Abstract
Chronic lymphocytic leukemia (CLL) is an incurable disease derived from the monoclonal expansion of CD5+ B lymphocytes. High expression levels of ZAP-70 or CD38 and deletions of 17p13 (TP53) and 11q22-q23 (ATM) are associated with poorer overall survival and shorter time to disease progression. DNA damage and p53 play a pivotal role in apoptosis induction in response to conventional chemotherapy, because deletions of ATM or p53 identify CLL patients with resistance to treatment. Forodesine is a transition-state inhibitor of the purine nucleoside phosphorylase with antileukemic activity. We show that forodesine is highly cytotoxic as single agent or in combination with bendamustine and rituximab in primary leukemic cells from CLL patients regardless of CD38/ZAP-70 expression and p53 or ATM deletion. Forodesine activates the mitochondrial apoptotic pathway by decreasing the levels of antiapoptotic MCL-1 protein and induction of proapoptotic BIM protein. Forodesine induces transcriptional up-regulation of p73, a p53-related protein able to overcome the resistance to apoptosis of CLL cells lacking functional p53. Remarkably, no differences in these apoptotic markers were observed based on p53 or ATM status. In conclusion, forodesine induces apoptosis of CLL cells bypassing the DNA-damage/ATM/p53 pathway and might represent a novel chemotherapeutic approach that deserves clinical investigation.
Introduction
Chronic lymphocytic leukemia (CLL) is a clinical heterogeneous disorder derived from the monoclonal expansion of CD5+ B lymphocytes with abnormal regulation of apoptosis.1,2 Unmutated status of the immunoglobulin variable heavy chain genes, expression of the ζ-chain associated protein 70 kDa (ZAP-70), CD38 antigen detection on tumoral cells, and the presence of cytogenetic abnormalities, especially deletions of 17p13 (TP53) and 11q22 to q23 (ATM), are associated with poorer prognosis and shorter time to disease progression.3-5 The combination of the purine analog fludarabine with other DNA-damaging agents, such as cyclophosphamide or mitoxantrone or both, or the monoclonal antibody anti-CD20 rituximab, is currently considered the treatment of choice for CLL.6-8 Recently, the antitumor activity of bendamustine, a hybrid agent with alkylating and also purine analog–like activity has been described in B-cell malignancies, including CLL.9-11 Despite recent advances, CLL remains an incurable disease, and many patients develop drug resistance
ATM (the upstream regulator of p53) and p53 are key players during the DNA-damage response induced by chemotherapeutic drugs in CLL cells.5 In view of the poor prognosis of patients with CLL with functional deficiency in the p53 pathway, it is important to find new agents that induce apoptosis through DNA damage–independent pathways, bypassing p53 and ATM alterations. However, a hallmark of CLL cells is their resistance to apoptosis induction, being the antiapoptotic members of the BCL-2 family proteins BCL-2 and MCL-1, critical regulators of apoptosis and survival in CLL cells.12 Activation of the mitochondrial apoptotic pathway is regulated by a tight balance between proapoptotic and antiapoptotic members of the BCL-2 family proteins. Several BH3-only members of this family, such as BID, BAD, BMF, PUMA, NOXA, and BIM proteins, promote apoptosis by modulating the interactions between the antiapoptotic BCL-2, MCL-1, and BCL-XL proteins and the proapoptotic effectors BAX and BAK.13 CLL cells express high levels of the antiapoptotic BCL-2 and MCL-1 proteins, being correlated with the failure to achieve complete response to conventional therapy.1,12,14
The purine nucleoside phosphorylase (PNP) is an enzyme in the purine salvage pathway that phosphorolysis (deoxy)nucleoside analogs to their respective bases and (deoxy)ribose phosphate.15 Forodesine (BCX-1777) is a highly specific and potent transition state analog inhibitor of PNP that inhibits T-cell proliferation, being in phase 1/2 clinical trials for patients with T-cell and B-cell acute lymphoblastic leukemia and cutaneous T-cell lymphoma. PNP inhibition results in elevation of plasma 2′deoxyguanosine (dGuo) levels and subsequent intracellular accumulation of deoxyguanosine triphosphate (dGTP) in those cells with high deoxynucleoside kinase activity, leading to cell death.15,16 The susceptibility of CLL cells to forodesine has been previously reported, and it has been proposed that this effect would be due to the high deoxycytidine kinase (dCK) activity observed in these cells.17 dCK is a primary enzyme for the conversion of dGuo to deoxyguanosine monophosphate, which is then converted to dGTP, making CLL cells susceptible to PNP inhibition. Our results show that forodesine as a single agent or in combination with bendamustine or rituximab is highly effective in CLL independently of the ZAP-70 or CD38 expression levels, p53 status, or cytogenetic abnormalities. Unlike others purine analogs, forodesine is not incorporated into DNA and represents a new class of selective antitumor agent with a novel and not fully understood mechanism of action. We also show that forodesine activates the mitochondrial apoptotic pathway by decreasing the levels of MCL-1 protein and induction of p73 and proapoptotic BIM proteins regardless of p53 status, suggesting a common apoptotic pathway independent of p53-mediated cell death.
Methods
Samples
Primary leukemic lymphocytes from patients diagnosed with CLL according to the World Health Organization classification and peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll/Hypaque sedimentation (Seromed). Informed consent was obtained from each patient in accordance with the Declaration of Helsinki and the Institutional Ethics Committee of the Hospital Clinic (Barcelona, Spain), which approved these studies. The percentage of tumoral cells (CD19+CD5+) and ZAP-70 and CD38 expression levels of CLL cells were quantified as previously described.3 Cytogenetic alterations were assessed by fluorescence in situ hybridization with the use of the Vysis LSI p53/LSI ATM and LSI D13S319/LSI 13q34/CEP 12-Multicolor Probe. The cutoff level for 13q, 17p, and 11q deletion was greater than 20% of cells (Table 1). P53 mutations were confirmed by direct sequencing according to the IARC TP53 consortium (http://p53.iarc.fr). Mutations of p53 gene are usually missense mutations, and the mutant protein has a prolonged half-life enabling its detection by Western blot (not shown). Cells (2 × 106 cells/mL) were cultured in RPMI 1640 (Gibco), supplemented with 10% human serum, 2 mM glutamine, and 50 μg/mL penicillin-streptomycin, in a humidified atmosphere at 37°C containing 5% carbon dioxide.
Patient characteristics
| CLL no. . | CD19* . | CD5† . | ZAP-70‡ . | CD38§ . | 11q (ATM) . | 17p (p53) . | 13q . |
|---|---|---|---|---|---|---|---|
| 1 | 95 | 87 | 19 | ND | ND | N | ND |
| 2 | 94 | 100 | 42 | 17 | N | N | N |
| 3 | 94 | 100 | 72 | 16 | N | N | del (95%) |
| 4 | 92 | 96 | 30 | ND | ND | ND | ND |
| 5 | 86 | 60 | 100 | 85 | N | N | N |
| 6 | 96 | 98 | 64 | 74 | del (90%) | N | N |
| 7 | 91 | 69 | 29 | 7 | N | N | N |
| 8 | 94 | 99 | 41 | 26 | ND | N | ND |
| 9 | 99 | 100 | 32 | 14 | ND | N | ND |
| 10 | 97 | 93 | 2 | ND | ND | N | ND |
| 11 | 87 | 95 | 33 | 38 | N | N | N |
| 12 | 86 | 92 | 29 | 52 | ND | N | ND |
| 13 | 97 | 69 | 2 | 59 | N | N | N |
| 14 | 92 | 75 | 12 | 0.2 | ND | ND | ND |
| 15 | 92 | 90 | 0.4 | 3 | N | N | del (89%) |
| 16 | 94 | 83 | 27 | 94 | N | N | del |
| 17 | 96 | 87 | 3 | 1 | N | N | N |
| 18 | 92 | 100 | 4 | 38 | N | N | del (14%) |
| 19 | 90 | 94 | 10 | 100 | ND | ND | ND |
| 20 | 92 | 98 | 15 | ND | ND | N | ND |
| 21 | 94 | 97 | 1 | 5 | N | N | del (94%) |
| 22 | 98 | 69 | 4 | 75 | N | N | del (96%) |
| 23 | 90 | 96 | 10 | 27 | N | N | del (40%) |
| 24 | 86 | 100 | 3 | 16 | N | N | del (79%) |
| 25 | 87 | 93 | 16 | 6 | ND | ND | ND |
| 26 | 87 | 97 | 7 | 12 | ND | ND | ND |
| 27 | 84 | 98 | ND | 2 | N | N | N |
| 28 | 92 | 96 | ND | ND | ND | ND | ND |
| 29 | 92 | 87 | 50 | 26 | N | del (90%) | ND |
| 30 | 99 | 90 | 60 | 50 | N | del/del (98%) | N |
| 31 | 92 | 93 | 3 | 80 | N | del (98%)‖ | N |
| 32 | 91 | 100 | 6 | 68 | N | del (92%)‖ | N |
| 33 | 86 | 100 | 1 | 96 | N | del (89%)‖ | N |
| 34 | 91 | 100 | 90 | 90 | N | del (96%)¶ | N |
| 35 | 60 | 98 | 36 | 71 | N | del (60%)‖ | del (29%) |
| 36 | 97 | 96 | 87 | 16 | N | del (93%)¶ | del (93%) |
| 37 | 82 | 100 | 53 | 85 | N | del (82%)‖ | del (60%) |
| 38 | 95 | 99 | 73 | ND | N | del (85%)¶ | N |
| 39 | 90 | 100 | 91 | 97 | N | del (88%)¶ | N |
| 40 | 97 | 100 | 68 | 0,1 | N | del (96%)‖ | N |
| 41 | 92 | 100 | 50 | 26 | N | del (87%)‖ | N |
| 42 | 96 | 100 | 30 | 85 | N | N | del |
| 43 | 90 | 95 | 12 | 45 | del (90%) | N | del (76%) |
| 44 | 92 | 92 | 5 | 10 | del (86%) | N | del (87%) |
| 45 | 91 | 89 | 58 | 4 | del (85%) | N | N |
| 46 | 97 | 99 | 62 | 9 | del (91%) | N | del (70%) |
| 47 | 90 | 95 | 10 | 78 | del (90%) | N | N |
| 48 | 92 | 99 | 52 | 65 | del (90%) | N | N |
| 49 | 97 | 100 | 2 | 45 | del (87%) | N | N |
| 50 | 99 | 98 | 42 | 57 | del (89%)l | N | del (60%) |
| CLL no. . | CD19* . | CD5† . | ZAP-70‡ . | CD38§ . | 11q (ATM) . | 17p (p53) . | 13q . |
|---|---|---|---|---|---|---|---|
| 1 | 95 | 87 | 19 | ND | ND | N | ND |
| 2 | 94 | 100 | 42 | 17 | N | N | N |
| 3 | 94 | 100 | 72 | 16 | N | N | del (95%) |
| 4 | 92 | 96 | 30 | ND | ND | ND | ND |
| 5 | 86 | 60 | 100 | 85 | N | N | N |
| 6 | 96 | 98 | 64 | 74 | del (90%) | N | N |
| 7 | 91 | 69 | 29 | 7 | N | N | N |
| 8 | 94 | 99 | 41 | 26 | ND | N | ND |
| 9 | 99 | 100 | 32 | 14 | ND | N | ND |
| 10 | 97 | 93 | 2 | ND | ND | N | ND |
| 11 | 87 | 95 | 33 | 38 | N | N | N |
| 12 | 86 | 92 | 29 | 52 | ND | N | ND |
| 13 | 97 | 69 | 2 | 59 | N | N | N |
| 14 | 92 | 75 | 12 | 0.2 | ND | ND | ND |
| 15 | 92 | 90 | 0.4 | 3 | N | N | del (89%) |
| 16 | 94 | 83 | 27 | 94 | N | N | del |
| 17 | 96 | 87 | 3 | 1 | N | N | N |
| 18 | 92 | 100 | 4 | 38 | N | N | del (14%) |
| 19 | 90 | 94 | 10 | 100 | ND | ND | ND |
| 20 | 92 | 98 | 15 | ND | ND | N | ND |
| 21 | 94 | 97 | 1 | 5 | N | N | del (94%) |
| 22 | 98 | 69 | 4 | 75 | N | N | del (96%) |
| 23 | 90 | 96 | 10 | 27 | N | N | del (40%) |
| 24 | 86 | 100 | 3 | 16 | N | N | del (79%) |
| 25 | 87 | 93 | 16 | 6 | ND | ND | ND |
| 26 | 87 | 97 | 7 | 12 | ND | ND | ND |
| 27 | 84 | 98 | ND | 2 | N | N | N |
| 28 | 92 | 96 | ND | ND | ND | ND | ND |
| 29 | 92 | 87 | 50 | 26 | N | del (90%) | ND |
| 30 | 99 | 90 | 60 | 50 | N | del/del (98%) | N |
| 31 | 92 | 93 | 3 | 80 | N | del (98%)‖ | N |
| 32 | 91 | 100 | 6 | 68 | N | del (92%)‖ | N |
| 33 | 86 | 100 | 1 | 96 | N | del (89%)‖ | N |
| 34 | 91 | 100 | 90 | 90 | N | del (96%)¶ | N |
| 35 | 60 | 98 | 36 | 71 | N | del (60%)‖ | del (29%) |
| 36 | 97 | 96 | 87 | 16 | N | del (93%)¶ | del (93%) |
| 37 | 82 | 100 | 53 | 85 | N | del (82%)‖ | del (60%) |
| 38 | 95 | 99 | 73 | ND | N | del (85%)¶ | N |
| 39 | 90 | 100 | 91 | 97 | N | del (88%)¶ | N |
| 40 | 97 | 100 | 68 | 0,1 | N | del (96%)‖ | N |
| 41 | 92 | 100 | 50 | 26 | N | del (87%)‖ | N |
| 42 | 96 | 100 | 30 | 85 | N | N | del |
| 43 | 90 | 95 | 12 | 45 | del (90%) | N | del (76%) |
| 44 | 92 | 92 | 5 | 10 | del (86%) | N | del (87%) |
| 45 | 91 | 89 | 58 | 4 | del (85%) | N | N |
| 46 | 97 | 99 | 62 | 9 | del (91%) | N | del (70%) |
| 47 | 90 | 95 | 10 | 78 | del (90%) | N | N |
| 48 | 92 | 99 | 52 | 65 | del (90%) | N | N |
| 49 | 97 | 100 | 2 | 45 | del (87%) | N | N |
| 50 | 99 | 98 | 42 | 57 | del (89%)l | N | del (60%) |
N indicates normal; and ND, not determined.
Percentage of cells positive for CD19 surface expression.
Percentage of cells positive for CD5 surface expression.
Percentage of cells positive for ZAP-70 expression.
Percentage of cells positive for CD38 expression.
p53 Mutational status: CLL no. 31, Trp91X; CLL no. 32, Arg248Gln; CLL no. 33, His179delinsGlnfsXI; CLL no. 35, His179Tyr; CLL no. 37, Pro190fsX56; CLL no. 40, Intron 5-6 GT-GC.
Mutation not found by direct sequencing, p53 protein expression confirmed by Western blot.
Culture of CLL primary cells and PBMCs from healthy donors and cell death detection
Cells were incubated with forodesine (BCX-1777/immucillin H; BioCryst Pharmaceuticals Inc) in the presence or absence of dGuo (Sigma-Aldrich). When indicated, cells were preincubated for 1 hour with the pan-caspase inhibitor z-VAD.fmk (Bachem), 2′-deoxycytidine, N-acetyl-l-cysteine (NAC), Tiron, or glutathione-reduced ethyl ester (GSH; Sigma-Aldrich). For the drug combination studies, cells were pretreated for 4, 12, or 24 hours with fludarabine (Schering), bendamustine hydrochloride (Treanda, provided by Cephalon Inc), mafosfamide (ASTA Medica AG), or the monoclonal antibody anti-CD20 rituximab (Roche) before adding forodesine and dGuo. CLL cells treated with rituximab were incubated in complete RPMI 1640 supplemented with 10% human AB serum (as a source of complement). Cell viability and apoptosis induction were analyzed by quantification of phosphatidyl serine exposure by staining with annexin V–FITC and propidium iodide (Bender Medsystems). Loss of mitochondrial transmembrane potential (ΔΨm) and reactive oxygen species (ROS) production were evaluated, respectively, by labeling with 20 nM DiOC6 (Molecular Probes) and with 2 μM dihydroethidine (Molecular Probes) and analyzed by flow cytometry. Conformational changes of BAX and BAK proteins were analyzed as previously described.10
Measurement of intracellular dGTP levels
Immunoblotting
Cells were lysed in RIPA buffer (137 mM NaCl, 20 mM Tris-HCl, pH 8, 1 mM MgCl2, 1 mM CaCl2, 10% glycerol, 1% NP-40, 0.5% Deoxicolate, 0.1% SDS) supplemented with protease and phosphatase inhibitors (leupeptine 10 μg/mL, aprotinine 10 μg/mL, 1 μM phenylmethanesulfonyl fluoride, 1 μM sodium ortovanadate, 1 μM sodium fluoride, and 2 μM sodium pyrophosphate decahydrate [Sigma-Aldrich]). Total cellular proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred to Immobilon-P (Millipore) membranes. Primary antibodies against the following proteins were used: BIM, BAK (Ab1), caspase-8 (Ab-3), p53 (Ab-2; Calbiochem); BID, caspase-9, FOXO1, and XIAP (Cell Signaling Technologies); BCL-2, dCK, MCL-1 (S-19), and p73 (clone 5B429; Santa Cruz Biotechnology); PARP (Roche Applied Science); caspase-3 and anti-BAX (clone 6A7; BD PharMingen); survivin (Abcam); β-actin and α-tubulin (Sigma-Aldrich); and FOXO3a (Upstate-Millipore). Rabbit antiphospho-dCK (Ser74) was provided by F.B. Blots were developed with horseradish peroxidase–labeled anti–mouse, anti–rabbit (Sigma-Aldrich), or anti–goat (Dako) antibodies using enhanced chemiluminescence reagents (Pierce).
mRNA quantification by real-time reverse transcription polymerase chain reaction
Total RNA was extracted with the use of TRIzol (Invitrogen) according to the manufacturer's instructions. Total RNA (1 μg) was retro-transcribed to cDNA with random primers and the M-MLV reverse transcriptase (Invitrogen). The p73 mRNA levels were determined in an ABI Prism 7900HT Sequence Detection System using predesigned Assay-on-demand probe (Hs00232088_m1 probe; Applied Biosystems). The relative expression of each gene was quantified by the comparative cycle threshold method (ΔΔCt), using β-glucuronidase as an endogenous control, and untreated samples as a calibrator.
Statistical analysis
Data are represented as mean plus or minus SD of 3 independent experiments. Statistical analyses were performed with GraphPad Prism 3.0 software (GraphPad Software Inc). Results were considered statistically significant when the P value was .05 or less (*P < .05, **P < .01, ***P < .001). Combination index (CI) was analyzed using Calcusyn software Version 2.0 (Biosoft). The interaction between 2 drugs was considered synergic when CI values were lower than 1, additive when equal to 1, and antagonistic when CI was higher than 1.
Results
Forodesine induced apoptosis in primary cells from patients with CLL independent of ZAP-70, CD38, and cytogenetic status
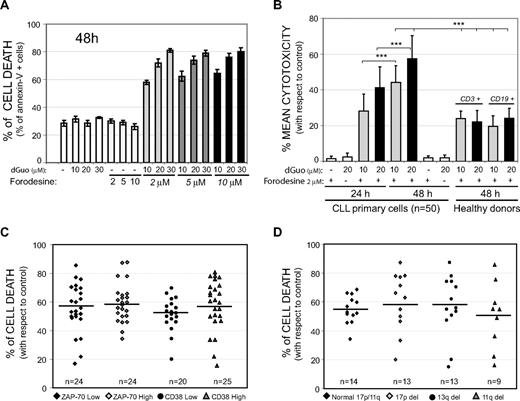
To analyze the in vitro efficacy of forodesine, it is necessary to add an external source of dGuo together with forodesine to mimic in vivo plasma elevation of dGuo levels after forodesine infusion.17 The pharmacokinetic and pharmacodynamic studies have shown that, after intravenous infusion of forodesine, plasma levels of forodesine reached values between 4 and 7.5 μM, whereas the elevation of dGuo plasma levels were in the range of 2.6 and 34 μM.19 Incubation of primary leukemic lymphocytes from patients with CLL with pharmacologic-achievable levels of forodesine (2, 5, and 10 μM) with increasing doses of dGuo (10, 20, or 30 μM) for 24 and 48 hours induced cell death (Figure 1A), whereas forodesine or dGuo alone was not cytotoxic at theseconcentrations. No differences on cell death were observed with the use of higher concentrations of forodesine (even up to 15 μM; not shown). On the contrary, increasing dGuo concentration together with the lowest forodesine concentration resulted in a higher induction of cell death. Consequently, the subsequent studies were performed with forodesine (2 μM) together with either 10 or 20 μM dGuo. The mean cytotoxicity observed in primary leukemic cells from 50 patients with CLL treated with 2 μM forodesine together with 20 μM dGuo was 43.1% (± 11.2%) at 24 hours and 55.9% (± 13%) at 48 hours (P < .001; Figure 1B). Cell death was higher than 60% in 23 cases (46% of total), between 40% and 60% in 20 cases (40% of total) and lower than 40% in only 7 cases (14% of total). Cell death induced by forodesine and dGuo was lower in PBMCs from healthy donors compared with CLL cells (***P < .001), both in T lymphocytes (CD3+ cells) and B lymphocytes (CD19+ cells; Figure 1B). We did not observe any significant differences (P = .99) in forodesine-induced cell death regarding the expression levels of ZAP-70 (ZAP-70 low, 57.1% ± 10%, vs ZAP-70 high, 55% ± 9% of mean cytotoxicity), expression levels of CD38 (CD38 low, 50.2% ± 10%, vs CD38 high, 57.2% ± 13% of mean cytotoxicity; P = .96; Figure 1C) or the presence of 13q deletion, 11q deletion, or 17p deletion (Figure 1D; P = .80).
Forodesine-induced cell death in primary CLL cells. (A) Cells from 1 representative patient with CLL were treated for 48 hours with forodesine (2, 5, or 10 μM) and dGuo (10, 20, or 30 μM). Cell death was analyzed by annexin V binding. (B) Primary cells from 50 patients with CLL and CD3+ cells or CD19+ cells from PBMCs of 4 healthy donors were incubated with 2 μM forodesine and dGuo (10-20 μM), and cell death was analyzed by annexin V binding at 24 and 48 hours. Mean cytotoxicities are given as the percentage of the apoptotic cells with respect to the viability of control cells. Data are given as the mean ± SD. (C) Cell death of forodesine 2 μM and dGuo 20 μM after 48 hours of incubation considering the expression levels of ZAP-70 or CD38. The cutoff point for high expression levels of ZAP-70 was 20% or greater and 30% or greater for CD38.3,4 (D) Forodesine 2 μM and dGuo 20 μM cell death at 48 hours in primary CLL cells regarding cytogenetic alterations. Black bars show the mean of cell death induced of each group.
Forodesine-induced cell death in primary CLL cells. (A) Cells from 1 representative patient with CLL were treated for 48 hours with forodesine (2, 5, or 10 μM) and dGuo (10, 20, or 30 μM). Cell death was analyzed by annexin V binding. (B) Primary cells from 50 patients with CLL and CD3+ cells or CD19+ cells from PBMCs of 4 healthy donors were incubated with 2 μM forodesine and dGuo (10-20 μM), and cell death was analyzed by annexin V binding at 24 and 48 hours. Mean cytotoxicities are given as the percentage of the apoptotic cells with respect to the viability of control cells. Data are given as the mean ± SD. (C) Cell death of forodesine 2 μM and dGuo 20 μM after 48 hours of incubation considering the expression levels of ZAP-70 or CD38. The cutoff point for high expression levels of ZAP-70 was 20% or greater and 30% or greater for CD38.3,4 (D) Forodesine 2 μM and dGuo 20 μM cell death at 48 hours in primary CLL cells regarding cytogenetic alterations. Black bars show the mean of cell death induced of each group.
In vitro combination of forodesine with fludarabine, bendamustine, rituximab, and cyclophosphamide
Next, we compared the cytotoxic response of 31 CLL cases, 11 of them with 17p (p53) deletions treated with either forodesine 2 μM and dGuo 20 μM, fludarabine 3.75 and 7.5 μM, and bendamustine 10 and 25 μM for 48 hours. In cells from CLL cases with no 17p deletion, the mean cytotoxicity was similar among the 3 drugs tested, being 54.7% (± 9%) for forodesine 2 μM and dGuo 20 μM, 50.1% (± 10%) for fludarabine 7.5 μM, and 44.6% (± 15%) for bendamustine 25 μM (Table 2). Remarkably, CLL cases with 17p deletion showed a high response to forodesine and dGuo (P = .004), with a mean cytotoxicity at 48 hours of 60.1% (± 21%; Table 2), even in those cells obtained from patients with chemorefractory CLL (CLL nos. 31, 33, 38, 41, and 50). In contrast, CLL cases with 17p deletion showed a lower response to fludarabine 7.5 μM (27.9% ± 12% of mean cytotoxicity; P < .001) compared with cells with no 17p deletions. However, no significant differences were observed on bendamustine cytotoxicity (44.6% of mean cytotoxicity in CLL cases with no 17p deletion vs 37.1% of mean cytotoxicity in CLL cases with 17p deletion; P = .14). These results agree with bendamustine induction of cell death irrespective of p53 status.10 We observed that CLL cases (CLL nos. 3, 5, 6, 8, 15, 30, 31, 34, 35, and 36) with in vitro response lower than 35% to bendamustine 25 μM or fludarabine 7.5 μM or both agents showed a high response to forodesine (mean cytotoxicity of 63.8% ± 12%; P < .001; Table 2).
Cytotoxic response to forodesine, fludarabine, and bendamustine
| . | Previous treatment . | Response . | Forod 2 μM + dGuo 20 μM . | Fludarab 7.5 μM . | Benda 25 μM . | Forod + Fluda 7.5 μM . | Benda 10 μM . | Forod + Benda 10 μM . |
|---|---|---|---|---|---|---|---|---|
| CLL No. | ||||||||
| 1 | No | — | 49.8 ± 3 | 64.7 ± 2 | 69.8 ± 5 | 58.1 ± 4 | 56.3 ± 4 | 78.9 ± 3 |
| 2 | No | — | 36.8 ± 5 | 67.6 ± 5 | 39.9 ± 2 | 55.9 ± 3 | 34.4 ± 3 | 61.2 ± 4 |
| 3 | No | — | 54.8 ± 3 | 33.2 ± 1 | 10.5 ± 2 | 33.5 ± 6 | 5.9 ± 6 | 64.9 ± 4 |
| 5 | No | — | 62.7 ± 4 | 40.2 ± 2 | 13.1 ± 4 | 45.6 ± 7 | 10.2 ± 3 | 69.9 ± 1 |
| 6 | No | — | 59.4 ± 2 | 53.5 ± 6 | 30.6 ± 3 | 50.6 ± 3 | 20.8 ± 3 | 72.5 ± 3 |
| 7 | No | — | 50.1 ± 3 | 68.1 ± 3 | 62.9 ± 3 | 65.1 ± 3 | 53.2 ± 3 | 78.6 ± 1 |
| 8 | No | — | 50.3 ± 6 | 38.3 ± 3 | 22.7 ± 4 | 35.6 ± 5 | 11.6 ± 5 | 62.6 ± 3 |
| 9 | No | — | 49.1 ± 3 | 68.3 ± 4 | 67.1 ± 2 | 55.8 ± 3 | 58.1 ± 3 | 69.9 ± 4 |
| 13 | No | — | 75.1 ± 3 | 44.3 ± 3 | 66.1 ± 3 | 68.7 ± 2 | 50.7 ± 2 | 87.2 ± 1 |
| 15 | No | — | 53.1 ± 2 | 44.4 ± 4 | 34.7 ± 5 | 45.3 ± 5 | 21.1 ± 4 | 62.6 ± 3 |
| 16 | No | — | 65.6 ± 3 | 53.4 ± 6 | 53.9 ± 3 | 49.2 ± 2 | 39.8 ± 2 | 75.3 ± 4 |
| 17 | No | — | 50.8 ± 5 | 48.8 ± 3 | 61.4 ± 5 | 44.9 ± 4 | 44.1 ± 4 | 74.1 ± 1 |
| 18 | No | — | 61.2 ± 3 | 50.4 ± 5 | 41.5 ± 2 | 50.7 ± 3 | 36.6 ± 2 | 85.3 ± 4 |
| 21 | No | — | 62.2 ± 2 | 52.3 ± 3 | 54.1 ± 2 | 58.9 ± 4 | 51.3 ± 3 | 79.6 ± 2 |
| 22 | No | — | 55.6 ± 5 | 43.5 ± 4 | 66.1 ± 3 | 50.6 ± 4 | 43.5 ± 4 | 70.2 ± 4 |
| 27 | No | — | 52.1 ± 2 | 68.3 ± 2 | 48.6 ± 5 | 57.2 ± 3 | 35.9 ± 3 | 68.5 ± 5 |
| 44 | FCM | Yes | 56.8 ± 2 | 42.2 ± 4 | 57.6 ± 4 | 55.1 ± 3 | 33.1 ± 5 | 67.2 ± 3 |
| 45 | No | — | 60.2 ± 1 | 32.2 ± 3 | 39.5 ± 2 | 52.3 ± 2 | 28.3 ± 4 | 77.2 ± 3 |
| 49 | CHOP | Yes | 44.1 ± 4 | 51.1 ± 3 | 47.2 ± 3 | 45.1 ± 4 | 35.2 ± 6 | 70.9 ± 2 |
| 50 | FCM | No | 44.9 ± 3 | 42.1 ± 2 | 46.4 ± 5 | 44.1 ± 3 | 30.9 ± 5 | 68.3 ± 4 |
| Mean | 54.7 ± 9 | 50.1 ± 10 | 44.6 ± 15 | 51.1 ± 8 | 35.1 ± 15 | 72.2 ± 8 | ||
| CLL p53 del | ||||||||
| 30 | CdA/FCM | Yes | 87.2 ± 3 | 5.1 ± 2 | 26.1 ± 4 | 65.3 ± 2 | ND | ND |
| 31 | CLB | No | 67.3 ± 5 | 19.9 ± 3 | 28.6 ± 3 | 38.3 ± 4 | 25.1 ± 3 | 74.5 ± 3 |
| 32 | R-CHOP | No | 33.4 ± 6 | 32.2 ± 5 | 37.3 ± 3 | 36.5 ± 4 | 30.5 ± 5 | 46.2 ± 3 |
| 33 | FC/R-F/CHOP | No | 78.4 ± 5 | 38.3 ± 3 | 41.8 ± 2 | 67.2 ± 1 | 28.7 ± 3 | 87.2 ± 2 |
| 34 | No | — | 46.5 ± 3 | 4.2 ± 3 | 15.8 ± 3 | 33.7 ± 2 | 10.9 ± 3 | 58.9 ± 5 |
| 35 | No | — | 65.1 ± 3 | 34.6 ± 5 | 26.4 ± 5 | 58.1 ± 2 | 24.4 ± 5 | 72.8 ± 4 |
| 36 | FCM/Camp/P | No | 20.1 ± 5 | 25.3 ± 4 | 23.1 ± 2 | 29.3 ± 2 | 19.2 ± 3 | 42.8 ± 2 |
| 37 | No | — | 79.6 ± 3 | 33.1 ± 5 | 40.6 ± 3 | 67.9 ± 3 | ND | ND |
| 38 | FCM/CHOP | No | 48.2 ± 4 | 42.1 ± 4 | 50.9 ± 4 | 47.7 ± 5 | 39.6 ± 4 | 59.6 ± 4 |
| 39 | FCM | Yes | 72.2 ± 4 | 40.2 ± 4 | 75.3 ± 2 | 56.1 ± 3 | ND | ND |
| 41 | R-CHOP | No | 63.1 ± 6 | 32.3 ± 3 | 41.4 ± 4 | 43.8 ± 5 | 30.5 ± 4 | 70.8 ± 5 |
| Mean | 60.1 ± 21 | 27.9 ± 12 | 37.1 ± 15 | 49.4 ± 14 | 26.1 ± 8 | 64.1 ± 11 |
| . | Previous treatment . | Response . | Forod 2 μM + dGuo 20 μM . | Fludarab 7.5 μM . | Benda 25 μM . | Forod + Fluda 7.5 μM . | Benda 10 μM . | Forod + Benda 10 μM . |
|---|---|---|---|---|---|---|---|---|
| CLL No. | ||||||||
| 1 | No | — | 49.8 ± 3 | 64.7 ± 2 | 69.8 ± 5 | 58.1 ± 4 | 56.3 ± 4 | 78.9 ± 3 |
| 2 | No | — | 36.8 ± 5 | 67.6 ± 5 | 39.9 ± 2 | 55.9 ± 3 | 34.4 ± 3 | 61.2 ± 4 |
| 3 | No | — | 54.8 ± 3 | 33.2 ± 1 | 10.5 ± 2 | 33.5 ± 6 | 5.9 ± 6 | 64.9 ± 4 |
| 5 | No | — | 62.7 ± 4 | 40.2 ± 2 | 13.1 ± 4 | 45.6 ± 7 | 10.2 ± 3 | 69.9 ± 1 |
| 6 | No | — | 59.4 ± 2 | 53.5 ± 6 | 30.6 ± 3 | 50.6 ± 3 | 20.8 ± 3 | 72.5 ± 3 |
| 7 | No | — | 50.1 ± 3 | 68.1 ± 3 | 62.9 ± 3 | 65.1 ± 3 | 53.2 ± 3 | 78.6 ± 1 |
| 8 | No | — | 50.3 ± 6 | 38.3 ± 3 | 22.7 ± 4 | 35.6 ± 5 | 11.6 ± 5 | 62.6 ± 3 |
| 9 | No | — | 49.1 ± 3 | 68.3 ± 4 | 67.1 ± 2 | 55.8 ± 3 | 58.1 ± 3 | 69.9 ± 4 |
| 13 | No | — | 75.1 ± 3 | 44.3 ± 3 | 66.1 ± 3 | 68.7 ± 2 | 50.7 ± 2 | 87.2 ± 1 |
| 15 | No | — | 53.1 ± 2 | 44.4 ± 4 | 34.7 ± 5 | 45.3 ± 5 | 21.1 ± 4 | 62.6 ± 3 |
| 16 | No | — | 65.6 ± 3 | 53.4 ± 6 | 53.9 ± 3 | 49.2 ± 2 | 39.8 ± 2 | 75.3 ± 4 |
| 17 | No | — | 50.8 ± 5 | 48.8 ± 3 | 61.4 ± 5 | 44.9 ± 4 | 44.1 ± 4 | 74.1 ± 1 |
| 18 | No | — | 61.2 ± 3 | 50.4 ± 5 | 41.5 ± 2 | 50.7 ± 3 | 36.6 ± 2 | 85.3 ± 4 |
| 21 | No | — | 62.2 ± 2 | 52.3 ± 3 | 54.1 ± 2 | 58.9 ± 4 | 51.3 ± 3 | 79.6 ± 2 |
| 22 | No | — | 55.6 ± 5 | 43.5 ± 4 | 66.1 ± 3 | 50.6 ± 4 | 43.5 ± 4 | 70.2 ± 4 |
| 27 | No | — | 52.1 ± 2 | 68.3 ± 2 | 48.6 ± 5 | 57.2 ± 3 | 35.9 ± 3 | 68.5 ± 5 |
| 44 | FCM | Yes | 56.8 ± 2 | 42.2 ± 4 | 57.6 ± 4 | 55.1 ± 3 | 33.1 ± 5 | 67.2 ± 3 |
| 45 | No | — | 60.2 ± 1 | 32.2 ± 3 | 39.5 ± 2 | 52.3 ± 2 | 28.3 ± 4 | 77.2 ± 3 |
| 49 | CHOP | Yes | 44.1 ± 4 | 51.1 ± 3 | 47.2 ± 3 | 45.1 ± 4 | 35.2 ± 6 | 70.9 ± 2 |
| 50 | FCM | No | 44.9 ± 3 | 42.1 ± 2 | 46.4 ± 5 | 44.1 ± 3 | 30.9 ± 5 | 68.3 ± 4 |
| Mean | 54.7 ± 9 | 50.1 ± 10 | 44.6 ± 15 | 51.1 ± 8 | 35.1 ± 15 | 72.2 ± 8 | ||
| CLL p53 del | ||||||||
| 30 | CdA/FCM | Yes | 87.2 ± 3 | 5.1 ± 2 | 26.1 ± 4 | 65.3 ± 2 | ND | ND |
| 31 | CLB | No | 67.3 ± 5 | 19.9 ± 3 | 28.6 ± 3 | 38.3 ± 4 | 25.1 ± 3 | 74.5 ± 3 |
| 32 | R-CHOP | No | 33.4 ± 6 | 32.2 ± 5 | 37.3 ± 3 | 36.5 ± 4 | 30.5 ± 5 | 46.2 ± 3 |
| 33 | FC/R-F/CHOP | No | 78.4 ± 5 | 38.3 ± 3 | 41.8 ± 2 | 67.2 ± 1 | 28.7 ± 3 | 87.2 ± 2 |
| 34 | No | — | 46.5 ± 3 | 4.2 ± 3 | 15.8 ± 3 | 33.7 ± 2 | 10.9 ± 3 | 58.9 ± 5 |
| 35 | No | — | 65.1 ± 3 | 34.6 ± 5 | 26.4 ± 5 | 58.1 ± 2 | 24.4 ± 5 | 72.8 ± 4 |
| 36 | FCM/Camp/P | No | 20.1 ± 5 | 25.3 ± 4 | 23.1 ± 2 | 29.3 ± 2 | 19.2 ± 3 | 42.8 ± 2 |
| 37 | No | — | 79.6 ± 3 | 33.1 ± 5 | 40.6 ± 3 | 67.9 ± 3 | ND | ND |
| 38 | FCM/CHOP | No | 48.2 ± 4 | 42.1 ± 4 | 50.9 ± 4 | 47.7 ± 5 | 39.6 ± 4 | 59.6 ± 4 |
| 39 | FCM | Yes | 72.2 ± 4 | 40.2 ± 4 | 75.3 ± 2 | 56.1 ± 3 | ND | ND |
| 41 | R-CHOP | No | 63.1 ± 6 | 32.3 ± 3 | 41.4 ± 4 | 43.8 ± 5 | 30.5 ± 4 | 70.8 ± 5 |
| Mean | 60.1 ± 21 | 27.9 ± 12 | 37.1 ± 15 | 49.4 ± 14 | 26.1 ± 8 | 64.1 ± 11 |
Primary cells from 31 patients with CLL, 11 with 17p (p53) deletion were incubated with forodesine (Forod) 2 μM + dGuo 20 μM, fludarabine (Fludarab) 7.5 μM, or bendamustine (Benda) 10 and 25 μM for 48 hours. Cell viability was determined by annexin V–FITC labeling by flow cytometry (expressed as the percentage of cytotoxicity with respect to control cells). Data are shown as the mean ± SD of triplicate points. In vivo response to previous treatment was determined as complete response after treatment.
F indicates fludarabine; C, cyclophosphamide; M, mitoxantrone; CHOP, cyclophosphamide-doxorubicin-vincristine-prednisone; CdA, cladribine; ND, not determined; CLB, chlorambucil; R, rituximab; Camp, campath-1; and P, prednisone.
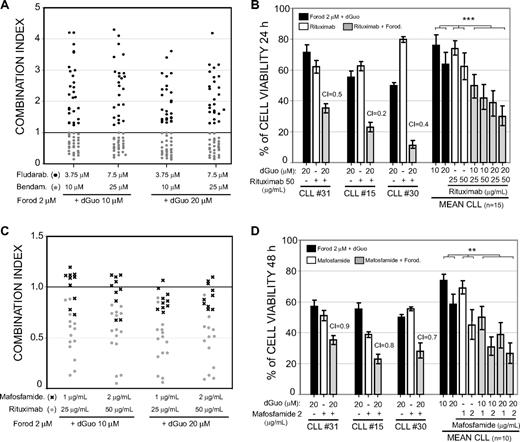
We also analyzed whether the combination of forodesine with fludarabine, bendamustine, or rituximab had a synergistic advantage. The combination of forodesine 2 μM and dGuo (10-20 μM) with fludarabine (3.75-7.5 μM) did not increase the cytotoxic effect of both drugs if compared with their single-agent activity (Table 2). In all CLL cases analyzed (n = 28), the CI values calculated for the fludarabine and forodesine combination was higher than 1 (Figure 2A black dots), indicating an antagonistic effect between these 2 agents. In contrast, the combination of bendamustine with forodesine clearly enhanced the cytotoxic response of both drugs (Table 2). The mean cytotoxicity achieved with a low dose of bendamustine (10 μM) as single agent in all CLL cases analyzed was 32.5%, whereas the combination with forodesine 2 μM and dGuo 20 μM (mean cytotoxicity of 55.3%) increased the cytotoxicity effect to 70.5% (P < .001). CI values lower than 1 were obtained for this combination, indicating a potent synergistic effect between forodesine and bendamustine (Figure 2A gray dots).
In vitro combination of forodesine with fludarabine, bendamustine, and rituximab in primary CLL cells. (A) CI for forodesine 2 μM and dGuo 10 to 20 μM, with fludarabine (3.75 and 7.5 μM) or bendamustine (10 and 25 μM) in primary cells from 28 patients with CLL. Cell death was determined at 48 hours by annexin V binding as described in “Methods.” The black horizontal line represents the threshold between synergism (CI < 1) and antagonism (CI > 1). (B) Primary cells from 15 patients with CLL were treated with forodesine 2 μM and dGuo 10 or 20 μM, rituximab (25 and 50 μg/mL), or the combination of rituximab and forodesine for 24 hours. Percentage of cell viability (with respect to control) of 3 different patients with CLL and the mean of cell viability observed in 15 patients with CLL are shown. (C) CI for forodesine 2 μM and dGuo 10 or 20 μM with rituximab (25 and 50 μg/mL) or mafosfamide (1 and 2 μg/mL) in primary cells from 12 patients with CLL incubated for 24 hours. The black horizontal line represents the threshold between synergism (CI < 1) and antagonism (CI > 1). (D) Primary cells from patients with CLL were treated with forodesine 2 μM and dGuo 20 μM, mafosfamide (1 and 2 μg/mL), or the combination of forodesine and mafosfamide for 24 hours. Percentage of cell viability (with respect to control) of 3 representative patients with CLL and the mean of cell viability observed in 10 patients with CLL are shown.
In vitro combination of forodesine with fludarabine, bendamustine, and rituximab in primary CLL cells. (A) CI for forodesine 2 μM and dGuo 10 to 20 μM, with fludarabine (3.75 and 7.5 μM) or bendamustine (10 and 25 μM) in primary cells from 28 patients with CLL. Cell death was determined at 48 hours by annexin V binding as described in “Methods.” The black horizontal line represents the threshold between synergism (CI < 1) and antagonism (CI > 1). (B) Primary cells from 15 patients with CLL were treated with forodesine 2 μM and dGuo 10 or 20 μM, rituximab (25 and 50 μg/mL), or the combination of rituximab and forodesine for 24 hours. Percentage of cell viability (with respect to control) of 3 different patients with CLL and the mean of cell viability observed in 15 patients with CLL are shown. (C) CI for forodesine 2 μM and dGuo 10 or 20 μM with rituximab (25 and 50 μg/mL) or mafosfamide (1 and 2 μg/mL) in primary cells from 12 patients with CLL incubated for 24 hours. The black horizontal line represents the threshold between synergism (CI < 1) and antagonism (CI > 1). (D) Primary cells from patients with CLL were treated with forodesine 2 μM and dGuo 20 μM, mafosfamide (1 and 2 μg/mL), or the combination of forodesine and mafosfamide for 24 hours. Percentage of cell viability (with respect to control) of 3 representative patients with CLL and the mean of cell viability observed in 10 patients with CLL are shown.
We also analyzed the combination of forodesine and the monoclonal antibody anti-CD20 rituximab. At 24 hours, the cell death observed with rituximab (25-50 μg/mL) as a single agent was low, and the combination of rituximab 25 μg/mL with forodesine 2 μM and dGuo 10 μM clearly enhanced the cytotoxic effect of both drugs individually (Figure 2B; P < .001), achieving CI values close to 0.5, indicating a highly synergistic effect (Figure 2C gray dots). Finally, we also analyzed the effect of forodesine in combination with the alkylating agent mafosfamide, the active form of cyclophosphamide in vitro (Figure 2D). An additive effect was found between both drugs, with a mean CI of 0.91 (Figure 2C black crosses).
Correlation of increase in intracellular dGTP levels and dCK phosphorylation at Ser-74 with forodesine-induced cell death
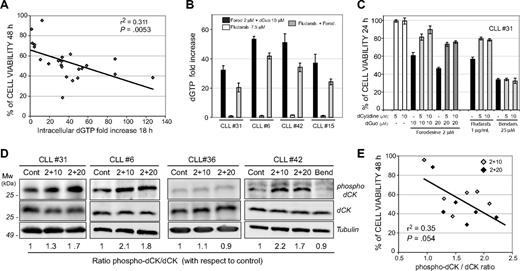
Because the increase in dGTP levels exerted by forodesine in CLL cells was postulated to be due to high dCK activity of CLL cells,17 we analyzed the intracellular levels of dGTP in primary cells from 26 patients with CLL treated with forodesine 2 μM and dGuo 10 μM for 18 hours. Forodesine induced up to a 96-fold increase in dGTP levels with respect to control basal levels (Figure 3A), reaching values between 6 and 129 pmoles of dGTP/106 cells. A significant correlation between the fold increase in the dGTP levels and forodesine-induced cell death was observed both at 24 hours (P < .05; supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and 48 hours (r2 = 0.31, P < .005; Figure 3A). Whole blood samples from patients with CLL treated ex vivo with forodesine and dGuo showed a similar increase in intracellular dGTP levels compared with their corresponding isolated CLL leukemic cells (supplemental Figure 1B). On the contrary, we found that fludarabine alone did not induce any increase in dGTP levels, whereas the combination of fludarabine with forodesine reduced the dGTP accumulation exerted by forodesine alone (Figure 3B), an effect that may explain the antagonistic effect observed between both drugs. To show that the increase on dGTP after forodesine treatment in CLL cells was mediated by the phosphorylation of dGuo by dCK, we analyzed forodesine-induced cell death in the presence of deoxycytidine, the primary substrate of dCK. If dGuo is also phosphorylated by dCK, then deoxycytidine should inhibit the apoptosis induction. Preincubation of CLL cells with deoxycytidine (5-10 μM) inhibited forodesine-induced cell death and also prevented fludarabine-induced cell death (Figure 3C). In contrast, deoxycytidine did not inhibit bendamustine-induced cell death, indicating that bendamustine induces cell death by a mechanism independent of the dCK activity.
Increase of intracellular dGTP and dCK phosphorylation at Ser-74 correlates with forodesine-induced cell death in CLL cells. (A) Cell viability (analyzed by annexin V binding) after forodesine 2 μM and dGuo 10 μM treatment for 48 hours was plotted against the intracellular dGTP fold increase observed after 18 hours of treatment as described in “Methods” in 26 CLL cases. (B) Cells from 4 patients with CLL were treated with forodesine and dGuo, fludarabine, or the combination of fludarabine and forodesine for 18 hours. Intracellular dGTP levels were quantified as described in “Methods.” (C) Primary CLL cells were incubated with forodesine 2 μM and dGuo (10 and 20 μM), fludarabine (7.5 μM), or bendamustine (25 μM) in the presence or absence of deoxycytidine (5 and 10 μM), and cell viability was analyzed at 24 hours. One representative CLL case of 5 cases analyzed is shown. (D) Cells from 4 representative patients with CLL were treated with forodesine 2 μM and dGuo (10 and 20 μM) or bendamustine (25 μM) for 10 (CLL no. 31) and 16 (CLL nos. 6, 36, and 42) hours. Protein extracts were subjected to Western blot with the antiphospho-Ser-74 dCK antibody and with the goat anti-dCK antibody as a control for dCK expression. α-Tubulin was used as loading control, and the ratio phospho dCK/dCK is shown. Four representative cases are shown. (E) Densitometric protein quantification of phospho dCK/dCK ratio was performed in 6 CLL cases treated with forodesine (2 μM) and dGuo (10 and 20 μM) and plotted against cell viability (analyzed by annexin V binding) after 48 hours of forodesine and dGuo treatment. Correlation coefficient and P value are shown.
Increase of intracellular dGTP and dCK phosphorylation at Ser-74 correlates with forodesine-induced cell death in CLL cells. (A) Cell viability (analyzed by annexin V binding) after forodesine 2 μM and dGuo 10 μM treatment for 48 hours was plotted against the intracellular dGTP fold increase observed after 18 hours of treatment as described in “Methods” in 26 CLL cases. (B) Cells from 4 patients with CLL were treated with forodesine and dGuo, fludarabine, or the combination of fludarabine and forodesine for 18 hours. Intracellular dGTP levels were quantified as described in “Methods.” (C) Primary CLL cells were incubated with forodesine 2 μM and dGuo (10 and 20 μM), fludarabine (7.5 μM), or bendamustine (25 μM) in the presence or absence of deoxycytidine (5 and 10 μM), and cell viability was analyzed at 24 hours. One representative CLL case of 5 cases analyzed is shown. (D) Cells from 4 representative patients with CLL were treated with forodesine 2 μM and dGuo (10 and 20 μM) or bendamustine (25 μM) for 10 (CLL no. 31) and 16 (CLL nos. 6, 36, and 42) hours. Protein extracts were subjected to Western blot with the antiphospho-Ser-74 dCK antibody and with the goat anti-dCK antibody as a control for dCK expression. α-Tubulin was used as loading control, and the ratio phospho dCK/dCK is shown. Four representative cases are shown. (E) Densitometric protein quantification of phospho dCK/dCK ratio was performed in 6 CLL cases treated with forodesine (2 μM) and dGuo (10 and 20 μM) and plotted against cell viability (analyzed by annexin V binding) after 48 hours of forodesine and dGuo treatment. Correlation coefficient and P value are shown.
dCK activity is positively regulated by phosphorylation of dCK at Ser-74 residue in CLL cells,20,21 so we analyzed the phosphorylation status of dCK on forodesine and dGuo treatment with the use of a specific antibody that recognizes the phosphorylation of dCK at the Ser-74 residue. Forodesine and dGuo treatment induced an increase of the phosphorylation of dCK on Ser-74 (Figure 3D), whereas no changes in total dCK levels were observed. Interestingly, when forodesine did not induce Ser-74 phosphorylation (as in CLL no. 36), no increase in dGTP levels was found (fold induction of 1.2), and the response to forodesine was very low (14% of cytotoxicity at 48 hours). These results suggest that forodesine and dGuo treatment produces an increase of dCK activity, which favors the conversion of dGuo into dGTP. A correlation between dCK phosphorylation assessed by densitometric analysis of phospho-dCK/dCK ratio after forodesine and dGuo treatment and forodesine-induced cell death both at 48 hours (r2 = 0.35, P = .05; Figure 3E) and 24 hours (P = .05; not shown) was found, confirming that dCK phosphorylation plays a role in forodesine-induced cell death. On the contrary, phosphorylation of dCK was not observed after bendamustine treatment (Figure 3D).
Forodesine induced activation of the mitochondrial apoptotic pathway independent of p53
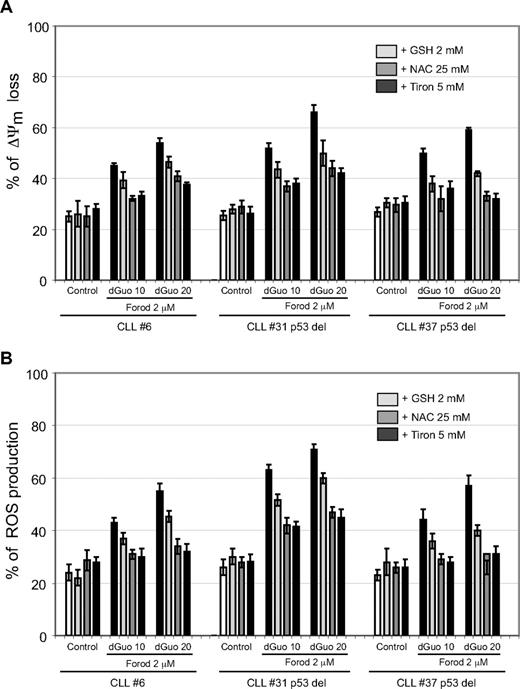
To elucidate the mechanism of action by which forodesine induced apoptosis, several hallmarks of the mitochondrial apoptotic pathway were analyzed. Forodesine and dGuo induced loss of ΔΨm and production of ROS at 16 hours, an effect that was independent of the p53 status (Figure 4). These mitochondrial apoptotic markers were observed at early time points (< 10 hours of forodesine incubation) and were related with apoptosis induction at 24 hours (not shown). Preincubation of CLL cells with several ROS scavengers (GSH, NAC, and Tiron) reduced ΔΨm loss and ROS production induced by forodesine (Figure 4A-B). NAC and Tiron (specific scavengers of O2) practically inhibited ROS production, whereas the effect of GSH, a scavenger selective for H2O2, was moderated.
Forodesine induced loss of ΔΨm and ROS production in CLL cells. Cells from patients with CLL were preincubated for 1 hour with the ROS scavengers GSH, NAC, or Tiron and treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 16 hours. Loss of ΔΨm (DIOC6 staining) and ROS production (dihydroethidine staining) were analyzed by flow cytometry. Data of 3 representative cases are shown as the mean value ± SD of triplicated points.
Forodesine induced loss of ΔΨm and ROS production in CLL cells. Cells from patients with CLL were preincubated for 1 hour with the ROS scavengers GSH, NAC, or Tiron and treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 16 hours. Loss of ΔΨm (DIOC6 staining) and ROS production (dihydroethidine staining) were analyzed by flow cytometry. Data of 3 representative cases are shown as the mean value ± SD of triplicated points.
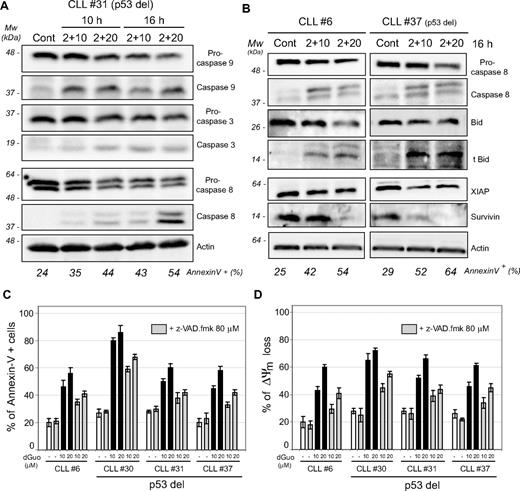
Next, we analyzed the pattern of caspase activation after forodesine exposure, because activation of the mitochondrial apoptotic pathway leads to caspase-dependent cell death. We observed a dose- and time-dependent activation of caspase-9, -8, and -3 (Figure 5A). The cleavage products of procaspase-9 and -8 were present almost simultaneously as early as 10 hours of forodesine incubation, being the p37 cleaved form of procaspase-9 first, followed by the increase in the p43/41 cleaved form of caspase-8 (Figure 5A). The cleavage of procaspase-3 and the presence of its active form were detected later. In correlation with caspase-8 activation, forodesine also decreased the levels of the BH3-only protein BID, a main substrate of caspase-8, to give its truncated proapoptotic form (Figure 5B). We observed also a decrease of the inhibitors of apoptosis XIAP and survivin in correlation with apoptosis induction (Figure 5B). Remarkably, all these events were observed independently of p53 status. Preincubation of cells with the broad range caspase inhibitor z-VAD.fmk partially reduced phosphatidyl serine exposure and ΔΨm loss after forodesine treatment for 24 hours (Figure 5C-D), implicating both caspase-dependent as well as -independent cell death.
Forodesine triggered caspase-9, -3, and -8 activation and processing of BID protein to its proapoptotic form tBID. (A) Cells from a representative patient with 17p-deleted CLL were treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 10 and 16 hours. Caspase-9, caspase-3, and caspase-8 were analyzed by Western blot in total protein extracts. β-Actin was used as internal loading control. (B) Cells from 2 representative patients with CLL (normal and 17p deleted) were treated with forodesine (2 μM) and dGuo (10-20 μM) for 16 hours, and caspase-8, BID, truncated BID, XIAP, and survivin levels were analyzed by Western blot in total protein extracts. Actin was used as internal loading control. Cell death was assessed by annexin V labeling as described in “Methods.” (C-D) Cells from 4 representative patients with CLL were preincubated for 1 hour with the broad-range caspase inhibitor z-VAD.fmk and treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 16 to 24 hours. Apoptosis induction (percentage of annexin V+ cells at 24 hours; C) and loss of ΔΨm (DIOC6 staining at 16 hours; D) were analyzed by flow cytometry.
Forodesine triggered caspase-9, -3, and -8 activation and processing of BID protein to its proapoptotic form tBID. (A) Cells from a representative patient with 17p-deleted CLL were treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 10 and 16 hours. Caspase-9, caspase-3, and caspase-8 were analyzed by Western blot in total protein extracts. β-Actin was used as internal loading control. (B) Cells from 2 representative patients with CLL (normal and 17p deleted) were treated with forodesine (2 μM) and dGuo (10-20 μM) for 16 hours, and caspase-8, BID, truncated BID, XIAP, and survivin levels were analyzed by Western blot in total protein extracts. Actin was used as internal loading control. Cell death was assessed by annexin V labeling as described in “Methods.” (C-D) Cells from 4 representative patients with CLL were preincubated for 1 hour with the broad-range caspase inhibitor z-VAD.fmk and treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 16 to 24 hours. Apoptosis induction (percentage of annexin V+ cells at 24 hours; C) and loss of ΔΨm (DIOC6 staining at 16 hours; D) were analyzed by flow cytometry.
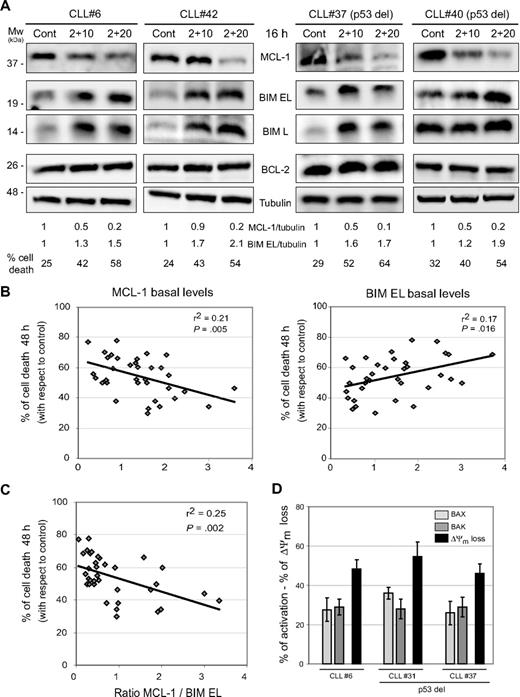
The outcome of the mitochondrial apoptotic pathway is controlled by several antiapoptotic and proapoptotic members of the BCL-2 family proteins.13 We observed a decrease of antiapoptotic MCL-1 protein levels, without affecting BCL-2 levels, and an increase in BIM levels, both extra-large (EL) and large (L) isoforms after treatment with forodesine and dGuo (Figure 6A). A significant correlation (P = .04) was found between the ratio of the decrease in MCL-1 and BIM EL induction and cell death induced by forodesine (supplemental Figure 2A). Basal levels of MCL-1, BIM EL and the ratio between MCL-1 and BIM EL levels were analyzed in 35 patients with CLL, and a significant correlation (P < .05) with forodesine-induced cell death was detected (Figure 6B-C). No correlation was observed between cell death exerted by forodesine and BCL-2 levels, BCL-2/BIM EL ratio, BCL-2/MCL-1 ratio (supplemental Figure 2B) and the levels of antiapoptotic proteins XIAP and survivin (not shown). Finally, forodesine also induced the conformational change of the proapoptotic effectors proteins BAX and BAK (Figure 6D).
Forodesine decreased the antiapoptotic MCL-1 protein levels and induced proapoptotic BIM protein independent of p53 status. (A) Cells from 4 representative CLL cases were treated with forodesine (2 μM) and dGuo (10-20 μM) for 16 hours, and MCL-1, BIM EL, BIM L, and BCL-2 proteins were analyzed by Western blot in total protein extracts. α-Tubulin was used as internal loading control. Cell viability was assessed by annexin V labeling as described in “Methods.” Densitometric protein quantification of MCL-1 and BIM EL protein levels with respect to control was performed. (B-C) Protein basal levels of MCL-1, BIM EL, BCL-2, and the ratio between MCL-1 and BIM EL were quantified by densitometric analysis on Western blot of total protein extracts in 35 CLL cases and plotted against the cytotoxicity of forodesine 2 μM and dGuo 20 μM at 48 hours. β-Actin was used as internal loading control. Correlation coefficient and P value are shown. (D) Primary cells from 3 representative patients with CLL were treated with forodesine (2 μM) and dGuo (20 μM) for 16 hours. Activation of BAX and BAK (conformational change) and loss of ΔΨm (DIOC6 staining) were analyzed by flow cytometry as described in “Methods.”
Forodesine decreased the antiapoptotic MCL-1 protein levels and induced proapoptotic BIM protein independent of p53 status. (A) Cells from 4 representative CLL cases were treated with forodesine (2 μM) and dGuo (10-20 μM) for 16 hours, and MCL-1, BIM EL, BIM L, and BCL-2 proteins were analyzed by Western blot in total protein extracts. α-Tubulin was used as internal loading control. Cell viability was assessed by annexin V labeling as described in “Methods.” Densitometric protein quantification of MCL-1 and BIM EL protein levels with respect to control was performed. (B-C) Protein basal levels of MCL-1, BIM EL, BCL-2, and the ratio between MCL-1 and BIM EL were quantified by densitometric analysis on Western blot of total protein extracts in 35 CLL cases and plotted against the cytotoxicity of forodesine 2 μM and dGuo 20 μM at 48 hours. β-Actin was used as internal loading control. Correlation coefficient and P value are shown. (D) Primary cells from 3 representative patients with CLL were treated with forodesine (2 μM) and dGuo (20 μM) for 16 hours. Activation of BAX and BAK (conformational change) and loss of ΔΨm (DIOC6 staining) were analyzed by flow cytometry as described in “Methods.”
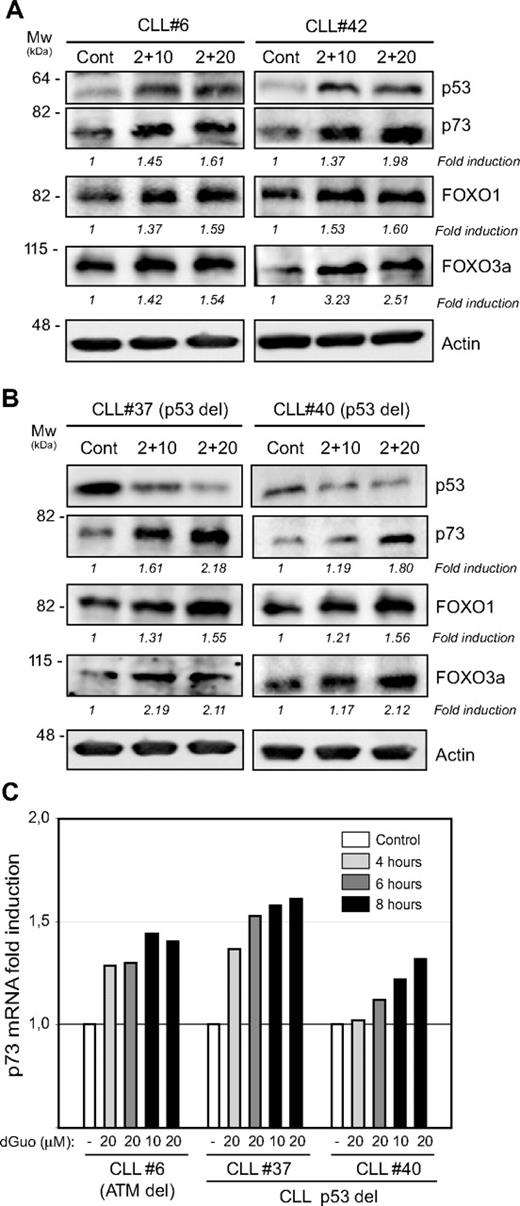
Forodesine treatment triggered an increase of p73 at mRNA and protein levels and the induction of FOXO1 and FOXO3A
Although forodesine induced apoptosis in CLL cases irrespective of the p53 status, forodesine induced stabilization of p53 protein in CLL cases with no 17p deletion (Figure 7A left). It has been described that the induction of the TAp73 protein, a p53-related protein needed for p53-mediated apoptosis,22 is able to overcome the resistance to apoptosis of CLL cells lacking functional p53.23 We observed that forodesine induced a clear up-regulation of p73 mRNA (Figure 7B) and TAp73 protein levels (Figure 7A), independent of the p53 status. It has been described that p73 regulates the induction of BIM through up-regulation of the transcription factors FOXO1 and FOXO3a, in a p53-dependent way but also p53-independent way.24 Therefore, we also analyzed the levels of FOXO1 and FOXO3a in CLL cells treated with forodesine. As shown in Figure 7A, forodesine induced an increase in both FOXO1 and FOXO3a in correlation with the increase in p73 observed. A time and dose relation between the increase in p73, FOXO1, and FOXO3a protein levels and BIM EL induction were observed in cell samples from patients with CLL treated with forodesine 2 μM and dGuo (10-20 μM) for 10 and 16 hours (not shown).
Forodesine treatment triggered an increase of p73 at mRNA and protein levels and the induction of FOXO1 and FOXO3a. (A-B) Cells from 4 representative patients with CLL were treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 16 hours, and p53, p73, FOXO1, and FOXO3a proteins were analyzed by Western blot in total protein extracts. β-Actin was used as internal loading control. Densitometric protein quantification was performed, and p73, FOXO1, and FOXO3a fold inductions with respect to control were calculated. (C) Analysis of mRNA expression by quantitative reverse transcription polymerase chain reaction of p73 in primary cells from 3 patients with CLL treated for 4 to 6 and 8 hours with forodesine (2 μM) and dGuo (10 and 20 μM). mRNA relative levels are given as arbitrary units, using untreated cells as a reference control.
Forodesine treatment triggered an increase of p73 at mRNA and protein levels and the induction of FOXO1 and FOXO3a. (A-B) Cells from 4 representative patients with CLL were treated with forodesine (2 μM) and dGuo (10 and 20 μM) for 16 hours, and p53, p73, FOXO1, and FOXO3a proteins were analyzed by Western blot in total protein extracts. β-Actin was used as internal loading control. Densitometric protein quantification was performed, and p73, FOXO1, and FOXO3a fold inductions with respect to control were calculated. (C) Analysis of mRNA expression by quantitative reverse transcription polymerase chain reaction of p73 in primary cells from 3 patients with CLL treated for 4 to 6 and 8 hours with forodesine (2 μM) and dGuo (10 and 20 μM). mRNA relative levels are given as arbitrary units, using untreated cells as a reference control.
Discussion
In the present study, we showed that forodesine, a purine nucleoside analog and a transition state inhibitor of PNP, induced a time- and dose-dependent cell death in primary CLL cells. No differences in the response were observed regarding deletions in 17p13 (TP53) and 11q22-q23 (ATM), genetic abnormalities acquired in advanced disease and associated with drug resistance and short survival.5 Forodesine displayed a high cytotoxic effect in cell samples from patients with CLL with p53 or ATM deletion, with high levels of cell death achieved in samples from patients with chemorefractory CLL and cases with low in vitro response to fludarabine. The combination of forodesine with clinical established antileukemic regimens enhances in vitro cytotoxic responses, with a strong synergistic effect observed with the combination of forodesine and bendamustine or rituximab and an additive effect with the alkylating agent cyclophosphamide. On the contrary, an antagonistic effect was found with fludarabine, an effect that could be explained by the competition between dGuo and fludarabine for phosphorylation by dCK. Fludarabine is phosphorylated by dCK to its active 5′-triphosphate metabolite,25 and the susceptibility of CLL cells to forodesine was postulated to be due to the high dCK activity observed in these cells.17 Our results have shown that dCK activity indeed controls the response of CLL cells to forodesine, because deoxycytidine, the primary substrate of dCK, reverted apoptosis induced by forodesine. Moreover, a positive correlation between phospho-dCK/dCK ratio, which regulates dCK activity,20,21 and forodesine-induced apoptosis was observed. The increase of phosphorylation of dCK on Ser-74 exerted by forodesine and dGuo could be due to forodesine or dGuo or both, because some nucleosides, such as 2-chlorodeoxyadenosine, increase Ser-74 phosphorylation and dCK activity in CLL cells.20,21 In line with previous results,17 forodesine leads to intracellular dGTP accumulation in CLL cells that correlates with cell death induced, suggesting that the dGTP levels reached after forodesine treatment would be a potential marker of the cytotoxic response. Furthermore, the reduction on accumulation of dGTP levels after combination of fludarabine with forodesine would explain the antagonistic effect observed. Altogether, these results indicate that dCK activity and the subsequent increase in dGTP play an important role as first step to cell death exerted by forodesine in CLL cells.
The lack of p53 function by deletion of P53 and mutation of the remaining allele increases in patients with CLL refractory to chemotherapy, being double-hits during the acquisition of drug resistance that abrogates the transcriptional and mitochondrial apoptotic activity of p53. Although fludarabine induces p53-dependent cell death in CLL cells and, therefore, is not an effective therapy for those patients with CLL with loss of p53 function,5 bendamustine shows clinical activity in patients with non-Hodgkin lymphomas refractory to conventional alkylator chemotherapy and in patients with heavily pretreated rituximab-refractory CLL.9,11 In this regard, we have previously reported that bendamustine induces apoptosis on CLL and mantle cell lymphoma (MCL) cells irrespective of p53 status.10 Bendamustine induces activation of DNA-damage stress response, G2 arrest, and induction of mitotic catastrophe.26 Taken together, our results support that forodesine as a single agent or in combination with bendamustine at low doses or rituximab might be highly effective in CLL regardless cytogenetic abnormalities.
Several mechanisms for dGTP-mediated cell death have been proposed. In PNP-deficient mice, accumulation of dGTP in T lymphocytes would lead to cell death involving mitochondrial damage.27 Accumulated deoxynucleosides can be phosphorylated in the mitochondria leading to abnormal accumulation of dNTPs, which might interfere with mitochondrial DNA synthesis and repair,28 increasing sensitivity to mitochondrial damage, p53 activation,29 and apoptosis.27,30 The imbalance in mitochondrial dGTP could also affect mitochondrial ATP synthesis or inactivation of antioxidant enzymes, such as the NADPH oxidase NOX2,31 leading to ROS production.32 Under severe oxidative stress, the increase in O2−, OH−, or H2O2 levels can rapidly provoke loss of Δψm and apoptosis.33 We have shown that forodesine-induced ROS production and Δψm loss, events reverted by preincubation of CLL cells with Tiron and NAC, specific scavengers of O2−, which supports the role of oxidative stress preceding the activation of mitochondrial apoptotic pathway characterized by a time-related activation of caspase-9, -8, and -3. In turn, caspase-8 induced the cleavage of BID protein to its proapoptotic-truncated form, tBID, that also activates the mitochondrial apoptotic pathway.13 A decrease of the inhibitors of apoptosis XIAP and survivin was also observed after forodesine treatment, being that all these events favor the enhancement of the mitochondrial apoptotic pathway.
A balance between antiapoptotic protein members of the BCL-2 family of proteins, such as BCL-2 and MCL-1, and proapoptotic protein members (BAX, BAK, and the BH3-only proteins BIM, PUMA, NOXA, BAD, BID, BMF, BIK, and HRK), controls the outcome of the mitochondrial apoptotic pathway.13 High levels of BCL-2 and MCL-1 proteins correlate with disease progression, poor survival, and the failure to achieve complete response to therapy with alkylating agents, nucleoside analogs, and rituximab in patients with CLL.1,14,34 Our results show that forodesine induces a decrease on MCL-1 protein levels together with an increase of BIM protein. In addition, basal levels of MCL-1 and BIM, but not BCL-2, as well as the ratio of MCL-1 to BIM EL, determined the sensitivity of CLL cells to forodesine. In CLL cells, BIM is associated with MCL-1,35 in consequence the decrease in MCL-1 levels would make CLL cells susceptible to this BH3-only protein. BIM, as well tBID, has dual functions as both inhibitors of antiapoptotic BCL-2 members and direct activators of proapoptotic effectors BAX and BAK, whose after activation insert into the outer mitochondrial membrane and provoke the mitochondrial membrane permeabilization.13 In this regard the increase in BIM levels and BID activation would lead to BAX and BAK activation, and together with MCL-1 decrease, the remaining antiapoptotic capacity of BCL-2 would be overwhelmed.
Although we and others have shown that forodesine induced stabilization of p53 in CLL cells,17 we showed that forodesine also activated the mitochondrial apoptotic pathway in cases with p53 alterations, indicating that forodesine acts by a different or additional mechanism independent of p53 in those cases with no functional p53. Phosphorylation and activation of p53 is induced by the DNA damage response but also by several stress signals.36 ROS generation and subsequent induction of DNA damage can activate both p53 and the mitochondrial apoptotic pathway.37,38 In this regard, ROS generation induced by forodesine may act as an upstream regulator of p53, which also activates the transcription of p73.36 Oxidative stress and ROS production also lead to activation of the transcription factor E2F-1 that is able to increase p53 phosphorylation at residues that are also phosphorylated in response to DNA damage.38 E2F-1 is implicated in activation of the mitochondrial apoptotic pathway through p53-dependent and -independent mechanisms39,40 by activation of the p53 homologue TAp7322,41,42 through transactivation of the p73 promoter.43 In this regard, it has been reported that TAp73 induction is able to overcome the resistance to apoptosis of CLL cells lacking functional p53.23 We have found that forodesine induced both p53 and TAp73 in CLL cells with functional p53, but, interestingly, up-regulation of p73 at transcriptional and translational level was also observed in CLL cells with p53 deletion. Induction of proapoptotic BIM independent of p53 status, as well as an increase in the levels of the transcription factors FOXO1 and FOXO3a, were observed. The FOXO family of transcription factors regulates the expression of many genes involved in apoptosis and can be activated by increased oxidative stress.44 FOXO1 and FOXO3a are transcriptional targets of E2F-145 and have been shown to be essential for ROS-induced apoptosis,46,47 as well as also being transcription factors of BIM.48-50 In this line, it has been described that the expression of both FOXO1a and BIM and subsequent apoptosis is regulated by p73 in tumoral cells defective for p53.24
In summary, this study shows that forodesine is highly active in primary tumor CLL cells, regardless of ZAP-70, CD38 expression levels, cytogenetic abnormalities, and ATM or p53 status, with a highly synergistic action in combination with bendamustine and rituximab. Our results also provide new insights into the mechanism involved in forodesine-induced apoptosis in CLL cells, showing that the oxidative stress and ROS production could provide a signal that up-regulates TAp73 and subsequent activation of the mitochondrial apoptotic pathway. Finally, on the basis of the results presented here, clinical trials with forodesine in patients with CLL of this new antileukemic agent are clearly warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Ministerio de Ciencia y Innovación (grant SAF 06/8850), Redes Temáticas de Investigacion Cooperativa de Cáncer (RTICC) from the Instituto de Salud Carlos III (grant RED 2006-20-014, to D.C., and grant FIS 080304). R.A. holds a postdoctoral contract with Sara Borrell from Instituto de Salud Carlos III. M.L.-G. is the recipient of a FI predoctoral fellowship from Generalitat de Catalunya.
Authorship
Contribution: R.A. contributed to the design of the study, performed the research, analyzed the data, and wrote the paper; M.L.-G. and N.V. participated in collecting CLL data and revising the manuscript; R.U. and S.B. contributed to technical assistance and performed the dGTP assays; C.S. and F.B. generated the antiphospho Ser74 dCK antibody; C.M. and T.M. gave scientific advice for this study; E.M. and E.C. revised the final version of the manuscript; and D.C. was the principal investigator and contributed to interpretation of data and to writing the paper. All authors revised the manuscript critically and approved the final version to be published.
Conflict-of-interest disclosure: R.U. and S.B. are employees of Biocryst Pharmaceuticals Inc. C.M. and T.M. are employees of Mundipharma International Ltd. The remaining authors declare no competing financial interests.
Correspondence: Dolors Colomer, Hematopathology Unit, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain; e-mail: dcolomer@clinic.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal