Parental cells were pretreated with ZVAD.fmk before treatment with ABT-737 to prevent apoptosis and subsequent proteolysis. We were able to show that BIM is displaced from BCL-2 in both parental and resistant cell lines after treatment with ABT-737 (Figure 4A-B,D-E). CHAPS is a useful detergent for these studies because it does not induce the artifactual conformation changes in BAX and BAK that result in complex formation with BCL-2.30 It is notable that in CHAPS lysates, BAX does not appear to be priming BCL-2, and thus is not displaced by ABT-737 treatment (Figure 4B,D). Note that Figure 4B, D, and E provide evidence of ABT-737 contacting BCL-2 in resistant cells, as treatment causes BIM displacement, arguing against low drug accumulation as a cause of resistance. Although total BIM levels decrease when cells are treated with ABT-737 (Figure 4A), this loss is abrogated by cotreatment with ZVAD.fmk, whereas BIM is still displaced from BCL-2. Thus, the decrease in BIM:BCL-2 complex is not due simply to loss of BIM in sensitive cells.
ABT-737 reaches its target, BCL-2, in sensitive and resistant cells, and increased MCL-1 and BFL-1 sequester BIM displaced from BCL-2 by ABT-737. (A) OCI-LY1 cells were treated with 1μM ABT-737 for 4 hours after pretreatment with 10μM ZVAD.fmk for 30 minutes as indicated. Cells were lysed with 1% CHAPS lysis buffer. Lysates were immunoprecipitated using BCL-2– and MCL-1–specific antibodies. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot alongside whole-cell lysates. *IgG heavy chain. (B) OCI-Ly1 R10 cells were treated with DMSO or 1μM ABT-737 (+) for 4 hours. Cells were lysed with 1% CHAPS lysis buffer. A total of 100 μg of lysate were immunoprecipitated with the BCL-2 (6C8) antibody. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot alongside whole-cell lysates. These results are representative of 3 independent experiments. (C) OCI-Ly1 R10 cells were treated and lysed as in panel B. A total of 100 μg of lysate were immunoprecipitated with an MCL-1–specific antibody. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot alongside whole-cell lysates. These results are representative of 3 independent experiments. (D) SU-DHL-4 cells were treated with DMSO or 1μM ABT-737 (+) for 12 hours after pretreatment with 10μM ZVAD.fmk for 1 hour. SU-DHL-4 R2 cells were treated with DMSO or 1μM ABT-737 (+) for 12 hours. Cells were lysed with 1% CHAPS lysis buffer. Lysates were immunoprecipitated using a BCL-2–specific antibody (6C8). The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot. (E) SU-DHL-4 and SU-DHL-4 R2 cells were treated and lysed as in panel D. Lysates were immunoprecipitated using a BIM-specific antibody. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot. (F) Immunoblot analysis of the whole-cell lysates used for immunoprecipitation in panels D and E.
ABT-737 reaches its target, BCL-2, in sensitive and resistant cells, and increased MCL-1 and BFL-1 sequester BIM displaced from BCL-2 by ABT-737. (A) OCI-LY1 cells were treated with 1μM ABT-737 for 4 hours after pretreatment with 10μM ZVAD.fmk for 30 minutes as indicated. Cells were lysed with 1% CHAPS lysis buffer. Lysates were immunoprecipitated using BCL-2– and MCL-1–specific antibodies. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot alongside whole-cell lysates. *IgG heavy chain. (B) OCI-Ly1 R10 cells were treated with DMSO or 1μM ABT-737 (+) for 4 hours. Cells were lysed with 1% CHAPS lysis buffer. A total of 100 μg of lysate were immunoprecipitated with the BCL-2 (6C8) antibody. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot alongside whole-cell lysates. These results are representative of 3 independent experiments. (C) OCI-Ly1 R10 cells were treated and lysed as in panel B. A total of 100 μg of lysate were immunoprecipitated with an MCL-1–specific antibody. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot alongside whole-cell lysates. These results are representative of 3 independent experiments. (D) SU-DHL-4 cells were treated with DMSO or 1μM ABT-737 (+) for 12 hours after pretreatment with 10μM ZVAD.fmk for 1 hour. SU-DHL-4 R2 cells were treated with DMSO or 1μM ABT-737 (+) for 12 hours. Cells were lysed with 1% CHAPS lysis buffer. Lysates were immunoprecipitated using a BCL-2–specific antibody (6C8). The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot. (E) SU-DHL-4 and SU-DHL-4 R2 cells were treated and lysed as in panel D. Lysates were immunoprecipitated using a BIM-specific antibody. The resulting immunoprecipitated and coimmunoprecipitated proteins were analyzed by immunoblot. (F) Immunoblot analysis of the whole-cell lysates used for immunoprecipitation in panels D and E.
If increased MCL-1 and BFL-1 expression play important roles in preventing ABT-737–induced death in resistant cells, one potential mechanism for this resistance is that the extra MCL-1 and BFL-1 sequester the BIM displaced from BCL-2. To test this hypothesis, we immunoprecipitated MCL-1 and immunoblotted for BIM (Figure 4C). As we were unable to successfully immunoprecipitate BFL-1, we examined the potential role of BFL-1 and MCL-1 in binding displaced BIM in SU-DHL-4 R2 cells by immunoprecipitating BIM and immunoblotting MCL-1, BFL-1, and BCL-2 (Figure 4E). These experiments suggest that BIM displaced from BCL-2 by ABT-737 in the resistant cells is indeed binding to BFL-1 and/or MCL-1 (Figure 4C,E). BIM displaced from BCL-2 in the SU-DHL-4 parental cells also appears to bind to MCL-1; however, there is presumably additional displaced BIM, and we did not detect any BIM bound to BFL-1 in the parental cells (Figure 4E). We were also unable to detect any binding of displaced BIM by MCL-1 in the OCI-LY1 parental cells (Figure 4A).
BFL-1 and/or MCL-1 are transcriptionally up-regulated in resistant cells
Next, we wanted to investigate the mechanism underlying the increased MCL-1 protein levels in the resistant cell lines. Because MCL-1 apparently plays a more singular role in the resistant OCI-LY-1 cells, we used these cells for further study. MCL-1 protein has a short half-life, on the order of an hour, which can be seen with translational interference by cycloheximide. Sensitive and resistant OCI-LY1 cell lines were treated with cycloheximide, harvested, lysed, and analyzed by Western blot (Figure 5A). Using this method, we saw no differences in MCL-1 half-life, indicating that increased stability of MCL-1 protein is not the cause of increased MCL-1 levels in the OCI-LY1–derived resistant lines.
MCL-1 and BFL-1 are transcriptionally up-regulated in resistant cells. (A) OCI-Ly1, OCI-Ly1 R7, and OCI-LY1 R10 cells were treated with 20 μg/mL CHX for the time indicated. Cells were lysed immediately after treatment and subject to immunoblot analysis. These results are representative of 3 independent experiments. (B) Cells were cultured in the absence of ABT-737 for 2 weeks. RNA was isolated and MCL-1 fold change was analyzed by quantitative PCR. (C) Cells were cultured in the absence of ABT-737 for 2 weeks and then treated with DMSO or 250nM ABT-737 for 16 hours. RNA was isolated and MCL-1 fold change was analyzed by quantitative PCR. (D) OCI-Ly1 and OCI-Ly1 R10 cells were cultured in the absence of ABT-737 for 3 weeks. Cells were then treated with 20μM ZVAD.fmk for 45 minutes before treatment with 1μM ABT-737 for the time indicated. RNA was isolated and MCL-1 fold change was analyzed by quantitative PCR. (E) SU-DHL-4 and SU-DHL-4 R2 cells were cultured in the absence of ABT-737 for 3 weeks and treated and analyzed as in panel D. (F) RNA from panel E was used to assay BFL-1 fold change by quantitative PCR. Error bars in panels B through F indicate the SEM. RNA levels from the untreated parental cell lines were set to 1.
MCL-1 and BFL-1 are transcriptionally up-regulated in resistant cells. (A) OCI-Ly1, OCI-Ly1 R7, and OCI-LY1 R10 cells were treated with 20 μg/mL CHX for the time indicated. Cells were lysed immediately after treatment and subject to immunoblot analysis. These results are representative of 3 independent experiments. (B) Cells were cultured in the absence of ABT-737 for 2 weeks. RNA was isolated and MCL-1 fold change was analyzed by quantitative PCR. (C) Cells were cultured in the absence of ABT-737 for 2 weeks and then treated with DMSO or 250nM ABT-737 for 16 hours. RNA was isolated and MCL-1 fold change was analyzed by quantitative PCR. (D) OCI-Ly1 and OCI-Ly1 R10 cells were cultured in the absence of ABT-737 for 3 weeks. Cells were then treated with 20μM ZVAD.fmk for 45 minutes before treatment with 1μM ABT-737 for the time indicated. RNA was isolated and MCL-1 fold change was analyzed by quantitative PCR. (E) SU-DHL-4 and SU-DHL-4 R2 cells were cultured in the absence of ABT-737 for 3 weeks and treated and analyzed as in panel D. (F) RNA from panel E was used to assay BFL-1 fold change by quantitative PCR. Error bars in panels B through F indicate the SEM. RNA levels from the untreated parental cell lines were set to 1.
Based on these results, we investigated whether MCL-1 levels are increasing due to increased transcript abundance. We isolated mRNA from sensitive and resistant OCI-LY1 cells, both cultured in the absence of ABT-737, and performed reverse transcription–polymerase chain reaction (RT-PCR) followed by quantitative real-time PCR (Figure 5B). Here we found a more than 5-fold increase of MCL-1 mRNA in resistant cells. Due to the transient induction of MCL-1 protein that follows ABT-737 treatment (Figure 2B-C), we also measured mRNA levels with and without ABT-737 treatment (Figure 5C). We found that MCL-1 transcript abundance is stably up-regulated in resistant cells, and that transcript abundance is further dynamically increased upon treatment with ABT-737. These results suggest that either increased transcription rate or increased transcript stability lay at the heart of increased MCL-1 levels in the resistant cells.
We wanted to test whether this dynamic change was a property distinct to the resistant cells. In Figure 5D, we used quantitative PCR to compare MCL-1 transcript levels in parental and resistant OCI-LY-1 cells treated with the caspase inhibitor ZVAD.fmk, necessary to prevent apoptosis in the parental cells. Whereas MCL-1 transcript levels were consistently higher in resistant cells, parental cells shared the property of increasing MCL-1 transcript levels after BCL-2 antagonism.
We also tested MCL-1 transcript levels in SU-DHL-4 parental and resistant cells. In this case, MCL-1 levels in the resistant line start higher than parental, and stay constant even after treatment, corresponding with protein levels observed in Figure 2C. Parental transcript levels increase after BCL-2 antagonism, however. We also examined BFL-1 transcript levels in the SU-DHL-4 parental and resistant cells. Transcript levels in the resistant cells are 20-fold higher than in parental cells before treatment. Parental cells demonstrate a steady increase in transcript after BCL-2 antagonism, whereas resistant cells show an increase at 8 hours. In summary, both parental and resistant cell lines have the capacity for a dynamic response to BCL-2 inhibition that includes an increase in transcript levels of other antiapoptotic proteins.
CDK-9 inhibition decreases MCL-1 and increases sensitivity in resistant cells
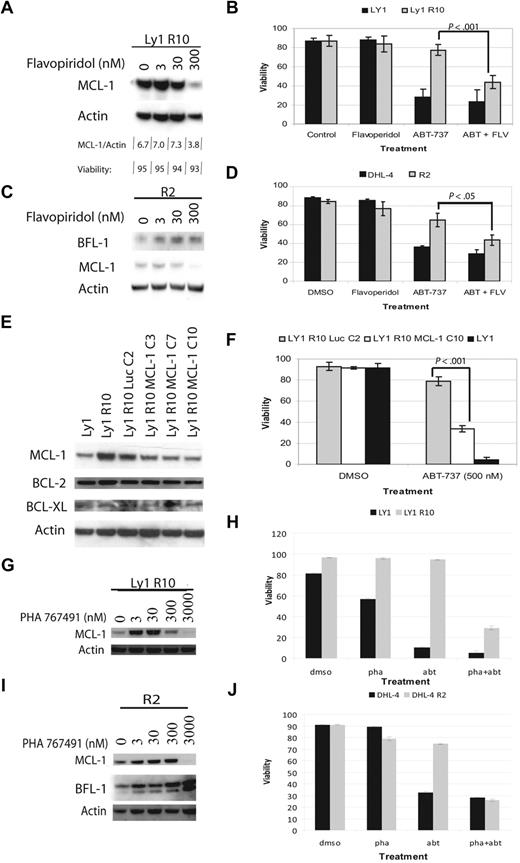
We have established that increased MCL-1 transcript and protein levels correlate with acquired resistance to ABT-737. To confirm that MCL-1 up regulation is a cause of resistance, we examined whether decreasing MCL-1 restores sensitivity to ABT-737–resistant cells. Other groups have shown that flavopiridol can be used to decrease MCL-1 protein levels.31,32 Flavopiridol is a cell-cycle inhibitor that inhibits CDK9 activity, which is required for RNA polymerase II phosphorylation and activation.33 Inhibitors of CDK9 activity are therefore suggested to have a disproportionate effect on short half-life proteins whose levels are most quickly reduced by inhibition of transcription or translation. It is worth noting, however, that flavopiridol doubtless causes other prodeath perturbations, because cells dependent on BCL-2 can also be killed, albeit somewhat less efficiently than MCL-1–dependent ones.34 We first attempted to find a dose of flavopiridol that reduced MCL-1 levels in resistant cells without inducing cell death (Figure 6A,C). We found that a 4-hour treatment with 300nM flavopiridol significantly decreased MCL-1 levels, but did not decrease BFL-1 levels or cell viability. Next, we treated cells with ABT-737, 300nM flavopiridol, or a combination of the 2 drugs (Figure 6B,D). Our results showed that ABT-737–resistant lines were sensitized to ABT-737 when MCL-1 was decreased by flavopiridol. Flavopiridol demonstrated little effect on parental cell lines. PHA 767491 is another inhibitor of CDK9.35 We tested the ability of this agent to lower MCL-1 levels, and found that it could do so at 3μM. Treatment with this agent, like flavopiridol, reversed resistance to ABT-737, while having little effect on parental cell lines (Figure 6G-J).
CDK9 inhibition or shRNA decrease MCL-1 levels and restore sensitivity to ABT-737. (A) Immunoblot analysis of OCI-LY1 R10 whole-cell lysates after 4 hours of flavopiridol treatment with different doses. Viability was assayed by annexin-V–FITC staining and flow cytometry. (B) OCI-LY1 and OCI-LY1 R10 cells were treated for 4 hours with DMSO or 300nM flavopiridol in combination with 0 or 250nM ABT-737, stained with annexin-V–FITC, and analyzed by flow cytometry. This experiment was performed in quadruplicate. (C) Immunoblot analysis of SU-DHL-4 R2 whole-cell lysates after 4 hours of flavopiridol treatment with different doses. (D) SU-DHL-4 and SU-DHL-4 R2 cells were treated for 12 hours with DMSO or 500nM ABT-737. During the last 4 hours of ABT-737 treatment, cells were treated with DMSO or 300nM flavopiridol. Cells were then stained with annexin-V–FITC and PI and analyzed by flow cytometry. The experiment was performed in triplicate. (E) Immunoblot analysis of MCL-1 knockdown by shRNA in single-cell clones. (F) Clones were chosen based on knockdown in panel E and tested for sensitivity by treatment with 500nM ABT-737 or DMSO. This experiment was performed in quadruplicate. All P values were determined by 2-tailed t tests. (G) Immunoblot analysis of OCI-LY1 R10 whole-cell lysates after 4 hours of PHA 767491 treatment with different doses. (H) OCI-LY1 and OCI-LY1 R10 cells were treated for 4 hours with DMSO or 3000nM PHA 767491 in combination with 0 or 1μM ABT-737, stained with annexin-V–FITC, and analyzed by flow cytometry. (I) Immunoblot analysis of SU-DHL-4 R2 whole-cell lysates after 4 hours of PHA 767491 treatment with different doses. (J) SU-DHL-4 and SU-DHL-4 R2 cells were treated for 12 hours with DMSO or 1μM ABT-737. During the last 4 hours of ABT-737 treatment, cells were treated with DMSO or 3000nM PHA 76741. Cells were then stained with annexin-V–FITC and PI and analyzed by flow cytometry. Error bars for panels B, D, and F represent SEM of independent quadruplicate, triplicate, and quadruplicate experiments, respectively. Error bars in panels H and J are representative of technical replicates, and the graphs presented are representative of 2 independent experiments.
CDK9 inhibition or shRNA decrease MCL-1 levels and restore sensitivity to ABT-737. (A) Immunoblot analysis of OCI-LY1 R10 whole-cell lysates after 4 hours of flavopiridol treatment with different doses. Viability was assayed by annexin-V–FITC staining and flow cytometry. (B) OCI-LY1 and OCI-LY1 R10 cells were treated for 4 hours with DMSO or 300nM flavopiridol in combination with 0 or 250nM ABT-737, stained with annexin-V–FITC, and analyzed by flow cytometry. This experiment was performed in quadruplicate. (C) Immunoblot analysis of SU-DHL-4 R2 whole-cell lysates after 4 hours of flavopiridol treatment with different doses. (D) SU-DHL-4 and SU-DHL-4 R2 cells were treated for 12 hours with DMSO or 500nM ABT-737. During the last 4 hours of ABT-737 treatment, cells were treated with DMSO or 300nM flavopiridol. Cells were then stained with annexin-V–FITC and PI and analyzed by flow cytometry. The experiment was performed in triplicate. (E) Immunoblot analysis of MCL-1 knockdown by shRNA in single-cell clones. (F) Clones were chosen based on knockdown in panel E and tested for sensitivity by treatment with 500nM ABT-737 or DMSO. This experiment was performed in quadruplicate. All P values were determined by 2-tailed t tests. (G) Immunoblot analysis of OCI-LY1 R10 whole-cell lysates after 4 hours of PHA 767491 treatment with different doses. (H) OCI-LY1 and OCI-LY1 R10 cells were treated for 4 hours with DMSO or 3000nM PHA 767491 in combination with 0 or 1μM ABT-737, stained with annexin-V–FITC, and analyzed by flow cytometry. (I) Immunoblot analysis of SU-DHL-4 R2 whole-cell lysates after 4 hours of PHA 767491 treatment with different doses. (J) SU-DHL-4 and SU-DHL-4 R2 cells were treated for 12 hours with DMSO or 1μM ABT-737. During the last 4 hours of ABT-737 treatment, cells were treated with DMSO or 3000nM PHA 76741. Cells were then stained with annexin-V–FITC and PI and analyzed by flow cytometry. Error bars for panels B, D, and F represent SEM of independent quadruplicate, triplicate, and quadruplicate experiments, respectively. Error bars in panels H and J are representative of technical replicates, and the graphs presented are representative of 2 independent experiments.
MCL-1 knockdown restores sensitivity in resistant OCI-LY1 cells
Because flavopiridol and PHA 767491 inhibit other kinases, it may affect proteins and processes other than MCL-1.36 We therefore tested another independent strategy for reducing MCL-1 levels, shRNA transfection. We transfected OCI-LY1 R10 cells with 3 different shRNA constucts targeting MCL-1 as well as a control construct targeting luciferase. After single-cell cloning, we were able to identify a construct that produced a subtotal knockdown of MCL-1 to levels comparable with those found in the parental line (Figure 6E). We selected the OCI-Ly1 R10 MCL-1 C10 clone, which displayed the largest knockdown of MCL-1, for further experiments. We compared its sensitivity to ABT-737 with the control OCI-Ly1 R10 Luc C2 and OCI-Ly1 parent cells. Although the C10 knockdown did not completely restore sensitivity to ABT-737 to parental levels, reduction of MCL-1 levels does result in the killing of most resistant cells (Figure 6F). These results suggest that while MCL-1 up-regulation is a key facet of the acquired resistance in OCI-LY1 R10 cells, other mechanisms may also participate.
Discussion
For even the most effective chemotherapies in cancer, acquired resistance is a clinical problem. In most cases, the biologic basis for such acquired resistance is poorly understood. When it is understood, the mechanism often differs from causes of inherent resistance that occur before treatment begins. To plan strategies to overcome acquired resistance, it is necessary first to understand its cause.
Novel small molecules that target BCL-2 and related proteins are now in clinical trials. ABT-263, an orally available derivative of ABT-737, is among them and is being currently tested in CLL, non-Hodgkin lymphoma, and small-cell lung cancer.37 Impressive single-agent responses have been reported, but based on the field's experience with other chemotherapies, it seems inevitable that even those tumors that respond best run some risk of acquiring resistance and recurring. This study is an attempt to understand the molecular basis for acquired resistance in a non-Hodgkin lymphoma model to anticipate its occurrence clinically.
In our lymphoma model of acquired resistance, we find that selection for increased expression of BFL-1 and/or MCL-1 is apparently the key feature in creating resistance. As MCL-1 and BFL-1 are antiapoptotic proteins that are not targeted by ABT-737, this is perhaps not surprising. In fact, it has been observed that de novo resistance to ABT-737 correlates with high levels of MCL-1 expression in acute myelogenous leukemia and small-cell lung cancer.21,23,38,39 In addition, stromal cell signaling–induced BFL-1 expression has been suggested as a significant source of de novo resistance in CLL.25 In this paper, we tested whether a distinct mechanism of resistance might be selected for in the case of acquired resistance. This would be particularly likely if the biologic effects of ABT-737 extended beyond its intended targets. The fact that mechanisms of acquired resistance are based on overexpression of antiapoptotic BCL-2 family proteins poorly targeted by ABT-737 suggests that we really have a useful understanding of how this drug kills. Furthermore, it suggests that, perhaps due to the proximity of the target to the commitment to cell death, the variety of mechanisms of resistance available to an initially sensitive cell may be quite limited.
We show by 3 methods, flavopiridol treatment, PHA 767491, and shRNA transfection, that decreasing MCL-1 levels restores sensitivity. Of these 3, only flavopiridol treatment is currently clinically relevant, as it is also being used in human clinical trials. However, given its myriad effects, caution must be used in interpreting flavopiridol as simply an MCL-1–lowering agent. Given the experience of sometimes severe side effects connected with its clinical use, including a surprisingly rapid onset of a syndrome resembling tumor lysis syndrome, in vivo studies of the combination would be prudent before further clinical exploration.40 We present it here simply as a useful in vitro tool to demonstrate a correlation between restoration of low MCL-1 levels and restoration of sensitivity. That this correlation is also seen in our shRNA experiments lends confidence to our conclusion that increased MCL-1 levels are indeed critical in causing the acquisition of resistance to ABT-737.
Further bolstering our confidence of the importance of the observed MCL-1 and BFL-1 increases in inducing resistance is our demonstration that the resistance is mitochondrially based. To perform this study, we made use of a technique we have found increasingly useful, BH3 profiling. We have found this technique useful in studying determinants of resistance in other systems, and this study bears out once again its utility. Following up on this study, we captured the displacement of BIM from BCL-2 to MCL-1 and BFL-1, confirming the participation of MCL-1 and BFL-1 in the mechanism of resistance.
Although the detection of increased BFL-1 and/or MCL-1 levels in cell lines that acquired resistance to ABT-737 may not have been very surprising, the mechanism of up-regulation was unexpected. Control of MCL-1 levels by modulation of protein half-life has been reported by several groups, and we were surprised not to see that occur in this model, particularly considering the short half-life of the MCL-1 protein.41,42 A stable increase in transcript abundance is perhaps not completely unexpected, but the dynamic component of it is completely novel. With our current knowledge of the functions of BCL-2 family proteins, there is no mechanism to explain how inhibition of BCL-2 with ABT-737 yields a dynamic increase in MCL-1 and BFL-1 transcript and protein levels. There appears to be an entirely new biologic pathway at work suggesting a novel connection of antiapoptotic protein function to transcript levels. Such a mechanism appears to be present in both resistant cells and parental cells that are temporarily preserved by caspase inhibition.
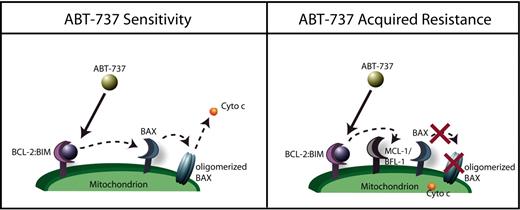
ABT-737 is nearly unique as a drug because we understand how it kills cells all the way from drug contacting target to commitment to cell death. The primary reason for this is that, unlike other drugs, there are very few steps between drug contacting target and the decision to commit to apoptosis. In Figure 7, we summarize what we have found in this study. In sensitive parental cells, ABT-737 displaces BIM from BCL-2, allowing BIM to activate BAX and BAK and committing the cell to death. Resistant cells express high levels of BFL-1 and/or MCL-1, allowing them to intercept the displaced BIM. In Figure 4, we are able to capture this “ping-pong” displacement and recapture of BIM after ABT-737 treatment as it occurs in resistant cells.
Proposed mechanism of acquired resistance. In sensitive cells, BIM is displaced from BCL-2, inducing BAX activation and apoptosis. In resistant cells, BIM is still displaced, but is captured by BFL-1 and/or MCL-1, preventing BAX activation and maintaining survival.
Proposed mechanism of acquired resistance. In sensitive cells, BIM is displaced from BCL-2, inducing BAX activation and apoptosis. In resistant cells, BIM is still displaced, but is captured by BFL-1 and/or MCL-1, preventing BAX activation and maintaining survival.
These studies once again support the essential role that priming of BCL-2 with activator proteins like BIM plays in determining sensitivity to ABT-737. This current work suggests that such priming is necessary, but it is not by itself sufficient to absolutely predict sensitivity to ABT-737. In cells that have not been subjected to continuous treatment with ABT-737, priming alone is likely a good index of response, as there has been no selection pressure to escape the consequences of priming. Indeed, when we previously examined a large group of lymphoma cell lines, the quantity of primed BCL-2:BIM complex quantitatively predicted in vitro response to ABT-737.18 However, after perturbation by selection due to exposure to ABT-737, priming can be maintained, but resistance is acquired by increased expression of “empty” antiapoptotic proteins unbound by ABT-737, in this case BFL-1 and/or MCL-1. Note that while identifying ABT-737 sensitive cells by looking for BIM:BCL-2 complex levels may miss resistance induced by MCL-1 and BFL-1 expression, the functionally driven BH3 profiling still correctly identifies cells with decreased sensitivity despite persistent BIM:BCL-2 complexes (Figure 3).
It has been suggested that sequestration of activator BH3-only proteins is not a mechanism by which antiapoptotic proteins prevent death.12,17 Rather, antiapoptotic proteins inhibit death solely due to their ability to sequester BAX or BAK. In this view, when any BH3-only protein binds an antiapoptotic protein, the effect is to promote apoptosis by displacing BAX or BAK. This model is inconsistent with the results we present here. Here, we could not detect sequestration of BAX by BCL-2 (Figure 4B,D), and yet the cell is nonetheless sensitive to antagonism of BCL-2. It is BIM, not BAX, that is displaced to cause death, and it is BIM that is sequestered by BFL-1 and/or MCL-1 in the resistant cells to preserve life. These results suggest that, at least in these cells, it is the sequestration of BIM rather than BAX that is the essential role that is played by BCL-2 in maintaining survival.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Elena Ivanovna and Alexei Protopopov for FISH and karyotypic analysis of cell lines.
The authors would like to gratefully acknowledge support from National Institutes of Health (NIH) grant nos. K08 CA10254 (A.L.) and R01CA129974, and Leukemia & Lymphoma Society grant SCOR no. 7391-07.
A.L. is a Leukemia & Lymphoma Society Scholar.
National Institutes of Health
Authorship
Contribution: D.Y., N.E.C., J.D., and A.L. contributed to the design and analysis of experiments; D.Y., N.E.C., and A.L. wrote the manuscript; D.Y., N.E.C., and J.D. conducted all of the experiments, performed statistical analysis, and made the figures; and A.L. supervised the study.
Conflict-of-interest disclosure: A.L. is a founder of Eutropics Pharmaceuticals and a member of its scientific advisory board and has served on an advisory board for Abbott Laboratories. A patent application covering the BH3 profiling technology has been filed with the United States Patent Office. The remaining authors declare no competing financial interests.
Correspondence: Anthony Letai, Department of Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: anthony_letai@dfci.harvard.edu.
References
Author notes
D.Y., N.E.C., and J.D. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal