Abstract
In a previously developed inducible transgenic mouse model of chronic myeloid leukemia, we now demonstrate that the disease is transplantable using BCR-ABL+ Lin−Sca-1+c-kit+ (LSK) cells. Interestingly, the phenotype is more severe when unfractionated bone marrow cells are transplanted, yet neither progenitor cells (Lin−Sca-1−c-kit+), nor mature granulocytes (CD11b+Gr-1+), nor potential stem cell niche cells (CD45−Ter119−) are able to transmit the disease or alter the phenotype. The phenotype is largely independent of BCR-ABL priming before transplantation. However, prolonged BCR-ABL expression abrogates the potential of LSK cells to induce full-blown disease in secondary recipients and increases the fraction of multipotent progenitor cells at the expense of long-term hematopoietic stem cells (LT-HSCs) in the bone marrow. BCR-ABL alters the expression of genes involved in proliferation, survival, and hematopoietic development, probably contributing to the reduced LT-HSC frequency within BCR-ABL+ LSK cells. Reversion of BCR-ABL, or treatment with imatinib, eradicates mature cells, whereas leukemic stem cells persist, giving rise to relapsed chronic myeloid leukemia on reinduction of BCR-ABL, or imatinib withdrawal. Our results suggest that BCR-ABL induces differentiation of LT-HSCs and decreases their self-renewal capacity.
Introduction
Chronic myeloid leukemia (CML) arises from the transformation of a hematopoietic stem cell (HSC).1,2 In vivo studies have elucidated the role of the oncogene BCR-ABL,3-7 and this has led to the development of tyrosine kinase inhibitors (TKIs), which have revolutionized the treatment of CML.8-10 Despite encouraging results with TKI in chronic phase, secondary resistance caused by BCR-ABL mutations as well as primary resistant disease (accelerated-phase CML or blast crisis) hamper the response to antileukemic treatment.11 Moreover, cell populations enriched for HSCs have been shown to persist despite TKI treatment in vitro and in vivo.12-14 However, it is currently unclear whether this is the result of insufficient inhibition of BCR-ABL by TKI or BCR-ABL–independent survival of these cells. In retroviral mouse models, BCR-ABL–mediated CML-like disease was conferred by HSCs but not progenitor cells,15,16 and this was most probably the result of limited self-renewal activity of the latter cell population. However, the role of more mature cells in the pathogenesis of BCR-ABL disease has not been resolved.
Several reports have suggested that CML stem cells may possess decreased self-renewal potential compared with their normal counterparts. This was seen in human17-20 as well as murine cells21 and depended on the phase of disease. Whereas bone marrow (BM) cells from blast crisis CML readily transferred the aggressive disease, BM cells from chronic-phase CML failed to transplant the disease.17,18 Interestingly, chronic-phase BM also showed inferior engraftment of NOD/SCID mice compared with Philadelphia chromosome–negative (Ph−) cells.17,18 Engraftment was improved using CML samples that were highly enriched for Ph+ long-term culture initiation cells (LT-CICs); nonetheless, these cells were unable to transplant the disease.19 Together, these results suggest that CML stem cells show reduced engraftment and self-renewal potential, which is in line with data showing reduced replating potential of Lin−CD34+CD38− cells from patients with CML.22 However, the effects of BCR-ABL on long-term HSCs (LT-HSCs) and how it affects their efficiency to repopulate irradiated hosts and transfer disease are still incompletely understood.
We have previously generated transgenic mice inducibly and reversibly expressing BCR-ABL, under the control of the 3′ enhancer of the murine stem cell leukemia (SCL) gene, thus targeting BCR-ABL expression mainly to the HSC population, and these mice develop a chronic myeloproliferative disorder resembling human CML.6 These mice offer the possibility to study leukemogenesis in vivo under steady-state conditions as well as the repopulation potential of BCR-ABL+ stem cells after transplantation. Here, we provide evidence that the use of sibling recipients allows transplantation of the disease via Lin−Sca-1+c-kit+ (LSK) cells, that the use of unfractionated bone marrow (ufBM) leads to a more severe disease phenotype, that BCR-ABL induces differentiation and thereby decreases self-renewal of the LSK compartment, and that abrogation of BCR-ABL activity does not lead to eradication of leukemic stem cells.
Methods
Real-time quantitative reverse-transcribed polymerase chain reaction
Isolation of DNase-treated RNA was performed using RNeasy Mini Kit or RNeasy Micro Kit for BM and spleen and QIAampRNA Blood Mini Kit (QIAGEN) for isolation from peripheral blood (PB). RNA isolation was followed by cDNA synthesis using the Moloney murine leukemia virus reverse transcriptase from Promega following the manufacturer's protocol. For detection of p210 BCR-ABL transcripts, a real-time TaqMan assay (Eurogentec) and PCR master (TaqMan Universal PCR Master Mix, Applied Biosystems) were used in combination with the ABI 7500 Fast Real-Time PCR system (Applied Biosystems). As an internal standard, the expression level of glyceraldehyde-3-phosphate dehydrogenase was used.
Mice and genotyping
Genotyping of SCLtTAxBCR-ABL double transgenic (dtg), SCLtTA single transgenic (stg), and BCR-ABL stg mice was described previously.6 Twelve-week-old wild-type (wt) FVB/N recipients were purchased from Charles River Laboratories. FVB/N CD45.2+ recipients have been previously described.23 Approval for the animal research was obtained from the local authorities of North Rhine–Westphalia.
BM transplantation
BM cells were harvested from the femurs and tibias of SCLtTA/BCR-ABL (dtg) and control mice (SCLtTA stg, BCR-ABL stg, or wt). For transplantation of ufBM, 1 × 106 cells were injected into the tail veins of 12-week-old FVB/N wt or CD45.2 FVB/N mice as indicated. For transplantation of LSK cells, 1200 or 2500 fluorescence-activated cell sorter (FACS)–sorted cells were used for injection, along with 1 × 105 wt BM cells; wt cells were also cotransplanted with 2500 progenitor cells (Lin−Sca-1−c-kit+). Transplantation of CD11b+Gr-1+ granulocytes was conducted using 3.6 × 105 cells, along with 8 × 104 wt BM cells. Donor mice had either been induced for 3 weeks before BM harvest or never been induced. All recipients were irradiated before transplantation using 9.5 or 10.5 Gy. For infection prophylaxis, mice were treated with cotrimoxazole (Ratiopharm) until 2 weeks after transplantation.
Imatinib treatment
Imatinib treatment was performed in transplanted recipients, starting 4 weeks after transplantation, over a 4-week period. Imatinib (LC Laboratories) was dissolved in water daily and applied by oral gavage, using 100 mg/kg body weight twice daily.
Analysis and noninvasive monitoring of disease
PB was collected from the retro-orbital plexus. The number of white blood cells (WBCs) was determined using a Hemavet counter (Drew Scientific). Light microscopy was performed with an Axioplan microscope (Zeiss) using a 25× Plan-Neofluar 0.80, 63× Plan-Apochromat 1.4 oil, or 100× Plan-Neofluar 1.30 oil lens. Images were captured using Axiovision (Zeiss), Photoshop Version 5.5 (Adobe Systems), and Microsoft PowerPoint 2000.
Flow cytometric analysis
PB, BM, and spleen cells were isolated as described previously.6 Lysis of enucleated red blood cells was achieved by applying ammonium-chloride-potassium buffer. After washing, cells were analyzed using a FACSCalibur or FACSAria machine (BD Biosciences) after incubation with the following antibodies: murine CD3, B220, Gr-1, CD41, Ter119, IgG2a, and IgG2b (BD Biosciences) and CD11b antibodies (Invitrogen).
Multicolor FACS sorting and analysis of HSCs
For analysis of LSK cells, red blood cell-lysed BM and spleen cells were stained with tricolor-conjugated rat antibodies specific for the following lineage markers: CD3, CD4, CD8a, CD45R (B220), and Ly-6G (Gr-1; Invitrogen), and CD11b and Ter119 (eBioscience). LSK cells were sorted using CD117 (c-kit)–allophycocyanin, Sca-1 (Ly-6A/E)–biotin, antistreptavidin-phycoerythrin-Cy7, (BD Biosciences) antibodies. LT-HSCs, short-term (ST)–HSCs, and multipotent progenitors (MPPs) were identified using CD34 and CD135 (Flt3) antibodies in addition (BD Biosciences).
Microarray analysis of HSCs
BM-derived cells were isolated from 3 induced dtg and 3 induced control mice (wt or stg). A total of 5000 LSK cells were FACS-sorted directly into RNA extraction buffer. After addition of carrier RNA, RNA was DNAse-treated, extracted, and frozen at −80°C. The yield of total RNA ranged between 10 and 20 ng. For linear amplification of less than 20 ng of total RNA, we applied a technique consisting of 2 cycles of reverse transcription plus T7 promoter-based in vitro transcription.24 The RNA yield from purified LSK cells of each mouse ranged between 15 and 25 μg of biotinylated cRNA. A total of 15 μg of the biotinylated cRNA was hybridized to Affymetrix Mouse Genome 430 2.0 GeneChips covering approximately 45 000 transcripts. After hybridization at 45°C for 16 hours, the GeneChips were analyzed using an Affymetrix GeneChip Scanner 3000. For evaluating the functional significance of altered gene expression, a pathway analysis was performed, using Ingenuity Pathway Analysis software 7.0 (IPA, Ingenuity Systems).
Statistical analysis
Statistical analyses were performed using Student t test (normal distribution) or Mann-Whitney U test (when normal distribution was not given). P less than .05 was considered statistically significant.
Results
Chronic-phase CML can be transplanted using BCR-ABL+ LSK cells, but the phenotype is more severe when ufBM is used
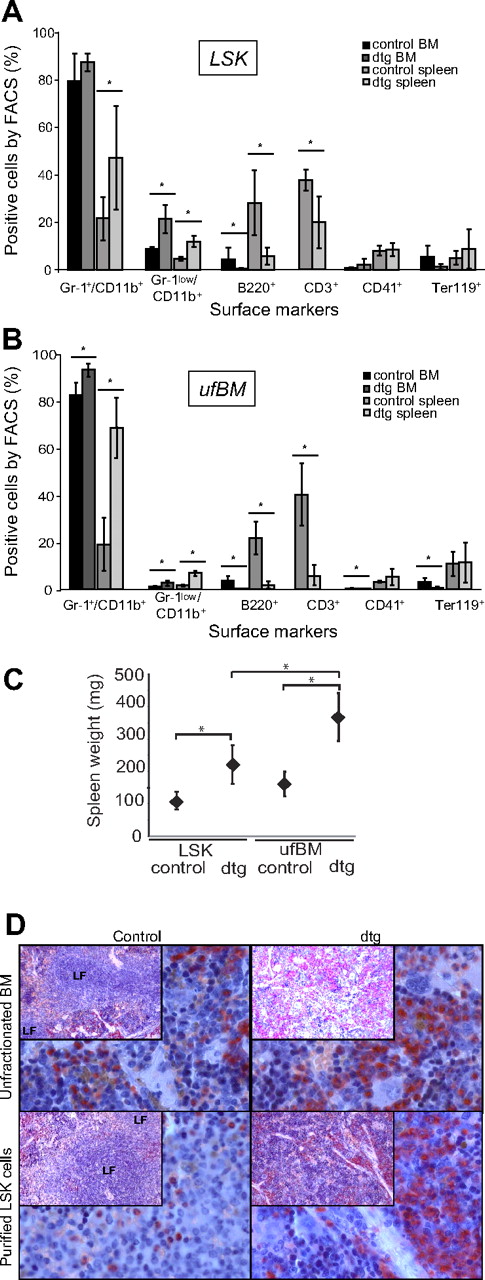
We have previously generated tet-off inducible transgenic mice reversibly expressing BCR-ABL under the control of the SCL 3′ enhancer, and these mice develop a chronic-phase CML-like disease.6 Transplantation of BM or spleen-derived SCLtTA/BCR-ABL (dtg) donor cells into NOD/SCID mice had previously resulted in premature loss of donor cells and no detectable disease in the engrafted mice.6 Here, we therefore decided to transplant the donor cells into 10.5-Gy irradiated littermate sibling FVB– animals. Transplantation of BCR-ABL+ FACS-sorted LSK cells from the BM of 3-week induced mice led to a chronic-phase CML-like disease, closely resembling the disease seen in primary mice, demonstrating that the disease is cell-autonomous to BM-derived stem cells (Figure 1A). However, when ufBM donor cells were transplanted, the disease was more severe as evident by the significant increase in leukocytes (WBCs) and absolute numbers of granulocytes in recipient mice, compared with LSK cell-transplanted mice, 7 weeks after transplantation (Table 1; Figure 1B). In addition, a shortened latency (moribund condition of LSK cell recipients at 11 weeks vs 8 weeks in ufBM recipients), but an increased extent of splenomegaly, was observed in ufBM recipients (Table 1; Figure 1C; P < .05). This was true even though the actual number of transplanted LSK cells was 3-fold lower in the ufBM-transplanted group compared with the LSK-transplanted group (Table 1). When we studied the disease phenotype, we found an increase of granulocytic cells, both in the BM and spleen, whereas erythropoiesis was suppressed only in BM (Figure 1A-B,D).
CML-like disease is transplantable using LSK or ufBM cells. For transplantation experiments, BM cells of 3-week–induced SCLtTA/BCR-ABL (dtg) or single transgenic (stg) control donor mice were used (n = 4 each). (A) Recipients of 1200 FACS-sorted LSK cells showed signs of disease 11 weeks after transplantation and were analyzed at that time point. Percentages of mature granulocytes (Gr-1+CD11b+), immature granulocytes (Gr-1lowCD11b+), B cells (B220+), megakaryocytes (CD41+), and erythroid cells (Ter119+) were measured in BM and spleen. In addition, the percentage of T cells (CD3+) was measured in spleen. (B) Recipients of 1 × 106 ufBM cells were moribund and thus analyzed 8 weeks after transplantation. FACS analysis was performed according to LSK transplanted mice in panel A above (stg n = 5, dtg n = 4). (C) Splenomegaly was more pronounced in mice transplanted with ufBM compared with LSK cells. (D) Histologic analyses of the spleen were performed using naphthyl acetate (chloro-)esterase (NACE)–stained slides and are shown at original magnifications of ×10 (insets) and ×40. Infiltration by myeloid cells as well as a disturbed lymph follicle architecture were evident in the spleen of recipients, transplanted with ufBM or LSK cells from dtg, but not control donors. All data are shown as mean ± SD. *P < .05.
CML-like disease is transplantable using LSK or ufBM cells. For transplantation experiments, BM cells of 3-week–induced SCLtTA/BCR-ABL (dtg) or single transgenic (stg) control donor mice were used (n = 4 each). (A) Recipients of 1200 FACS-sorted LSK cells showed signs of disease 11 weeks after transplantation and were analyzed at that time point. Percentages of mature granulocytes (Gr-1+CD11b+), immature granulocytes (Gr-1lowCD11b+), B cells (B220+), megakaryocytes (CD41+), and erythroid cells (Ter119+) were measured in BM and spleen. In addition, the percentage of T cells (CD3+) was measured in spleen. (B) Recipients of 1 × 106 ufBM cells were moribund and thus analyzed 8 weeks after transplantation. FACS analysis was performed according to LSK transplanted mice in panel A above (stg n = 5, dtg n = 4). (C) Splenomegaly was more pronounced in mice transplanted with ufBM compared with LSK cells. (D) Histologic analyses of the spleen were performed using naphthyl acetate (chloro-)esterase (NACE)–stained slides and are shown at original magnifications of ×10 (insets) and ×40. Infiltration by myeloid cells as well as a disturbed lymph follicle architecture were evident in the spleen of recipients, transplanted with ufBM or LSK cells from dtg, but not control donors. All data are shown as mean ± SD. *P < .05.
Phenotype of mice transplanted with unfractionated BM or FACS-purified LSK cells
| . | Unfractionated BM . | LSK cells . | ||
|---|---|---|---|---|
| Control . | SCLtTA/BCR-ABL . | Control . | SCLtTA/BCR-ABL . | |
| Total no. of transplanted cells per mouse | 1 × 106 | 1 × 106 | 1200 (+1 × 105 wt) | 1200 (+1 × 105 wt) |
| No. of transplanted LSK cells per mouse | 230 | 398 | 1200 | 1200 |
| WBCs, ×109/L | 5.2 ± 0.09 | 18.8 ± 9.9* | 4.82 ± 1.2 | 5.88 ± 1.9† |
| Gr-1+CD11b+ cells in the PB, percentage | 20 ± 6.1 | 68.8 ± 14.52* | 28.5 ± 14.8 | 53.6 ± 16.1 |
| Absolute no. of Gr-1+CD11b+ cells in the PB, ×103/μL | 0.9 ± 0.3 | 12.7 ± 7.4* | 1.5 ± 1.1 | 3.4 ± 2.1† |
| Moribund appearance after BM transplantation, wk | Never | 8 | Never | 11 |
| . | Unfractionated BM . | LSK cells . | ||
|---|---|---|---|---|
| Control . | SCLtTA/BCR-ABL . | Control . | SCLtTA/BCR-ABL . | |
| Total no. of transplanted cells per mouse | 1 × 106 | 1 × 106 | 1200 (+1 × 105 wt) | 1200 (+1 × 105 wt) |
| No. of transplanted LSK cells per mouse | 230 | 398 | 1200 | 1200 |
| WBCs, ×109/L | 5.2 ± 0.09 | 18.8 ± 9.9* | 4.82 ± 1.2 | 5.88 ± 1.9† |
| Gr-1+CD11b+ cells in the PB, percentage | 20 ± 6.1 | 68.8 ± 14.52* | 28.5 ± 14.8 | 53.6 ± 16.1 |
| Absolute no. of Gr-1+CD11b+ cells in the PB, ×103/μL | 0.9 ± 0.3 | 12.7 ± 7.4* | 1.5 ± 1.1 | 3.4 ± 2.1† |
| Moribund appearance after BM transplantation, wk | Never | 8 | Never | 11 |
WBC and Gr-1+CD11b+ cells were assessed 7 weeks after transplantation.
P < .05 vs control group.
P < .05 vs ufBM SCLtTA/BCR-ABL group. ufBM, n = 5 (stg) or 4 (dtg); LSK, n = 4 each.
Hematopoietic progenitor and CD11b+Gr-1+ cells do not initiate or contribute to the CML phenotype
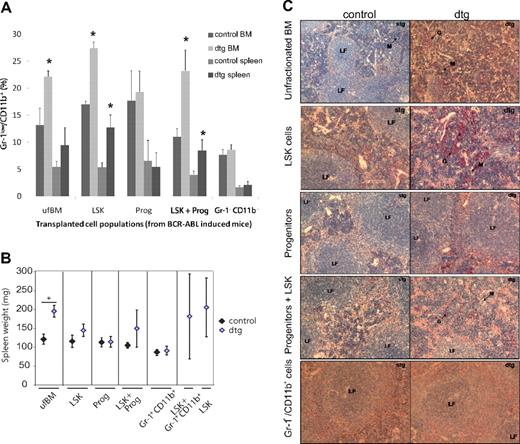
Although expression of BCR-ABL is decreased in CML progenitor compared with LSK cells in our SCLtTA/BCR-ABL mouse model, BCR-ABL is still readily expressed within the progenitor cell population (M. Schemiouek and S.K., unpublished data, November 2008). To assess their contribution to the disease phenotype, we transplanted 1 × 106 ufBM cells, 2500 LSK cells, 2500 Lin−Sca-1−c-kit+ progenitor cells, or LSK cells in combination with progenitors, into 9.5-Gy irradiated recipients. All donor mice had been induced for 3 weeks before BM harvest. Progenitor cells were unable to give rise to CML-like disease on transplantation. In addition, they were unable to alter the disease phenotype in transplantation recipients when added back to BCR-ABL+ LSK cells (Figure 2). These results suggest that a phenotypically more mature cell population is responsible for the enhanced disease seen after transplantation of ufBM. To assess the contribution of granulocytic cells, we transplanted 3.6 × 105 CD11b+Gr-1+ cells, which still express low levels of BCR-ABL, into 9.5-Gy irradiated recipients (M. Schemiouek and S.K., unpublished data, November 2008). In addition, the same number of granulocytes was transplanted along with 2500 LSK cells. However, these cells were also unable to induce CML-like disease when transplanted alone, or to enhance the disease phenotype with LSK cells, in the transplanted recipients (Figure 2). In addition, cotransplantation of 9 × 103 CD45−Ter119− cells, which harbor cells from the BM microenvironment and the megakaryocytic lineage, did not further increase the disease phenotype when transplanted with LSK cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Although the percentage of Gr-1lowCD11b+ granulocytes did increase on transplantation of dtg LSK cells, we could not detect any difference on cotransplantation of CD45−Ter119− cells (supplemental Figure 1).
Leukemic granulocytes or progenitors do not initiate or enhance the CML-like disease. Hematopoietic subpopulations were isolated by FACS sorting from BM of 3-week-induced leukemic SCLtTA/BCR-ABL (dtg) and wt or stg control mice. Purification of cell populations was performed according to surface markers expressed by LSK cells, progenitors (Lin−Sca-1−c-kit+), or granulocytes (Gr-1+CD11b+). Recipients were transplanted with 1 × 106 ufBM, 2500 LSK cells, 2500 progenitors, or 3.6 × 105 granulocytes. In addition, LSK cells were added back to additional groups of mice receiving granulocytes or progenitors. Each error bar represents the mean ± SD. (A) The percentage of immature granulocytes (Gr-1low/CD11b+) measured in BM and spleen of mice transplanted with different subpopulations of cells. (B) Spleen weight was unchanged in mice receiving granulocytes or progenitors only. (C) Histologic analyses of spleen were performed by NACE stain. Infiltration with granulocytes and disturbed follicles were only evident in spleens of recipients transplanted with ufBM, LSK cells, or LSK cells added back to granulocytes or progenitors. Slides are depicted at original magnifications of ×10 or ×20. Mice receiving ufBM, LSK, progenitors, or LSK+ progenitors isolated from stg or dtg mice were analyzed between 49 to 55 days after transplantation. Mice receiving granulocytes from dtg or stg donors were analyzed 77 days after transplantation. Recipients of dtg LSK cells or LSK cells plus granulocytes were analyzed 126 days after transplantation. n = 4 each for ufBM, LSK, progenitors, or Gr1+CD11b+ cells. n = 4 (stg) or 3 (dtg) for LSK+ progenitors; n = 3 dtg for LSK and LSK+ Gr1+CD11b+ cells. *P < .05.
Leukemic granulocytes or progenitors do not initiate or enhance the CML-like disease. Hematopoietic subpopulations were isolated by FACS sorting from BM of 3-week-induced leukemic SCLtTA/BCR-ABL (dtg) and wt or stg control mice. Purification of cell populations was performed according to surface markers expressed by LSK cells, progenitors (Lin−Sca-1−c-kit+), or granulocytes (Gr-1+CD11b+). Recipients were transplanted with 1 × 106 ufBM, 2500 LSK cells, 2500 progenitors, or 3.6 × 105 granulocytes. In addition, LSK cells were added back to additional groups of mice receiving granulocytes or progenitors. Each error bar represents the mean ± SD. (A) The percentage of immature granulocytes (Gr-1low/CD11b+) measured in BM and spleen of mice transplanted with different subpopulations of cells. (B) Spleen weight was unchanged in mice receiving granulocytes or progenitors only. (C) Histologic analyses of spleen were performed by NACE stain. Infiltration with granulocytes and disturbed follicles were only evident in spleens of recipients transplanted with ufBM, LSK cells, or LSK cells added back to granulocytes or progenitors. Slides are depicted at original magnifications of ×10 or ×20. Mice receiving ufBM, LSK, progenitors, or LSK+ progenitors isolated from stg or dtg mice were analyzed between 49 to 55 days after transplantation. Mice receiving granulocytes from dtg or stg donors were analyzed 77 days after transplantation. Recipients of dtg LSK cells or LSK cells plus granulocytes were analyzed 126 days after transplantation. n = 4 each for ufBM, LSK, progenitors, or Gr1+CD11b+ cells. n = 4 (stg) or 3 (dtg) for LSK+ progenitors; n = 3 dtg for LSK and LSK+ Gr1+CD11b+ cells. *P < .05.
BCR-ABL expression decreases the repopulating potential of leukemic cells by decreasing LT-HSC numbers
To directly assess the effect of BCR-ABL induction on the repopulation capacity of LSK cells and on the disease phenotype, we compared transplantation of BM cells from mice in which BCR-ABL had been induced for 3 weeks before donor cell harvest with transplantation of noninduced cells. Tetracycyline was withdrawn from all transplantation recipients to induce BCR-ABL expression in the donor cells after transplantation. These experiments showed that disease in recipient mice occurred independent of the induction status of the cells before transplantation. Total ufBM as well as FACS-purified LSK cells were able to induce the disease in recipients (Figure 3A-C).
Serial transplantation reduces CML-like disease. Recipients were transplanted with 1 × 106 ufBM cells or 2500 LSK cells, isolated from dtg or stg mice. Donors had either been induced for 3 weeks (preinduced) or never been induced (noninduced) before BM harvest. (A) FACS analyses of BM and spleen showed increasing percentages of immature granulocytes in mice that had been transplanted with either preinduced or noninduced ufBM or LSK cells from dtg mice. Graphs show mean ± SD; n = 4 per group. (B) Serial transplantation of 1 × 106 ufBM cells isolated from diseased primary recipients of ufBM (first-degree transplantation) did not lead to CML-like phenotype in BM of secondary recipients (second-degree transplantation). Depicted is the percentage of immature granulocytes in BM and spleen of serial transplanted mice; n = 4. (C) Splenomegaly was only evident in primary recipients but was not observed on serial transplantation. (D) Histologic analysis by NACE stain of the spleen of secondary recipients that had received ufBM from preinduced donors showed no alterations of spleen morphology. *P < .05.
Serial transplantation reduces CML-like disease. Recipients were transplanted with 1 × 106 ufBM cells or 2500 LSK cells, isolated from dtg or stg mice. Donors had either been induced for 3 weeks (preinduced) or never been induced (noninduced) before BM harvest. (A) FACS analyses of BM and spleen showed increasing percentages of immature granulocytes in mice that had been transplanted with either preinduced or noninduced ufBM or LSK cells from dtg mice. Graphs show mean ± SD; n = 4 per group. (B) Serial transplantation of 1 × 106 ufBM cells isolated from diseased primary recipients of ufBM (first-degree transplantation) did not lead to CML-like phenotype in BM of secondary recipients (second-degree transplantation). Depicted is the percentage of immature granulocytes in BM and spleen of serial transplanted mice; n = 4. (C) Splenomegaly was only evident in primary recipients but was not observed on serial transplantation. (D) Histologic analysis by NACE stain of the spleen of secondary recipients that had received ufBM from preinduced donors showed no alterations of spleen morphology. *P < .05.
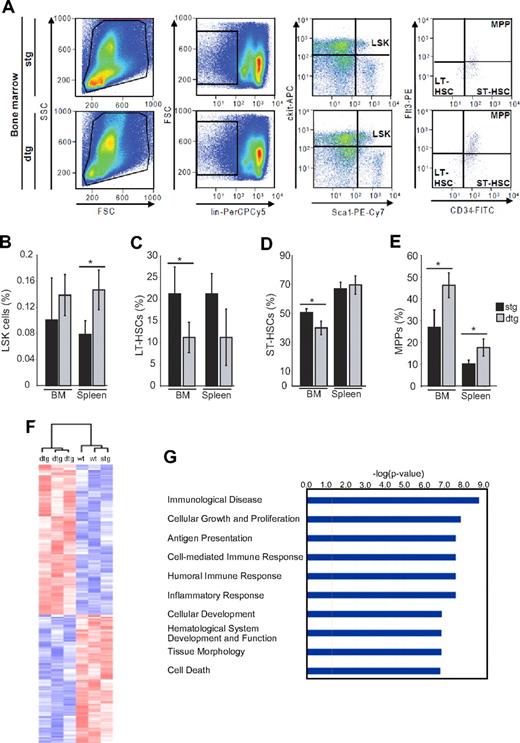
Interestingly, when we retransplanted equal numbers of ufBM cells from the mice that had received induced or noninduced BM cells, neither group was able to generate CML-like disease in secondary recipients, indicating that leukemic stem cells in the primary transplantation recipients had lost significant self-renewal and disease-inducing properties (Figure 3B-D). Quantitative reverse-transcribed polymerase chain reaction analysis showed that BCR-ABL was expressed in the BM and spleen of the secondary recipients from both groups, albeit at approximately 10-fold lower levels than in primary recipients tested at the same time point (data not shown). We reasoned that BCR-ABL had altered the composition of the LSK and progenitor cell compartment. Therefore, we investigated the distribution of LT-HSCs, ST-HSCs, and MPPs within the LSK cell compartment in primary induced dtg versus control mice (Figure 4A). The percentage of overall LSK cells in the BM was not significantly altered by BCR-ABL, whereas there was a 2-fold increase of spleen LSK cells (Figure 4B). Intriguingly, we found an increase in the percentage of MPP and a decrease in the percentage of LT-HSCs and ST-HSC in the BM of dtg versus control mice (Figure 4C-E). The absolute number of MPP was increased 1.8-fold in dtg mice, and the number of LT-HSCs and ST-HSCs was decreased 1.9- and 1.2-fold, respectively. With the splenomegaly present in dtg mice, the absolute numbers of MPP in the spleen were projected to be increased, whereas absolute LT-HSC numbers were maintained in the spleen. Taken together, LT-HSC numbers were significantly decreased in the BM of leukemic mice, whereas MPP were increased, suggesting that BCR-ABL increases the differentiation of LT-HSCs.
Expression of BCR-ABL enhances differentiation of LT-HSCs. Analysis of LSK composition was performed in the BM and spleen isolated from 4-week-induced SCLtTA/BCR-ABL (dtg) and single transgenic (stg) controls. (A) For identification of LSK subpopulations, LT-HSCs (CD34−Flt3−), ST-HSCs (CD34+Flt3−), and MPPs (CD34+Flt3+) were further distinguished according to their immunphenotype. Depicted are the percentages of (B) LSK cells in BM and spleen, (C) LT-HSCs, (D) ST-HSCs, and (E) MPP cells within the LSK compartment. Error bars represent the mean ± SD. FSC indicates forward scatter; and SSC, sideward scatter. n = 4 per group. *P < .05. (F) Expression pattern of leukemic LSK cells was determined by microarray analyses of 3 leukemic dtg mice and 3 controls (wt or stg). The figure shows the clustering of the 6 mice according to 300 genes found to be at least 1.5-fold changed in expression (P < .05). The same clustering was achieved when unselected data were used or when the genes were analyzed using the SAM method. (G) A pathway analysis using Ingenuity software shows pathways with most significant changes in the LSK cell compartment of dtg mice.
Expression of BCR-ABL enhances differentiation of LT-HSCs. Analysis of LSK composition was performed in the BM and spleen isolated from 4-week-induced SCLtTA/BCR-ABL (dtg) and single transgenic (stg) controls. (A) For identification of LSK subpopulations, LT-HSCs (CD34−Flt3−), ST-HSCs (CD34+Flt3−), and MPPs (CD34+Flt3+) were further distinguished according to their immunphenotype. Depicted are the percentages of (B) LSK cells in BM and spleen, (C) LT-HSCs, (D) ST-HSCs, and (E) MPP cells within the LSK compartment. Error bars represent the mean ± SD. FSC indicates forward scatter; and SSC, sideward scatter. n = 4 per group. *P < .05. (F) Expression pattern of leukemic LSK cells was determined by microarray analyses of 3 leukemic dtg mice and 3 controls (wt or stg). The figure shows the clustering of the 6 mice according to 300 genes found to be at least 1.5-fold changed in expression (P < .05). The same clustering was achieved when unselected data were used or when the genes were analyzed using the SAM method. (G) A pathway analysis using Ingenuity software shows pathways with most significant changes in the LSK cell compartment of dtg mice.
The leukemic stem cell compartment shows decreased expression of self-renewal associated genes
To identify the molecular mechanisms underlying enhanced differentiation of BCR-ABL+ LSK cells, we performed gene expression profiling. LSK cells from 3 BCR-ABL+ and 3 control mice were FACS-isolated, RNA was extracted and linearly amplified, and microarray analysis was carried out on Affymetrix Mouse Genome 430 2.0 GeneChips covering approximately 45 000 transcripts. Individual genes were analyzed using the MicroArraySuite 5.0 software for calculation of the presence or absence calls and the D-Chip Software. In a first analysis, 300 genes were found to be changed at least 1.5-fold (P < .05, Mann-Whitney test) in expression when 3 dtg mice were compared with 3 control mice. Using these 300 genes, hierarchical clustering led to a clear distinction between the 2 mouse cohorts (Figure 4F). Clustering of the unselected data or using the SAM method (score > 3 or < 3 and fold change < 2.5, yielding 52 induced and 37 repressed genes) also led to correct clustering of the 2 groups of mice (data not shown). Unbiased pathway analysis using Ingenuity Software revealed highly significant changes in pathways related to cellular growth and proliferation, inflammatory response and immunity, hematopoietic development, and cell death (Figure 4G). A list of selected genes with more than 1.5-fold changes in expression levels is shown in Table 2. Interestingly, many of the regulated genes are implicated in hematopoietic cell differentiation, proliferation, and tumorigenesis (Table 2). Genes involved in myeloid differentiation were up-regulated, whereas erythroid genes were down-regulated; and proliferative genes were up-regulated, whereas genes involved in self-renewal were down-regulated.
Selected differentially expressed genes in LSK cells (leukemic dtg/controls)
| Gene symbol: name . | Probe set ID . | Fold change . | P . |
|---|---|---|---|
| Differentiation | |||
| RelB: avian reticuloendotheliosis viral (v-rel) oncogene-related B | 1417856_at | 2.85 | .048 |
| FcgR2b: Fc receptor, IgG, low affinity IIb | 1451941_a_at | 2.79 | .005 |
| Ccr2: chemokine (C-C motif) receptor 2 | 1421186_at | 2.5 | .049 |
| Itgax: integrin α x (CD11c/CD18) | 1419128_at | 2.21 | .011 |
| CD48: CD48 molecule | 1427301_at | 2.07 | .037 |
| SOCS3: suppressor of cytokine signaling 3 | 1416576_at | 2.03 | .024 |
| Irf8: interferon regulatory factor 8 | 1448452_at | 1.8 | .049 |
| Csf1r: colony-stimulating factor 1 receptor | 1423593_a_at | 1.6 | .045 |
| Hba-a1: hemoglobin-α, adult chain 1 | 1452757_s_at | −6.59 | .021 |
| β-Globin | 1427866_x_at | −3.44 | .019 |
| HoxB8: homeobox B8 | 1452493_s_at | −1.57 | .005 |
| Proliferation | |||
| Ccnd1: cyclin D1 | 1417419_at | 1.98 | .013 |
| Cdkn1a: cyclin-dependent kinase inhibitor 1A (P21) | 1424638_at | 1.89 | .009 |
| Rras2/TC21: related RAS viral oncogene homolog 2 | 1417398_at | 1.86 | .020 |
| Set: SET translocation | 1421819_a_at | 1.85 | .022 |
| Bin1: bridging integrator 1 | 1423618_at | −35.55 | .035 |
| Ptpn14: protein tyrosine phosphatase, nonreceptor type 14 | 1436442_at | −1.59 | .033 |
| Self-renewal | |||
| Lifr: leukemia inhibitory factor receptor | 1450207_at | −1.80 | .014 |
| Evi1: ectropic viral integration site 1 | 1438325_at | −1.76 | .039 |
| Mpl: myeloproliferative leukemia virus oncogene | 1421461_at | −1.53 | .010 |
| Gene symbol: name . | Probe set ID . | Fold change . | P . |
|---|---|---|---|
| Differentiation | |||
| RelB: avian reticuloendotheliosis viral (v-rel) oncogene-related B | 1417856_at | 2.85 | .048 |
| FcgR2b: Fc receptor, IgG, low affinity IIb | 1451941_a_at | 2.79 | .005 |
| Ccr2: chemokine (C-C motif) receptor 2 | 1421186_at | 2.5 | .049 |
| Itgax: integrin α x (CD11c/CD18) | 1419128_at | 2.21 | .011 |
| CD48: CD48 molecule | 1427301_at | 2.07 | .037 |
| SOCS3: suppressor of cytokine signaling 3 | 1416576_at | 2.03 | .024 |
| Irf8: interferon regulatory factor 8 | 1448452_at | 1.8 | .049 |
| Csf1r: colony-stimulating factor 1 receptor | 1423593_a_at | 1.6 | .045 |
| Hba-a1: hemoglobin-α, adult chain 1 | 1452757_s_at | −6.59 | .021 |
| β-Globin | 1427866_x_at | −3.44 | .019 |
| HoxB8: homeobox B8 | 1452493_s_at | −1.57 | .005 |
| Proliferation | |||
| Ccnd1: cyclin D1 | 1417419_at | 1.98 | .013 |
| Cdkn1a: cyclin-dependent kinase inhibitor 1A (P21) | 1424638_at | 1.89 | .009 |
| Rras2/TC21: related RAS viral oncogene homolog 2 | 1417398_at | 1.86 | .020 |
| Set: SET translocation | 1421819_a_at | 1.85 | .022 |
| Bin1: bridging integrator 1 | 1423618_at | −35.55 | .035 |
| Ptpn14: protein tyrosine phosphatase, nonreceptor type 14 | 1436442_at | −1.59 | .033 |
| Self-renewal | |||
| Lifr: leukemia inhibitory factor receptor | 1450207_at | −1.80 | .014 |
| Evi1: ectropic viral integration site 1 | 1438325_at | −1.76 | .039 |
| Mpl: myeloproliferative leukemia virus oncogene | 1421461_at | −1.53 | .010 |
Expression pattern was analyzed in LSK cells of 3 leukemic mice vs 3 controls.
Transplanted CML-like disease can be reverted by abrogation of BCR-ABL expression and is reinducible on BCR-ABL reinduction
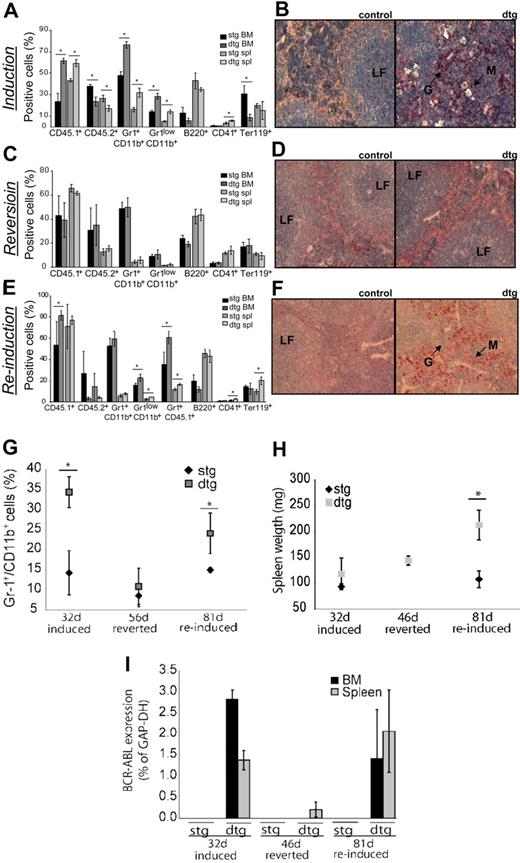
We next assessed whether the disease remains reversible after transplantation and whether it can subsequently be reinduced. To distinguish between donor and recipient cells in the transplanted animals, we used FVB– strains expressing either CD45.1 (donor) or CD45.2 (recipient). After transplantation and induction of BCR-ABL for 32 days, recipients displayed a CML-like phenotype, including neutrophilia in BM and spleen, decreased erythropoiesis, as well as decreased B and T cells in BM (Figure 5A-B). Overall, CD45.1 BCR-ABL+ donor cells were increased more than 2-fold in the BM of dtg mice versus controls. In addition, infiltration of myeloid cells into the spleen of dtg mice was evident (Figure 5A-B). After readministration of tetracycline to the drinking water of transplanted mice, the CML-like phenotype was reverted completely (Figure 5C-D). BCR-ABL expression was completely shut off in the BM and spleen (1 of 3 mice), whereas there was very low-level residual BCR-ABL positivity detectable in the spleen of the remaining 2 mice (Figure 5I). Donor cells were reduced by 20% in the BM but still present as demonstrated by the percentage of CD45.1+ cells (Figure 5C). The phenotypic reversion was further confirmed by histologic analyses of spleens, showing no sign of disease in reverted dtg mice, compared with controls (Figure 5D). After 42 to 56 days of reversion, CML-like disease was again reinducible, albeit with an increased latency of disease onset to 81 (vs 32 days at first induction). Reinduction again led to an increase of BCR-ABL+ donor cells as well as immature granulocytes (Figure 5E). Diseased mice showed neutrophilia in PB (Figure 5G) as well as splenomegaly and infiltration of myeloid cells into the spleen (Figure 5F,H). BCR-ABL was expressed in the BM and spleen at approximately the same level as before reversion (Figure 5I). Moreover, the number of LSK cells after reinduction was significantly increased in the spleen (Figure 6A right panel), but not BM of dtg mice compared with controls, with 80% to 90% of cells being of donor origin (CD45.1+; Figure 6C). These results indicate that CML stem cells persisted in the spleen after abrogation of BCR-ABL expression and were reinduced to generate CML on reexpression of the oncogene. In addition, the fact that the percentage of BM LSK cells was unchanged but was made up of 80% CD45.1 donor cells in dtg mice (Figure 6C) indicated that normal (BCR-ABL−) hematopoiesis was suppressed by BCR-ABL+ cells.
CML-like disease in transplanted mice is reversible and reinducible. Recipients were transplanted with 1 × 106 ufBM cells isolated from noninduced SCLtTA/BCR-ABL (dtg) or noninduced stg donors. Expression of BCR-ABL was induced directly after transplantation for 32 days, reverted for 46 days, and reinduced for 81 days. At each time point, at least 3 dtg and 3 control mice were analyzed. (A) CML-like disease was observed 32 days after transplantation. Average percentages of donor cells (CD45.1+), recipient cells (CD45.2+), mature granulocytes (Gr-1+CD11b+), immature granulocytes (Gr-1lowCD11b+), B cells (B220+), megakaryocytes (CD41+), and erythroid cells (Ter119+) are shown (mean ± SD). (B) Histologic analyses of the spleen show infiltration of granulocytes and disturbed follicles in recipients transplanted with dtg, but not control BM. Slides are depicted with an original magnification of ×20. (C) After reversion of BCR-ABL expression for 46 days, CML-like disease was undetectable in the BM or spleen of all recipients. (D) Histologic analyses of spleens showed no alterations of spleen morphology in reverted animals. (E) Reappearance of disease was evident by increasing percentages of dtg donor cells (CD45.1+) and increases in the percentage of immature granulocytes, 81 days after reinduction. (F) Infiltration of granulocytes into the spleen of reinduced recipients of dtg BM was evident by NACE staining and is shown at original magnification ×20. (G) Percentage of Gr-1+/CD11b+cells in the PB of mice that had been induced for 32 days, reverted for 56 days, and reinduced for 81 days showing neutrophilia is dependent on BCR-ABL activity. (H) Splenomegaly was observed on reinduction of BCR-ABL expression. (I) Relative expression of BCR-ABL was analyzed by quantitative reverse-transcribed polymerase chain reaction and was assessed in relation to glyceraldehyde-3-phosphate dehydrogenase in BM and spleen of induced, reverted, and reinduced mice. *P < .05.
CML-like disease in transplanted mice is reversible and reinducible. Recipients were transplanted with 1 × 106 ufBM cells isolated from noninduced SCLtTA/BCR-ABL (dtg) or noninduced stg donors. Expression of BCR-ABL was induced directly after transplantation for 32 days, reverted for 46 days, and reinduced for 81 days. At each time point, at least 3 dtg and 3 control mice were analyzed. (A) CML-like disease was observed 32 days after transplantation. Average percentages of donor cells (CD45.1+), recipient cells (CD45.2+), mature granulocytes (Gr-1+CD11b+), immature granulocytes (Gr-1lowCD11b+), B cells (B220+), megakaryocytes (CD41+), and erythroid cells (Ter119+) are shown (mean ± SD). (B) Histologic analyses of the spleen show infiltration of granulocytes and disturbed follicles in recipients transplanted with dtg, but not control BM. Slides are depicted with an original magnification of ×20. (C) After reversion of BCR-ABL expression for 46 days, CML-like disease was undetectable in the BM or spleen of all recipients. (D) Histologic analyses of spleens showed no alterations of spleen morphology in reverted animals. (E) Reappearance of disease was evident by increasing percentages of dtg donor cells (CD45.1+) and increases in the percentage of immature granulocytes, 81 days after reinduction. (F) Infiltration of granulocytes into the spleen of reinduced recipients of dtg BM was evident by NACE staining and is shown at original magnification ×20. (G) Percentage of Gr-1+/CD11b+cells in the PB of mice that had been induced for 32 days, reverted for 56 days, and reinduced for 81 days showing neutrophilia is dependent on BCR-ABL activity. (H) Splenomegaly was observed on reinduction of BCR-ABL expression. (I) Relative expression of BCR-ABL was analyzed by quantitative reverse-transcribed polymerase chain reaction and was assessed in relation to glyceraldehyde-3-phosphate dehydrogenase in BM and spleen of induced, reverted, and reinduced mice. *P < .05.
BCR-ABL donor cells increase within the LSK compartment on reinduction. (A) Analyses of LSK composition in reinduced mice showed doubling of LSK cells in the spleen of transplanted recipients of dtg, but not stg BM cells, on reinduction. (B) FACS analysis of donor (CD45.1+) and recipient (CD45.2+) cells within the LSK compartment. (C) Increase of dtg donor cells in LSK and progenitor cells was observed in BM and spleen of dtg recipients (mean ± SD); n = 3 per group. *P < .05. (D) Wild-type recipients were transplanted with 2 × 106 BM cells of noninduced dtg or stg donors. Expression of BCR-ABL was induced by removal of tetracycline from the drinking water directly after transplantation. Mice were treated twice daily, using imatinib or water for 4 weeks. WBCs and percentages of granulocytes (Gr-1+/CD11b+) in the PB were analyzed before treatment, during treatment (2 weeks and 4 weeks), and 2 weeks after completion of imatinib treatment. Error bars represent the mean ± SD; n(stg/dtg) before treatment = 3/3; n(stg/dtg IM or stg/dtg water) during treatment = (4/6/3/5); n(stg/dtg) 2 weeks after treatment = (4/4). *P < .05.
BCR-ABL donor cells increase within the LSK compartment on reinduction. (A) Analyses of LSK composition in reinduced mice showed doubling of LSK cells in the spleen of transplanted recipients of dtg, but not stg BM cells, on reinduction. (B) FACS analysis of donor (CD45.1+) and recipient (CD45.2+) cells within the LSK compartment. (C) Increase of dtg donor cells in LSK and progenitor cells was observed in BM and spleen of dtg recipients (mean ± SD); n = 3 per group. *P < .05. (D) Wild-type recipients were transplanted with 2 × 106 BM cells of noninduced dtg or stg donors. Expression of BCR-ABL was induced by removal of tetracycline from the drinking water directly after transplantation. Mice were treated twice daily, using imatinib or water for 4 weeks. WBCs and percentages of granulocytes (Gr-1+/CD11b+) in the PB were analyzed before treatment, during treatment (2 weeks and 4 weeks), and 2 weeks after completion of imatinib treatment. Error bars represent the mean ± SD; n(stg/dtg) before treatment = 3/3; n(stg/dtg IM or stg/dtg water) during treatment = (4/6/3/5); n(stg/dtg) 2 weeks after treatment = (4/4). *P < .05.
In addition, we tested whether imatinib was able to revert the disease in the same way as abrogation of BCR-ABL expression. We therefore treated transplanted leukemic mice and control mice with either imatinib or water, starting 3 weeks after transplantation. PB monitoring showed that neutrophilia was completely reversed after 4 weeks of treatment (Figure 6D) and reappeared on imatinib withdrawal, as early as 2 weeks after completion of imatinib therapy (Figure 6D). When we analyzed the BM of these mice, we observed that percentages of mature donor granulocytes (CD45.1+Gr-1+) were completely reverted to control levels by imatinib treatment, whereas water-treated mice showed a nearly 2-fold increase in these cells, compared with controls (data not shown). However, the leukemic phenotype was less affected by imatinib treatment than by abrogation of BCR-ABL expression, with continued presence of immature granulocytes (CD45.1+Gr-1low) in the BM and persistent splenomegaly in the treated mice (data not shown).
Discussion
Although CML is one of the best-studied diseases, the biology of CML-initiating and CML-maintaining stem cells is largely unknown. Here we have demonstrated, in an inducible transgenic mouse model, that CML-like disease can be transplanted using FACS-purified LSK cells. However, on serial transplantation, LSK cells lost their ability to initiate the disease in secondary recipients, although BCR-ABL was expressed at low levels. Detailed analyses showed that expression of BCR-ABL enhanced differentiation of LT-HSC in BM of transgenic leukemic mice. In addition, we showed that CML-like disease was reversible in transplanted recipients. However, on reinduction of BCR-ABL expression, CML-like disease reappeared, suggesting that CML-initiating cells persisted, despite abrogation of BCR-ABL activity. In addition, this is the first report to describe the donor versus recipient CD45.1 and CD45.2 FVB– syngeneic system in the LSK population.
Although the present model cannot reflect human disease completely, it does mimic the human disease very closely. In our model, expression of BCR-ABL is driven by the 3′ enhancer of the SCL gene. Although naturally occurring expression of BCR-ABL is driven by 5′ regulatory elements of the BCR promoter, the SCLtTA/BCR-ABL mice do develop a chronic myeloproliferative disorder, which mimics human CML.6 Our results in the transgenic mice showed that BCR-ABL expression was 7-fold decreased in progenitors compared with LSK cells (M. Schemiouek and S.K., unpublished data, November 2008), in line with previous human data demonstrating that expression of BCR-ABL was highest in leukemic stem cells but decreased in more mature cell subsets.13,25
Previously, reports on the transplantability of CML have been conflicting. Although most groups found that the disease was readily transplantable, either in retroviral mouse models mimicking blast crisis CML26 or by transplanting cells from patients with CML blast crisis into NOD/SCID mice,17 cells from patients with chronic-phase CML did not give rise to disease in recipients.17 In addition, our own data had shown that CML-like disease was not transferable into NOD/SCID mice, although engraftment of BCR-ABL+ donor cells was demonstrated.6 In part, these conflicting data may be explained by the increase of myeloid cells in the BM of CML mice and patients, resulting in a lower frequency of true stem cells. Indeed, when chronic-phase CML patients were selected for a high frequency of Ph+ LT-CICs, these cells efficiently engrafted NOD/SCID/β2-microglobulin–deficient mice, but they were still unable to initiate disease.19 In addition, xenograft mouse models of human disease may have been hampered by residual graft rejection mechanisms as well as suboptimal engraftment of more mature cell subsets.27 In the present study, we have circumvented these problems using a transgenic, syngeneic transplantation approach and have shown that the disease phenotype observed in the primary mice, including splenomegaly, neutrophilia, and granulocytic organ infiltration,6 is closely recapitulated in recipient animals. This clearly demonstrates that CML-like disease and the infiltrations seen in nonhematopoietic organs are cell-autonomous and result from effects of BCR-ABL in BM-derived hematopoietic cells. Through transplantation of FACS-sorted LSK cells into recipient mice, we showed that the leukemia-initiating cells are contained within the LSK cell fraction. When we compared these results with mice receiving ufBM cells, we observed an increase in disease severity. Nonetheless, the disease was clearly fatal in both groups of recipients. Importantly, the number of transplanted LSK cells was not higher, but reduced, in ufBM compared with purified LSK cells, suggesting that disease severity was enhanced by other cells present in the BM. Because it had been reported that mature cells might also contribute to the leukemic phenotype at least in AML,28 we speculated that more mature myeloid progenitor cells were responsible for this effect. However, neither Lin−Sca-1−c-kit+ progenitors nor CD11b+Gr-1+ cells were able to induce disease in the recipients or to enhance the disease phenotype in add-back experiments. In addition, cotransplantation of CD45−Ter119− cells, which may contain most of the nonhematopoietic microenvironmental cells and megakaryocytes, along with LSK cells showed that a substantial support of leukemogenesis by these cells is doubtful. We did not find evidence for decreased viability or impaired engraftment ability of the cells after FACS purification (data not shown). These findings indicate that only a small population of hematopoietic cells, residing in the LSK cell compartment, is capable of initiating CML-like disease (supplemental Figure 2) and is in line with lack of disease-inducing potential of hematopoietic progenitor cells observed in retroviral transplantation models.15 This was not the result of the lack of BCR-ABL expression in progenitors or granulocytes of the leukemic mice because BCR-ABL expression in our model was detectable at low levels, even in mature myeloid cells. Because we have not tested the effect of lymphocytes, monocytes, natural killer cells, or BM-derived fibroblasts, which may also express CD45,29 we cannot rule out the possibility that these cells might support leukemogenesis on transplantation in our model, possibly by the synthesis of growth factors and cytokines. Nevertheless, our data suggest that the LSK cell compartment contains the leukemic stem cells during chronic-phase CML and that this cell population should be targeted to eradicate the disease and prevent genomic instability by BCR-ABL30 and disease progression.
Induction of BCR-ABL in donor cells 3 weeks before transplantation did not significantly alter the disease phenotype in primary transplantation recipients. However, the fact that CML-like disease was not transplantable into secondary recipients suggests that the function of BCR-ABL+ LSK cells had been impaired. Therefore, we assessed whether BCR-ABL expression altered the composition of the LSK cell compartment and found an increase of MPP at the expense of LT-HSC. These data are in line with reports of diminished engraftment,17,18 serial replating,22 and induction of CML-like disease19,21 in CML cells of both murine and human origin. Moreover, BCR-ABL+, but not normal LT-CICs, were reported to be selectively lost during long-term culture, which prompted experiments to evaluate in vitro “purging” of CML cells.20,31 There are several explanations for our observed effects. (1) BCR-ABL may impair the engraftment or self-renewal potential of LT-HSC. However, because preinduced LSK cells do engraft and induce disease in primary animals, this explanation is unlikely. Whether BCR-ABL decreases the self-renewal potential remains to be investigated by transplantation of purified LT-HSCs. (2) Alternatively, only the percentage of LT-HSCs decreases with increasing proliferation of ST-HSCs and MPPs, resulting in transfer of less LT-HSCs per transplanted cell dose compared with normal cells. This hypothesis can be tested in the future by increasing the number of donor LSK cells. Mechanistically, BCR-ABL may induce asymmetric cell division of LT-HSCs to favor differentiation over self-renewal. Although this hypothesis contradicts findings showing no influence of BCR-ABL on asymmetric cell division,32 one has to take into account that those experiments were performed in vitro. BCR-ABL effects may be different in vivo, given that the microenvironment probably contributes to these effects.33 In addition, our gene expression profiling data from BM-derived LSK cells confirmed that BCR-ABL regulates pathways associated with differentiation, proliferation, and survival. Specifically, BCR-ABL induced myeloid genes (CD11c/CD18, Fcgr2b, Csf1r, Irf8) and suppressed erythroid genes (hemoglobin-α, β-globin), stimulated proliferation (up-regulation of cyclin D1 and Rras2, down-regulation of BIN1), and decreased self-renewal-associated genes (up-regulation of CD48, down-regulation of Evi1, Lifr, and c-mpl) in the LSK compartment (Table 2). Interestingly, SET was up-regulated by BCR-ABL, and this has already been described for human CML, resulting in suppression of the tumor suppressor PP2A.34
The finding that BCR-ABL induces differentiation of LT-HSCs raises the question why the CML clone does not exhaust in untreated patients. In our opinion, there are several possibilities to explain this discrepancy. First, not all LT-HSCs differentiated in response to BCR-ABL expression as shown by the 50% (but not 100%) decrease of LT-HSCs. In addition, BCR-ABL enhances survival of hematopoietic cells,35,36 yielding an overall increase and extended life span of MPPs as well as more mature myeloid cells. Interestingly, this has been predicted by mathematical models of CML.37 Second, the transplantation procedure itself may have accelerated cell-cycle entry and differentiation of LT-HSCs. Third, even though our results clearly suggest this, it is currently unknown whether human CML chronic-phase stem cells show a similar inability to induce disease on serial transplantation. Finally, cells from patients in blast crisis have acquired self-renewal capacities leading to disease progression.22 As has been described for various oncogenes, expression of BCR-ABL induces genomic instability.38-40 Thus, further genetic aberrations induced by BCR-ABL might either induce self-renewal or lead to the neutralization of inhibiting mechanisms within a leukemic stem cell. Together, these data suggest that BCR-ABL expression in stem cells leads to enhanced granulocytic differentiation and decreased LT-HSC, but without completely abolishing the repopulating ability of HSCs.
One unique aspect of our model is its reversibility by readministration of tetracycline,6 and we have now shown that the disease remains reversible on transplantation. Moreover, reinduction of BCR-ABL and of CML-like disease was observed after transplantation. Similar effects were observed on imatinib treatment of transplanted induced mice, although reversion of the disease phenotype was less pronounced compared with the abrogation of BCR-ABL expression. Together with our previous results showing repeated reinducibility of neutrophilia in primary dtg mice,6 these data suggest that neither abrogation of BCR-ABL expression nor imatinib treatment induces apoptosis in CML-initiating stem cells and that at least a fraction of these cells remain sensitive to BCR-ABL–induced transformation. These results may in part explain the persistence of CML stem cells in vitro13 and in vivo14,16 during TKI treatment in patients with CML. Together with the fact that CML frequently relapses in patients who pause TKI treatment,41-43 our data suggest that inhibition of BCR-ABL in stem cells by kinase inhibitors may halt BCR-ABL–induced alterations of these cells but does not eradicate these cells. It is of interest that, although the phenotype and BCR-ABL expression were completely reversed in the BM, 2 of 3 mice still showed low-level BCR-ABL expression in the spleen, although there was no sign of disease in the spleen. This may have been the result of minimal genomic DNA contamination because our transgene contains the cDNA of BCR-ABL and DNase treatment might not have been 100% efficient. Alternatively, spleen HSCs in these 2 mice may have become unresponsive to tetracycline and may have survived because of low-level BCR-ABL expression. Our data also clearly show that the mature cells are depleted on reversion of BCR-ABL expression.
Our transgenic system is the first CML model allowing assessment of the described experiments on LT-HSC differentiation, and this could be exploited during future studies when evaluating different treatment modalities. In particular, our data suggest that strategies enhancing LT-HSC differentiation and/or cell cycle entry may be pursued to eradicate these cells independently of BCR-ABL inhibition by TKI. Recently, interferon-α treatment has been demonstrated to force LT-HSCs into cycle44 ; and because it is already being used clinically for CML, it may be very interesting to investigate whether combined treatment of interferon and imatinib can eradicate CML stem cells in this model.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Linda Kamp for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (KO2155/1-1 and KO2155/2-1), the Thyssen Foundation (Az. 10.05.2.178), the Medical Research Council (G0600782), the Deutsche Carreras Leukämie-Stiftung (DJCLS R 0608v), the IZKF Münster, and the National Institute of Health (grants CA66996 and CA118316; D.G.T.).
National Institutes of Health
Authorship
Contribution: M. Schemionek, N.B., C.S.H., D.G.T., T.L.H., C.M.-T., H.S., and S.K. designed research; M. Schemionek, C.E., U.S., N.B., T.S., J.R.G., M. Stehling, and S.K. performed research and collected the data; M. Schemionek, U.S., M. Stehling, T.L.H., and S.K. analyzed the data; A.W., A.H., and L.T. contributed vital new reagents or analytical tools; and M. Schemionek, W.E.B., T.L.H., C.M.-T., and S.K. wrote the paper.
Conflict-of-interest disclosure: S.K. has served as advisory board member for Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Steffen Koschmieder, Department of Medicine/Hematology and Oncology, University of Münster, 48149 Münster, Germany; e-mail: Steffen.Koschmieder@ukmuenster.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal