Abstract
In older patients with acute myeloid leukemia (AML), the prevention of relapse has remained one of the major therapeutic challenges, with more than 75% relapses after complete remission. The anti-CD33 immunotoxin conjugate gemtuzumab ozogamicin (GO) has shown antileukemic remission induction activity in patients with relapsed AML. Patients with AML or refractory anemia with excess blasts in first complete remission attained after intensive induction chemotherapy were randomized between 3 cycles of GO (6 mg/m2 every 4 weeks) or no postremission therapy (control) to assess whether GO would improve outcome. The 2 treatment groups (113 patients receiving GO vs 119 control patients) were comparable with regard to age (60-78 years, median 67 years), performance status, and cytogenetics. A total of 110 of 113 received at least 1 cycle of GO, and 65 of 113 patients completed the 3 cycles. Premature discontinuation was mainly attributable to incomplete hematologic recovery or intercurrent relapse. Median time to recovery of platelets 50 × 109/L and neutrophils 0.5 × 109/L after GO was 14 days and 20 days. Nonhematologic toxicities were mild overall, but there was 1 toxic death caused by liver failure. There were no significant differences between both treatment groups with regard to relapse probabilities, nonrelapse mortality, overall survival, or disease-free survival (17% vs 16% at 5 years). Postremission treatment with GO in older AML patients does not provide benefits regarding any clinical end points. The HOVON-43 study is registered at The Netherlands Trial Registry (number NTR212) and at http://www.controlled-trials.com as ISRCTN77039377.
Introduction
The majority of patients with acute myeloid leukemia (AML) are 60 years of age or older. Older age is a major negative determinant of outcome in AML, independent of the greater prevalence of unfavorable cytogenetic features in older patients. Long-term survival (at 5 years) of intensively treated patients is approximately 10%.1-10 In those in whom intensive chemotherapy is applied for remission induction, the complete response (CR) rate is on average in the range of 40% to 50%. It is striking that almost all remissions are subsequently lost within 3 years. It is necessary to develop more active therapeutic regimens for the elimination of residual leukemia in remission and thereby stabilize these remissions more effectively.
The results of previous randomized studies concerned with the challenge of postremission treatment in patients ages 60 years and older have been highly unsatisfactory. Studies11,12 performed on the basis of the use of cytarabine according to various dose-intensification schedules did not produce therapeutic benefits. Two studies3,13 have produced an improvement of disease-free survival (DFS) in a comparison between low-dose cytarabine and no maintenance chemotherapy, but overall survival outcome was not different. A comparison between one additional cycle of combination chemotherapy for remission consolidation with 4 additional cycles showed no differences in survival.5 When 3 versus 4 successive cycles of intensive chemotherapy were directly compared, no differences in outcome were noted.10 In addition, the use of interferon after CR did not result in any significant positive effect on outcome.5
Two studies14,15 in which the authors compared one single intensive consolidation cycle in remission versus low-dose chemotherapy delivered in an outpatient setting produced contradictory results The first study in complete responders after intensive chemotherapy in which the authors compared a single additional intensive cycle of chemotherapy during hospitalization with 6 repeated cycles of lower-dose ambulatory combination chemotherapy suggested a survival and DFS advantage at 2 years for the ambulatory regimen, but whether this benefit was maintained during a longer follow-up period remains to be seen.14 The results of a second study,15 of smaller size, has suggested that one single additional intensive course of chemotherapy is better than a 1-year oral schedule of combination chemotherapy.
Although the use of high-dose cytarabine offers effective postremission treatment in young and middle-aged adults, it appears to be too toxic in patients older than the age of 60 years, and therefore the use of high-dose regimens of cytarabine generally is discouraged in older patients.11 Allogeneic stem cell transplantation has been applied in highly selected subgroups and awaits critical evaluation in prospective trials. Hence, there is no generally accepted postremission treatment in elderly patients with AML with firmly established efficacy. It remains a challenge to consolidate remissions more effectively in patients ages 60 years or older.
Gemtuzumab ozogamicin (GO) has shown antileukemic activity in remission induction in patients with AML who are older.16-18 GO is a conjugate of an anti-CD33 antibody and the toxin calicheamicin. The application of GO targets CD33 antigen-positive leukemic cells and after binding to the surface, the toxin is internalized, leading to cell death. Here we present the results of a randomized cooperative group study in which after remission induction chemotherapy in first CR, a maintenance regimen of 3 cycles of GO at 6 mg/m2 at 4-week intervals was compared with no further chemotherapy.
Methods
Study design and treatment
Patients in the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON-43)/Swiss Group for Clinical Cancer Research Collaborative Group (SAKK)/German-Austrian AML Study Group (AMLSG) study who had entered CR after 2 cycles of remission-induction chemotherapy had the option to be randomly assigned to 3 cycles of GO at 6 mg/m2 per 2-hour infusion at 4-week intervals or no further treatment.19 GO was provided free of charge by Wyeth Pharmaceuticals. Randomizations were balanced by minimization with the factors hospital, type of leukemia (AML vs myelodysplastic syndrome [MDS]), the allocated treatment at induction randomization, and whether CR was reached after cycle I or later. At diagnosis patients registered for the HOVON-43 trial had been randomized to a first cycle of induction treatment with daunorubicin (at a “conventional” dose of 45 mg/m2 or an “escalated” dose 90 mg/m2 per 3-hour infusion on each of days 1-3) and cytarabine at 200 mg/m2 per continuous infusion for 7 days and a second cycle of single-agent cytarabine (at 1000 mg/m2 by 6-hour infusion twice a day on days 1-6). The results of the induction treatment have been reported elsewhere.19 A total of 242 patients were randomized for the postremission question, of whom 9 patients were not eligible because they had not attained CR and for 1 patient no data were sent after randomization. This report compiled on the basis of 232 evaluable and eligible patients. The study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All participants provided their informed consent. HOVON-43 study is registered at The Netherlands Trial Registry (number NTR212) and as ISRCTN77039377.
Eligibility
Previously untreated patients with a cytopathologically confirmed diagnosis of AML (M0-M2 and M4-M7) and a minimum of 20% blast infiltrate in the bone marrow who were 61 years of age or older were eligible provided they had a World Health Organization (WHO) performance status of 2 or less and had provided informed consent. Eligibility also included a subtype of the MDS, that is, refractory anemia with excess of blasts (RAEB) with the International Prognostic Scoring System20 score of 1.5 or greater; however, patients with acute promyelocytic leukemia and blastic crisis of chronic myeloid leukemia were excluded. Patients with a concurrent other active malignancy or those previously treated with induction therapy for AML or MDS or those with AML after chronic myeloid leukemia, primary myelofibrosis, or polycythemia rubra vera were not eligible, nor were patients with severe heart, lung, or neurologic disease (for details, see Löwenberg et al19 ). For randomization, postremission patients were required to have attained a CR and completed the first and second induction cycles with daunorubicin and cytarabine and have a serum bilirubin of less than two times normal value, hematologic values of absolute neutrophil count greater than 1.5 × 109/L, and platelets greater than 100 × 109/L.
Patient characteristics and classification
On the basis of karyotype, patients were classified at diagnosis into distinct prognostic categories. Favorable risk applied to patients with core binding factor (CBF) abnormalities, that is, with t(8;21)(q22;q22), inv(16)(p13.1q22), or t(16;16)(p13.1;q22). Patients without cytogenetic abnormalities or with loss of X or Y as the only abnormality were classified as normal cytogenetics. Leukemia with 2 distinct autosomal monosomies or 1 autosomal monosomy in combination with at least one structural abnormality (other than CBF) was classified as monosomal karyotype.19,21 Patients without CBF abnormalities and without monosomal karyotype but with abnormalities usually considered as unfavorable (complex, −5/7(q), abn 3q, t(6;9), t(9;22) or abn 11q23) were classified as unfavorable, whereas the remaining cytogenetic abnormalities were designated as “other.”19 Therapy-related leukemia as well as leukemia after an antecedent hematologic condition was classified as secondary AML. The presence of hepatomegaly and splenomegaly assessed on physical examination, WHO performance status, extramedullary disease, and white blood cell count were registered at diagnosis.
Statistical analysis, criteria of response, and evaluation of outcome
The primary objectives of the study were to assess in a randomized comparison the value of GO (anti-CD33-calicheamycin) as postremission therapy (in direct comparison with control patients, ie, no further chemotherapy) in older patients with AML and RAEB in regard to DFS. Secondary objectives were the evaluation of the effects of GO therapy after remission with respect to toxicity, DFS with failure either as the result of relapse or death in first CR, the competing risk probabilities of relapse and death in first CR, and overall survival. CR had been defined as a cellular marrow with less than 5% blasts, no Auer rods, no evidence of extramedullary leukemia, and peripheral granulocyte and platelet counts of at least 1.0 × 109/L and 100 × 109/L, respectively. DFS and overall survival are measured from the time of postremission randomization until the occurrence of relapse or death (DFS), death (overall survival), or until censoring at last contact. Relapse is a recurrence of leukemia after a first CR.
We had expected that approximately 30% of all patients (or n = 240) would be randomized between GO after remission treatment and no further treatment. This number of patients would give a power of 78% with a 2-sided test at 5% significance level to detect an increase of the duration of DFS from second randomization with 50%, which corresponds with a hazard ratio of 0.67 and an expected 12-month DFS in the nonmaintenance arm of 40% compared with 54% in the GO postinduction arm
All analyses were performed according to intention to treat irrespective of protocol compliance, except for patients who were ineligible for randomization because they had not reached CR (2 in the control group and 7 in the GO treatment group) and for 1 patient in the control arm who was lost to follow-up after the date of randomization. The log-rank test and Cox regression analysis were applied to analyze the differences between both groups with respect to overall survival and DFS. These analyses were performed without and with adjustment for age and cytogenetic risk classification. All P values reported are 2-tailed.
Results
Randomization to postremission treatment
Between October 27, 2000, and June 9, 2006, 814 evaluable and eligible patients were entered in the study at diagnosis. Of 481 patients reaching CR on induction, a total of 232 evaluable and eligible patients (30% of registered patients and 48% of CRs) were randomized for postremission treatment, that is, 113 patients (14%) for GO and 119 patients (15%) for no further treatment. The median follow-up time of patients still alive at the date of last contact since the date of postremission randomization was 45 months. The major reasons why patients in CR were not randomized included poor performance status or residual organ toxicities after remission induction treatment (n = 53), incomplete platelet recovery or delayed hematopoietic recovery after remission induction therapy (n = 28), death (n = 41), relapse (n = 21), choice for allogeneic (n = 47) or autologous (n = 5) hematopoietic stem cell transplantation, refusal (n = 37), or other/unclear (n = 16). One patient in CR was randomized but was lost to follow-up since the date of randomization and was excluded as not evaluable.
Of the patients assigned to GO or no postremission therapy, 70% had previously achieved CR already after remission induction cycle I (same for GO and control study arms). Among the group assigned to GO, 3 patients did not receive GO. Among the control patients, none received GO, but 1 patient received an allograft and 1 patient received an autologous stem cell graft. These patients are included in their respective arms for the analysis according intention to treat. Of the randomized patients, 109 patients (47%) had received induction therapy with the 45 mg/m2 dose schedule of daunorubicin (days 1, 2, 3) and cytarabine (200 mg/m2; days 1-7), and 123 (53%) patients were induced with the high-dose regimen of daunorubicin at 90 mg/m2. Table 1 19 presents the characteristics of the patients enrolled in both postremission treatment arms. There were no differences between the 2 treatment groups with respect to age; sex; performance status; proportion of patients with RAEB or AML; secondary leukemia; the presence of extramedullary disease, hepatomegaly, or splenomegaly at diagnosis; and cytogenetic risk distribution.
Patient characteristics at diagnosis (no further therapy vs GO postremission therapy)
| . | Postremission arm, no. (%) . | |
|---|---|---|
| None . | GO . | |
| Total | 119 (100) | 113 (100) |
| Age, y | ||
| ≤ 65 | 49 (41) | 44 (39) |
| 66-70 | 45 (38) | 46 (41) |
| > 70 | 25 (21) | 23 (20) |
| Mean ± SD | 67 ± 4 | 67 ± 4 |
| Median (range) | 66 (60-78) | 67 (60-77) |
| Sex | ||
| Male | 65 (55) | 63 (56) |
| Female | 54 (45) | 50 (44) |
| WHO performance status | ||
| 0 | 41 (35) | 44 (39) |
| 1-2 | 77 (65) | 68 (61) |
| Type of AML | ||
| De novo | 102 (86) | 94 (83) |
| Previous MDS | 10 (8) | 12 (11) |
| Previous chemo/RT | 7 (6) | 7 (6) |
| AML/MDS | 100/19 (84/16) | 100/13 (88/12) |
| Extramedullary disease | 9 (8) | 14 (12) |
| Hepatomegaly | 8 (7) | 14 (12) |
| Splenomegaly | 9 (8) | 9 (8) |
| Cytogenetics | ||
| CBF | 6 (5) | 9 (8) |
| CN-X-Y | 58 (49) | 52 (46) |
| CA other | 23 (19) | 23 (20) |
| Unfavorable | 9 (8) | 11 (10) |
| Monosomal karyotype | 7 (6) | 8 (7) |
| ND/fail | 16 (13) | 10 (9) |
| Induction arm | ||
| Conventional dose daunorubicin (45 mg/m2)* | 56 (47) | 53 (47) |
| High-dose daunorubicin (90 mg/m2)† | 63 (53) | 60 (53) |
| CR reached | ||
| After cycle I | 84 (71) | 78 (69) |
| After cycle II | 35 (29) | 35 (31) |
| . | Postremission arm, no. (%) . | |
|---|---|---|
| None . | GO . | |
| Total | 119 (100) | 113 (100) |
| Age, y | ||
| ≤ 65 | 49 (41) | 44 (39) |
| 66-70 | 45 (38) | 46 (41) |
| > 70 | 25 (21) | 23 (20) |
| Mean ± SD | 67 ± 4 | 67 ± 4 |
| Median (range) | 66 (60-78) | 67 (60-77) |
| Sex | ||
| Male | 65 (55) | 63 (56) |
| Female | 54 (45) | 50 (44) |
| WHO performance status | ||
| 0 | 41 (35) | 44 (39) |
| 1-2 | 77 (65) | 68 (61) |
| Type of AML | ||
| De novo | 102 (86) | 94 (83) |
| Previous MDS | 10 (8) | 12 (11) |
| Previous chemo/RT | 7 (6) | 7 (6) |
| AML/MDS | 100/19 (84/16) | 100/13 (88/12) |
| Extramedullary disease | 9 (8) | 14 (12) |
| Hepatomegaly | 8 (7) | 14 (12) |
| Splenomegaly | 9 (8) | 9 (8) |
| Cytogenetics | ||
| CBF | 6 (5) | 9 (8) |
| CN-X-Y | 58 (49) | 52 (46) |
| CA other | 23 (19) | 23 (20) |
| Unfavorable | 9 (8) | 11 (10) |
| Monosomal karyotype | 7 (6) | 8 (7) |
| ND/fail | 16 (13) | 10 (9) |
| Induction arm | ||
| Conventional dose daunorubicin (45 mg/m2)* | 56 (47) | 53 (47) |
| High-dose daunorubicin (90 mg/m2)† | 63 (53) | 60 (53) |
| CR reached | ||
| After cycle I | 84 (71) | 78 (69) |
| After cycle II | 35 (29) | 35 (31) |
Cytogenetics: CBF: AML with CBF chromosomal abnormalities, ie, (t8;21) or inv(16)/t(16;16). CN-X-Y: normal cytogenetics or -X or -Y as single abnormalities only.
Unfavorable refers to complex abnormalities or −5/7(q), abn 3q, t(6;9), t(9;22), abn 11q23, and no CBF and no MK (for details, see Löwenberg et al19 ).
CA other indicates other cytogenetic abnormalities; CR, complete remission; GO, gemtuzumab ozogamicin; MDS, myelodysplastic syndrome; ND, not determined; RT, radiation therapy; and WHO, World Health Organization.
Remission induction treatment arm with a “conventional” daunorubicin dose of 45 mg/m2 3-hour infusion on days 1 to 3 and cytarabine 200 mg/m2 continuously intravenously days 1 to 7.
Remission induction treatment arm with high-dose daunorubicin dose of 90 mg/m2 3-hour infusion on days 1 to 3 and cytarabine 200 mg/m2 continuously intravenously days 1 to 7.
Postremission therapy
Of 113 patients assigned to the 3 monthly cycles of GO (6 mg/mg2) treatment, 110 completed postremission cycle 1, 89 completed GO cycle 2 at a median interval of 30 days, and 65 patients received the third cycle of GO after a median interval of 35 days after the second cycle. The reasons for not completing the GO treatment with 3 cycles were hematologic cytopenia/bone marrow suppression/toxicity (n = 27), relapse (n = 12), and noncompliance (n = 6).
Treatment outcome according postremission randomization and prognostic significance of cytogenetics
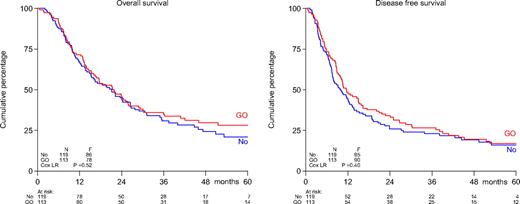
There were no differences in outcome (survival, DFS, relapse probability) between the 2 treatment arms (Table 2; Figure 1). Induction randomization between 2 dose levels of daunomycin (45 mg/m2 or 90 mg/m2) did not exert an effect on survival or remission duration in either postremission treatment arm. Among various diagnostic factors (age, WHO performance, sex, therapy-related AML and/or previous MDS, cytogenetics) only cytogenetics expressed prognostic value in univariate and multivariate analysis in regard to survival and DFS. The prognostic effect of cytogenetics was apparently independent of GO or no further postremission therapy.
Survival estimates by postremission treatment arm (randomization to no further therapy vs GO postremission therapy)
| . | No therapy . | GO . | ||
|---|---|---|---|---|
| At 24 mo . | At 60 mo . | At 24 mo . | At 60 mo . | |
| Overall survival | 45 ± 5 | 21 ± 4 | 45 ± 5 | 28 ± 5 |
| Disease-free survival | 26 ± 4 | 16 ± 4 | 34 ± 4 | 17 ± 4 |
| Relapse probability | 72 ± 4 | 82 ± 4 | 62 ± 5 | 76 ± 4 |
| Nonrelapse mortality | 2 ± 1 | 2 ± 1 | 4 ± 2 | 7 ± 3 |
| . | No therapy . | GO . | ||
|---|---|---|---|---|
| At 24 mo . | At 60 mo . | At 24 mo . | At 60 mo . | |
| Overall survival | 45 ± 5 | 21 ± 4 | 45 ± 5 | 28 ± 5 |
| Disease-free survival | 26 ± 4 | 16 ± 4 | 34 ± 4 | 17 ± 4 |
| Relapse probability | 72 ± 4 | 82 ± 4 | 62 ± 5 | 76 ± 4 |
| Nonrelapse mortality | 2 ± 1 | 2 ± 1 | 4 ± 2 | 7 ± 3 |
Survival estimates are given as mean percentage ± SE. GO indicates gemtuzumab ozogamicin; and mo, months.
Survival of patients aged 60 years or older with AML: comparison of treatment with GO vs no postremission treatment. Shown are overall survival (left) and disease-free survival (right). Patients in CR were randomized to 3 cycles of GO at 6 mg/m2 at 4-week intervals or no therapy. No indicates no postremission treatment.
Survival of patients aged 60 years or older with AML: comparison of treatment with GO vs no postremission treatment. Shown are overall survival (left) and disease-free survival (right). Patients in CR were randomized to 3 cycles of GO at 6 mg/m2 at 4-week intervals or no therapy. No indicates no postremission treatment.
Toxicities
Median time toward neutrophil recovery (0.5 × 109/L) after the GO cycles was 20 days (Table 3). At day 30, 89% of patients had shown neutrophil recovery to a minimum value of 0.5 × 109/L. The median time interval to platelet recovery (50 × 109/L) was 14 days, and 79% of the patients had recovered their platelet counts to 50 × 109/L or more on day 30. Hematologic recovery did not differ in relation to age or sequence number of GO cycle.
Supportive care and side effects after GO treatment
| . | Patients . | Cycles . |
|---|---|---|
| Total | 110 (100) | 264 (100) |
| Nights in hospital | ||
| 0 | 35 (32) | 135 (51) |
| 1 or more | 75 (68) | 129 (49) |
| Mean ± SD | 7 ± 8 | 4 ± 6 |
| Median (range) | 3 (1-35) | 1 (1-34) |
| No. of platelet transfusions | ||
| 0 | 60 (55) | 196 (74) |
| 1 or more | 50 (45) | 68 (26) |
| Mean ± SD | 3 ± 3 | 3 ± 3 |
| Median (range) | 2 (1-14) | 2 (1-14) |
| No. of red blood cell transfusions | ||
| 0 | 97 (88) | 248 (94) |
| 1 or more | 13 (12) | 16 (6) |
| Mean ± SD | 4 ± 3 | 3 ± 2 |
| Median (range) | 2 (1-10) | 2 (1-8) |
| Median no. of days until ANC > 0.5 × 109/L | 20 | |
| Median no. of days until platelets > 50 × 109/L | 14 | |
| Side effects with CTC grade 2-4 | ||
| Any | 62 (56) | 94 (36) |
| GI | 21 (19) | 29 (11) |
| Hepatic | 19 (17) | 25 (9) |
| Constitutional symptoms | 16 (15) | 20 (8) |
| Infections with CTC grade 2-4 | ||
| Any | 47 (43) | 68 (26) |
| Fever of unknown origin | 28 (25) | 35 (13) |
| Blood | 11 (10) | 15 (6) |
| GI tract | 11 (10) | 14 (5) |
| No. of deaths in CR* | ||
| Overall | 7 | |
| During first year | 4 |
| . | Patients . | Cycles . |
|---|---|---|
| Total | 110 (100) | 264 (100) |
| Nights in hospital | ||
| 0 | 35 (32) | 135 (51) |
| 1 or more | 75 (68) | 129 (49) |
| Mean ± SD | 7 ± 8 | 4 ± 6 |
| Median (range) | 3 (1-35) | 1 (1-34) |
| No. of platelet transfusions | ||
| 0 | 60 (55) | 196 (74) |
| 1 or more | 50 (45) | 68 (26) |
| Mean ± SD | 3 ± 3 | 3 ± 3 |
| Median (range) | 2 (1-14) | 2 (1-14) |
| No. of red blood cell transfusions | ||
| 0 | 97 (88) | 248 (94) |
| 1 or more | 13 (12) | 16 (6) |
| Mean ± SD | 4 ± 3 | 3 ± 2 |
| Median (range) | 2 (1-10) | 2 (1-8) |
| Median no. of days until ANC > 0.5 × 109/L | 20 | |
| Median no. of days until platelets > 50 × 109/L | 14 | |
| Side effects with CTC grade 2-4 | ||
| Any | 62 (56) | 94 (36) |
| GI | 21 (19) | 29 (11) |
| Hepatic | 19 (17) | 25 (9) |
| Constitutional symptoms | 16 (15) | 20 (8) |
| Infections with CTC grade 2-4 | ||
| Any | 47 (43) | 68 (26) |
| Fever of unknown origin | 28 (25) | 35 (13) |
| Blood | 11 (10) | 15 (6) |
| GI tract | 11 (10) | 14 (5) |
| No. of deaths in CR* | ||
| Overall | 7 | |
| During first year | 4 |
Data are n (%) unless otherwise noted. No significant trends of more or higher-grade side effects or infections with sequence of GO cycle or with age of the patient.
The duration of recovery showed no relation with the sequence number of the GO cycle or with age.
ANC indicates absolute neutrophil count; CR, complete remission; CTC, common terminology criteria; GI, gastrointestinal; and GO, gemtuzumab ozogamicin.
There were 2 deaths in CR in the control arm at 3 months and 21 months after randomization attributable to intracerebral hemorrhage and unknown cause. There were 7 deaths in CR in the GO arm attributable to acute liver failure (at 0.6 months after randomization and 11 days after first cycle of GO), infection (at 4 months after randomization and 53 days after GO), hemorrhage (at 9 months), infections after surgery for colon adenocarcinoma (at 10 months), secondary malignancy (at 30 months), and 2 unknown causes at 41 months and 52 months. Both hemorrhages appeared after platelet recovery. In addition, another patient died from veno-occlusive disease; however, he died not in first remission but after additional chemotherapy for relapse. He had attained a CR after cycle 1 and relapsed at 20 months. He died at 25 months after diagnosis.
Fever developed in 43% of patients, and sepsis in 10%. Hepatic (bilirubin) toxicities were noted in 17% (common terminology criteria grade 2-4), and gastrointestinal toxicities in 19% of patients (grades 2-4) of cases. There were 2 versus 7 deaths in CR in the control and GO treatment groups, respectively, of which 1 versus 4 occurred during the first year (P = NS). One patient died as the result of acute liver failure after a first cycle of GO treatment, an apparent toxic treatment-related death. Another patient died from veno-occlusive disease; however, it occurred not in first remission but after additional chemotherapy for relapse. He had attained a CR after cycle 1 and had relapsed at 20 months. He died at 25 months after diagnosis. A summary of toxicities is given in Table 3. The actuarial nonrelapse mortality was slightly but nonsignificantly greater in the GO treatment arm (Table 2).
Discussion
In general, CRs obtained after remission-induction chemotherapy in patients of 60 years and older are short lived, which ultimately results in an overall survival probability of approximately 10% at 5 years from diagnosis.1-10 In recent years, various efforts have been undertaken in CR to prevent relapse, but unfortunately these attempts have met with only limited success. Here we present the results of a prospective comparison with mature follow-up (the median follow up of the patients still alive is 45 months, whereas 88% of these patients have a follow-up longer than 2 years) regarding the use of GO in complete remitters who had first received 2 induction cycles of intensive cytarabine-daunorubicin–based chemotherapy (see Löwenberg et al19 ).
GO has been considered an interesting postremission agent because the drug as a single compound when given in 2 doses 14 days apart has shown clinical antileukemic activity in induction in older patients with AML.16-18 The current study was designed with a no further treatment arm as the comparator to allow for an absolute comparison. Thus, we assumed that 3 successive cycles of GO when applied in CR, that is, in conditions of subclinical disease, might exert a positive effect upon disease outcome. However, treatment results in regard to any of the major clinical end points (relapse probability, DFS, overall survival) between the GO postremission and no further treatment groups appear virtually identical. In the meantime, a phase 3 study with a naked anti-CD33 antibody (lintuzumab) applied in a different setting of induction therapy in patients with AML in relapse has not shown indications of clinical improvements, either.22 The use of GO as postremission treatment apparently added various hematologic and organ toxicities, usually mild, although there was one death caused by liver failure that was considered drug related (Table 3). The limitations of patient numbers precluded a meaningful exploratory analysis in regard to possible positive effect of GO in particular prognostic subgroups, for instance, with regard to age or cytogenetics.
Although there is no apparent survival advantage for GO therapy, it should also be noted that the current study carries some of the usual limitations that appear inherent to any postremission study in older patients with AML. Only 48% of the 481 patients who reached a CR after induction cycle I or II were randomized for the randomization question. This phenomenon is a common one in prospective studies in older patients with AML. In various other study reports' randomizations, postremission in the elderly generally showed a reduced compliance at levels of 50% or less. Notably, most of the patients in CR who entered this study represented an intermediate cytogenetic risk, which indicates that comparatively large numbers of unfavorable-risk patients dropped out early (more frequently no CR and more frequently early relapse). In addition, of those actually randomized to GO, a fraction of 58% received the full series of 3 cycles of treatment as planned in the protocol. It is not uncommon for elderly patients, after recovery from the toxicities associated with the remission induction treatment, to withdraw from continued postremission therapy. Medical factors (eg, early relapse) and toxicities (eg, cytopenia) prohibited the continuation of therapy in a significant fraction of patients. Hematologic toxicity associated with GO treatment was the most frequent cause that urged for premature discontinuation.
For the time being, postremission treatment has shown limited, if any, value in older patients with AML. Of the limited series of available studies addressing the question of different forms of remission maintenance, almost all have produced negative results or results of a very moderate magnitude only. The explanation for a lack of benefit of GO as maintenance treatment despite the ability of GO to induce remissions relates to the notoriously chemotherapy-unresponsive nature of AML emerging in the elderly. More patients in this age category express adverse cytogenetic characteristics, and as a consequence the responses that are observed are often of short duration only. According this notion, a significant load of residual resistant leukemia remains, and the surviving subpopulation of drug-resistant leukemia cells rapidly generates recurrence. If the latter explanation applies, novel types of drugs with entirely different modes of action (eg, epigenetic approaches) are needed to target these unresponsive subsets of the leukemic clone or other modalities of treatment (eg, allogeneic stem cell transplantation23 ) be advanced in this context in efforts to sustain CR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the local and central data managers for collecting patient data, in particular Petra Cornelisse, Ine Meulendijks, Anneke Ammerlaan, Silvia Verelst, and Christel van Hooije (HOVON Data Center); Bettina Adamo and Brigitte Fuellgraf (AMLSG); and Christina Biaggi (SAKK) and Yvette van Norden for statistical assistance.
Data management was supported by the Dutch Cancer Society Queen Wilhelmina Foundation.
Authorship
Contribution: The study was designed by the study group, of which the authors are members. The authors represent institutions contributing to the study. B.L. and W.v.P. wrote the first and subsequent drafts of the manuscript; W.v.P. coordinated the statistical analysis at the HOVON Data Center; and all authors provided feedback to the manuscript and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.F.V. is Isala Klinieken Hospital, Zwolle, The Netherlands.
A complete listing of the participating HOVON/SAKK institutes and investigators appears below in the Appendix.
Correspondence: Bob Löwenberg, Department of Hematology (L413), Erasmus University Medical Center, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: b.lowenberg@erasmusmc.nl.
Appendix
The following HOVON/SAKK institutes and investigators participated in the clinical studies described in this publication.
Belgium (B): Brussels, St Luc, A. Ferrant; Haine, St Paul Jolimont, A. Delannoy; Leuven, Gasthuisberg, J. Maertens and G. Verhoef; Roeselaere, Heilig Hart, H. Demuynck; Yvoir, Mont Godinne, A. Bosly and C. Graux; Antwerp, Ziekenhuis Netwerk, D. A. Breems and P. Zachee.
Germany (D): Frankfurt am Main, Nordwest, E. Jaeger; Mainz, Johannes-Gutenberg, J. Beck and Th. Fischer; Universität Bonn, Marie von Lilienfeld-Toal and A. Glasmacher; Homburg, University Hospital Saarland, F. Hartmann; Stuttgart, Buerger Hospital, W. Grimminger; University Hospital Ulm, H. Döhner.
Switzerland (CH): Aarau, Kantonsspital, M. Bargetzi and M. Wernli; Basel, University Hospital, A. Gratwohl; Bern, Inselspital, M. F. Fey and T. Pabst; Geneva, Cantonal University, J. Passweg and Y. Chalandon; Lausanne, CHUV, A. Herr; Luzern, Kantonsspital, W. A. Wuillemin; Zürich, University Hospital, G. Stuessi and U. Schanz.
The Netherlands (NL): Amsterdam, Academic Medical Center, J. Van Der Lelie and B. J. Biemond; Amsterdam, Hospital OLVG, B. De Valk; Amsterdam, VU University Medical Center, G. J. Ossenkoppele and P.C. Huijgens; The Hague, Leyenburg, P. W. Wijermans; Dordrecht, Albert Schweitzer Hospital, M. D. Levin; Enschede, Medisch Spectrum Twente, M. R. Schaafsma; Groningen, University Medical Center, S. M. G. J. Daenen and E. Vellenga; Heerlen, Atrium, P. J. Voogt; Maastricht, University Hospital, H. C. Schouten; Nieuwegein, Antonius, D. H. Biesma; Rotterdam, Erasmus University Medical Center, P. Sonneveld, J. Zijlmans, M. Jongen-Lavrencic, G. E. De Greef and B. Löwenberg; Rotterdam, Utrecht, University Hospital Utrecht, L. F. Verdonck and J. Kuball; Zwolle, Isala Hospital, M. Van Marwijk Kooy.
United Kingdom (UK): Birmingham, Heartlands Hospital, D. W. Milligan; Canterbury, Eastham Hospital, C. Pocock; Gillingham, Medway Hospital M. Aldouri.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal