Abstract
Already 20 years have passed since the cloning of the granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor α-chain, the first member of the GM-CSF/interleukin (IL)–3/IL-5 family of hemopoietic cytokine receptors to be molecularly characterized. The intervening 2 decades have uncovered a plethora of biologic functions transduced by the GM-CSF receptor (pleiotropy) and revealed distinct signaling networks that couple the receptor to biologic outcomes. Unlike other hemopoietin receptors, the GM-CSF receptor has a significant nonredundant role in myeloid hematologic malignancies, macrophage-mediated acute and chronic inflammation, pulmonary homeostasis, and allergic disease. The molecular mechanisms underlying GM-CSF receptor activation have recently been revealed by the crystal structure of the GM-CSF receptor complexed to GM-CSF, which shows an unexpected higher order assembly. Emerging evidence also suggests the existence of intracellular signosomes that are recruited in a concentration-dependent fashion to selectively control cell survival, proliferation, and differentiation by GM-CSF. These findings begin to unravel the mystery of cytokine receptor pleiotropy and are likely to also apply to the related IL-3 and IL-5 receptors as well as other heterodimeric cytokine receptors. The new insights in GM-CSF receptor activation have clinical significance as the structural and signaling nuances can be harnessed for the development of new treatments for malignant and inflammatory diseases.
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF) and the related cytokines interleukin (IL)–3 and IL-5 regulate the production and functional activation of hemopoietic cells, with GM-CSF acting on monocyte/macrophages and all granulocytes.1 GM-CSF also controls dendritic cell and T-cell function,2 thus linking innate and acquired immunity. Because of the widespread expression of the GM-CSF receptor in hematopoietic cells, it was assumed that both GM-CSF and its receptor were key players in the regulation of steady-state functions. Whereas this turned out to be true in terms of lung physiology,3-7 deletion of either the GM-CSF gene or its receptor showed no obvious deficiency in myeloid cell numbers or production.4,8,9 Rather, a growing body of evidence now suggests that GM-CSF plays a key role in signaling emergency hemopoiesis (predominantly myelopoiesis) in response to infection, including the production of granulocytes and macrophages in the bone marrow and their maintenance, survival, and functional activation at sites of injury or insult.10-13
The role of GM-CSF and its receptor in pathology, on the other hand, arises largely as a result of abnormal signaling leading to deregulated myelopoiesis with enhanced proliferation and survival of myeloid precursors, a common feature of myeloproliferative disorders and myeloid leukemias. For example, juvenile myelomonocytic leukemia (JMML), a rare, but potentially fatal myeloproliferative disorder of children clinically presenting with monocytosis, thrombocytopenia, and malignant infiltration of nonhematologic organs by clonal macrophages,14 exhibits hypersensitivity to GM-CSF in vitro,15,16 and a mouse model of JMML shows an absolute requirement for GM-CSF and its receptor in establishment and maintenance of the disease.17,18 Similarly, progenitor cells derived from patients with chronic myelomonocytic leukemia (CMML), a preleukemic disorder of clonal monocytosis somewhat related to JMML, but occurring in older adults, specifically require GM-CSF for spontaneous growth in vitro and in vivo.19
Although no activating mutations of the GM-CSF receptor itself have been discovered in human leukemias, activating mutations in downstream components of the GM-CSF receptor signaling pathway occur in myeloproliferative disorders: Janus kinase (Jak)2V617F in polycythemia rubra vera (PV), myelofibrosis, and essential thrombocythemia20 and acute myeloid leukemia (N-Ras, H-Ras).21 Interestingly, the GM-CSF receptor is constitutively activated on Ser585 in acute myeloid leukemia,22 and the presence of the GM-CSF receptor may be important in the pathogenesis of chronic myeloid leukemia and myeloproliferative diseases by propagating survival and proliferation signals promoted by the abnormal expression of Bcr-Abl and Jak2 mutations, respectively,23,24 thus highlighting the therapeutic potential of inhibiting the GM-CSF signaling axis. In addition to its role in hematologic neoplasia, studies with human patients and animal models of disease have now confirmed that GM-CSF is a central player in the cytokine network associated with inflammatory and autoimmune conditions,12,25 suggesting that its historical designation as a colony-stimulating factor belies other important pathophysiologic functions. In particular, murine models of rheumatoid arthritis and glomerulonephritis show a nonredundant role for GM-CSF indetermining disease severity.26-28 This dual role of the GM-CSF receptor in health and disease underscores the wide interest it has attracted in understanding its mechanism of action.
The GM-CSF receptor
The GM-CSF receptor, first identified on cells of the myelomonocytic lineage by ligand-binding studies,29,30 is a heterodimer that comprises a major binding subunit (GMRα)31 and a major signaling subunit (βc).32 The receptor subunits are always coexpressed on the surface of leukocytes, with βc being expressed at lower levels than GMRα. Certain nonhemopoietic cell types have also been reported to express the GM-CSF receptor33 and to respond to GM-CSF stimulation in vitro,34-36 although the in vivo significance of these observations remains uncertain. The closely related IL-3 and IL-5 receptors (IL-3R and IL-5R) also use a ligand-specific α-chain and share βc with the GM-CSF receptor.
The GM-CSF receptor system is analogous in composition to the IL-6, IL-4/IL-13, and IL-2 receptor systems, each of which uses a shared signaling subunit, suggesting an evolutionary conserved structural and functional arrangement whereby a single polypeptide receptor chain can recognize more than one cytokine to mediate multiple biologic activities. Like most other cytokine receptors, the GM-CSF receptor can signal an astonishing variety of cellular functions, including protection from apoptosis, entry and progression through the cell cycle, early commitment to myelopoiesis, differentiation/maturation of committed progenitors, and multiple activation and motility functions in mature cells. This plethora of biological phenomena has been termed cytokine pleiotropy or multifunctionality,1 an important property of cytokine receptors, the molecular basis of which is not well understood. Although βc is absolutely required for signaling in cells expressing GM-CSF, IL-3, or IL-5 receptors, there is evidence that the functional specificity of signaling may be fine-tuned by the presence of different α-chains. This could explain why the GM-CSF receptor appears to preferentially induce differentiation, whereas the IL-3R largely promotes proliferation,37 why IL-3 cannot replace GM-CSF in the generation of Langerhan cells from human monocytes,11 and why basophil survival is supported by IL-3, but not GM-CSF, despite these cells expressing both receptors.38
Clearly, there are events occurring both outside and inside the cell that contribute to the diverse range of biological properties transduced by the GM-CSF receptor. This review focuses on recent unexpected findings arising from the determination of the crystal structure of the extracellular GM-CSF ligand-receptor complex that39 provides evidence for the existence of higher order dodecameric (12-subunit) receptor complexes and sheds light upon the mechanism of receptor activation. In addition, we review the implications of intracellular phosphorylation motifs in the cytoplasmic domain of the GM-CSF receptor that are differentially activated in response to various concentrations of ligand and associated with distinct functional outcomes. Together, these and other published observations highlight the unique and complex nature of GM-CSF receptor signaling, reveal new potential molecular targets for intervention in GM-CSF–dependent pathology, and suggest that much of the mystery of cytokine pleiotropy may lie within the primary and 3-dimensional structures of the receptor itself.
Structure of the GM-CSF receptor complex
To understand the mechanism of activation of the GM-CSF receptor, structure-function studies used monoclonal antibodies (mAbs) to the ligand or the receptor itself40-42 ; mutational analyses of GM-CSF, GMRα, and βc43-49 ; and structural determinations of the ligand or the receptor subunits.50-53 However, it was not until the whole extracellular ternary complex was assembled in solution,54 crystallized,55 and its structure solved39 that a clear picture of how the receptor is activated began to emerge.
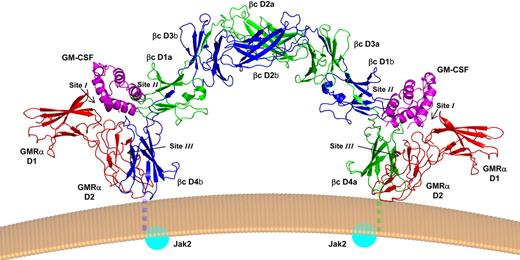
The crystal structure revealed a hexameric complex consisting of 2 GM-CSF molecules, 2 GMRα chains, and 2 βc chains (Figure 1). The new crystal structure recapitulates the intertwined βc dimer structure observed in the isolated βc molecule,53,56 a highly unusual structure not seen in other class I cytokine receptors to date. The βc configuration keeps the 2 βc cytoplasmic domains and their associated Jak2 molecules more than 100 Å apart, a separation that may prevent undue transphosphorylation and activation of the receptor. The hexamer complex reveals 3 sites of interaction, which follows the general rules set by the structure of the growth hormone:growth hormone receptor complex.57 The site 1 interaction between GM-CSF and GMRα shows the expected structural arrangement and is consistent with functional studies of both GM-CSF43 and GMRα,46,47 with helix D of the cytokine binding the elbow region defined by domains 1 and 2 of GMRα, reminiscent of the binding mode seen in other class I cytokine receptors.58
Crystal structure of the GM-CSF receptor ternary complex. Cartoon ribbon picture showing the hexamer complex as it would sit on a cell surface. One monomer of βc is shown in green (chain a) and the other in dark blue (chain b). GM-CSF is shown in magenta, and GMRα in red. Labels denote the protein domains, whereas the location of the interacting surfaces (sites I-III) is indicated. The transmembrane regions, missing in the structure, are shown stylistically as dashed lines. The Jak2 molecules, which are attached to the cytoplasmic tails and require transphosphorylation for receptor activation, are shown as blue spheres. This and subsequent figures were prepared with PyMOL.144
Crystal structure of the GM-CSF receptor ternary complex. Cartoon ribbon picture showing the hexamer complex as it would sit on a cell surface. One monomer of βc is shown in green (chain a) and the other in dark blue (chain b). GM-CSF is shown in magenta, and GMRα in red. Labels denote the protein domains, whereas the location of the interacting surfaces (sites I-III) is indicated. The transmembrane regions, missing in the structure, are shown stylistically as dashed lines. The Jak2 molecules, which are attached to the cytoplasmic tails and require transphosphorylation for receptor activation, are shown as blue spheres. This and subsequent figures were prepared with PyMOL.144
The site 2 interaction between GM-CSF and βc is particularly interesting because it is mostly provided by helix A of GM-CSF interacting with a composite βc surface comprising the A-B and E-F loops of domain 1 from 1 βc chain and the B-C and F-G loops of domain 4 of the second βc chain (Figure 1). The composite nature of the interface makes it highly unusual because in other ternary class I receptor complexes different regions of the cytokine interact with just 1 receptor chain at site 2 (Figure 2). Previous predictions from mutagenesis44,45,49 and structural studies51,53,56 are largely validated in the hexameric receptor structure, in particular the βc interaction with the essential glutamate at position 21 of GM-CSF.44,45,49,59-61 This is an important observation because Glu21 of GM-CSF is analogous to Glu22 in IL-3 and Glu13 in IL-5, and in all cases, this conserved glutamate is essential for cytokine binding to βc and for full agonist activity.48,59,60,62,63 The structure therefore provides a molecular basis to account for βc recognition of different cytokines using a conserved glutamate in helix A of the cytokine. As such, site 2 becomes an attractive target for the development of agents that can simultaneously block the action of all 3 cytokines such as mAbs, exemplified by BION-1,42 or small molecules that would take advantage of the distinct pocket in βc that envelopes helix A of the cytokine.39
Comparison of heteromeric cytokine receptor complexes. Cytokine receptor complexes for GM-CSF, IL-2, IL-4, IL-6, and IL-13. In all cases, the cytokine is shown in magenta, although the receptor subunits are colored according to the order in which they bind cytokine, red for the first subunit and blue or green for the second subunit, whereas the IL-2Rα subunit is yellow. Receptors are named, their domains are numbered D1 to D4, and the interacting surfaces are numbered I, II, and III. The inset panel at the bottom highlights the conserved interaction motif observed for GM-CSF/βc (site II), IL-2/IL-2Rα (site I), and IL-4/IL-4Rα (site I). Components are colored as above, and the key residues are shown in stick fashion, whereas cytokine helices A and C are labeled (Ha, Hc).
Comparison of heteromeric cytokine receptor complexes. Cytokine receptor complexes for GM-CSF, IL-2, IL-4, IL-6, and IL-13. In all cases, the cytokine is shown in magenta, although the receptor subunits are colored according to the order in which they bind cytokine, red for the first subunit and blue or green for the second subunit, whereas the IL-2Rα subunit is yellow. Receptors are named, their domains are numbered D1 to D4, and the interacting surfaces are numbered I, II, and III. The inset panel at the bottom highlights the conserved interaction motif observed for GM-CSF/βc (site II), IL-2/IL-2Rα (site I), and IL-4/IL-4Rα (site I). Components are colored as above, and the key residues are shown in stick fashion, whereas cytokine helices A and C are labeled (Ha, Hc).
The site 3 interaction between domain 2 of GMRα and domain 4 of βc occurs through a predominantly hydrophobic interface surrounded by a rim of charged residues. Site 3 may provide the strong interaction between GMRα and βc detectable in biochemical and functional studies as a constitutive association between GMRα and βc in the absence of GM-CSF (albeit at low stoichiometry64 ). The site 3 interaction also provides the possibility of βc dimer association with different α-chains leading to a combination of biologic activities, for example, IL-3R–mediated proliferation allied to GM-CSF receptor–mediated differentiation.37
Cytokine recognition by shared receptors
GM-CSF binds to the 2 subunits of its receptor complex via the elbow regions between receptor domains and in a sequential order, a process that is conserved among other heterodimeric receptors (reviewed by Garcia and colleagues65 ; Figure 2) such as the IL-6R complex66 (that has a shared signaling subunit, glycoprotein [gp]130 used by IL-6, IL-11, leukemia inhibitory factor, oncostatin M, cardiotrophin 1, and ciliary neurotrophic factor), the IL-2R and IL-4R complexes67-69 (that have a shared signaling subunit, common γ-chain, used by IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), and the IL-4 and IL-13 type II receptor complexes69 (that have a shared signaling subunit, IL-13Rα1, related to common γ-chain). The binding always occurs first through site 1, except for IL-13, which binds first through site 2 of the IL-13 type II receptor complex.
It is interesting to note that within this broadly conserved architecture of cytokine:receptor interactions, the cytokines typically use a conserved acidic residue in helix A to bind their receptors. However, whereas in GM-CSF the acidic residue is used for the site 2 interaction, in the case of IL-2, IL-4, and IL-13, the acidic residue is used for the site 1 interaction (Figure 2). Thus, the site 2 interaction in the GM-CSF receptor occurs through GM-CSF helix A Glu21 and loops in the elbow region of βc between domain 1 and domain 4.39 In contrast, the site 1 interaction in the IL-2R, IL-4R, and IL-13R occurs through the acidic residue in helix A of the cytokine forming polar contacts with hydrophobic residues in the elbow region of the receptor.67-70 Additional interactions involving helix C of IL-2, IL-4, and IL-13 also appear to be present in GM-CSF site 2, adding to the overall similarity of the binding site (Figure 2). Thus, the GM-CSF interaction with βc appears to use a mechanism common to other cytokine systems, but the context has been changed from a site 1 to a site 2 interaction.
Although the GM-CSF and IL-6 receptors form hexameric complexes with the same 2:2:2 stoichiometry, their assembly at the cell membrane is different, largely due to the different structures of the major signaling subunits βc and gp130, and this difference has important implications for their mechanism of activation. In the IL-6R system, the domains that bind cytokines in gp130 are far apart in the hexamer; however, the 3 extra membrane-proximal Ig-like domains take the form of “bending legs”71 that bring the 2 tails of gp130 in close proximity as they traverse the cell membrane. In this way, the Jak kinases associated with gp130 come close to each other inside the cell and can transphosphorylate each other and gp130 when ligand binds the receptor. In contrast, in the GM-CSF receptor system, βc has its cytokine-binding domain 4 proximal to the cell membrane, so that given the arch formed by the intertwined polypeptides, the tails, as they cross the cell membrane, remain more than 100 Å apart, indicating that alternative mechanisms of receptor activation need to be used.
How is the GM-CSF receptor activated?
GM-CSF receptor activation follows general rules observed for other class I cytokine receptors that invoke receptor dimerization and tyrosine transphosphorylation of cytoplasmic domains.72 The GM-CSF receptor does not have intrinsic tyrosine kinase activity, but associates with the tyrosine kinase Jak2 that is required for βc transphosphorylation and the initiation of signaling and biological activity. The cytoplasmic domains of both GMRα and βc are essential for receptor activation,73,74 but only βc associates with Jak2.39,75-77 Given that the structure of βc keeps its tails more than 100 Å apart, a distance presumably too large to allow Jak2 transphosphorylation, an alternative mechanism must bring βc-associated Jak2 together. A solution to this puzzle comes from the crystal lattice of the GM-CSF receptor that shows a dodecamer complex (Figure 3) consisting of 2 hexameric complexes related by a 2-fold axis and interacting through a new and unexpected site 4.39 Significantly, the dodecamer assembles in a head-to-head orientation, bringing the C-terminal, membrane-proximal domains of neighboring βc and GMRα molecules into close proximity. The dodecamer structure brings Jak2-associated βc close enough (∼ 10 Å) to allow functional dimerization and transphosphorylation of the receptor and initiation of signal transduction. Assembly of the dodecamer complex also promotes the interaction of 2 GMRα chains that are essential for signal transduction.74,77,78
The dodecamer complex. View of the dodecamer from above the membrane surface. Components are labeled and colored as in Figure 1, and each hexamer complex is indicated.
The dodecamer complex. View of the dodecamer from above the membrane surface. Components are labeled and colored as in Figure 1, and each hexamer complex is indicated.
The site 4 interaction occurs predominantly between βc domain 4 of each hexamer, with the rest contributed by an interaction between the GMRα of 1 hexamer and βc domain 4 of the other (Figure 4A). Functional studies using polyalanine βc site 4 mutants as well as polyclonal antibodies directed to βc site 4 support a critical functional role for site 4, and hence, dodecamer assembly in GM-CSF and IL-3 receptor activation.39 The relatively high level of sequence conservation in βc site 4 residues among many species suggests that this mechanism of receptor activation is conserved in this cytokine receptor family (Figure 4B). In addition, there may be a modest physical interaction between 2 GM-CSF molecules in the dodecamer complex, but its functional significance is unclear. The GMRα contribution to site 4 is consistent with a cytokine-dependent mechanism of dodecamer assembly preceded by cytokine-mediated heterodimerization of GMRα and βc. The structural and functional details of how GMRα and in particular the unseen N-terminal domain contribute to dodecamer assembly remain only partially resolved. Interestingly, an IL-3Rα isoform lacking the entire N-terminal domain has reduced signaling capacity,79 indicating that this domain contributes to receptor function, and intriguingly, may subtly influence signaling outcomes.
The site 4 interaction surface of the dodecamer complex. Dodecamer assembly occurs through the site 4 interaction surface at the hexamer:hexamer interface. (A) View of the site 4 interface, demonstrating that the residues are predominantly from βc domain 4 (green), but include some from GMRα domain 2 (red). Coloring as in Figure 1. (B) Comparison of the sequence of the βc site 4 region from different species. The blue bars denote the region located at the site 4 interface.
The site 4 interaction surface of the dodecamer complex. Dodecamer assembly occurs through the site 4 interaction surface at the hexamer:hexamer interface. (A) View of the site 4 interface, demonstrating that the residues are predominantly from βc domain 4 (green), but include some from GMRα domain 2 (red). Coloring as in Figure 1. (B) Comparison of the sequence of the βc site 4 region from different species. The blue bars denote the region located at the site 4 interface.
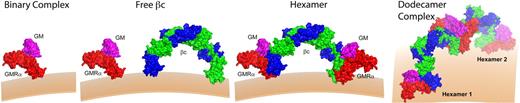
Summation of the structural and functional data enables us to propose a sequential model of GM-CSF receptor activation (Figure 5) that is initiated by the low-affinity interaction of GM-CSF with GMRα chain to form a binary complex. Recruitment of the binary complex to preformed βc dimers generates a 2:2:2 hexameric complex and converts GM-CSF binding to high affinity. Oligomerization of the hexamer complex generates a higher order dodecamer complex that is required for receptor tyrosine phosphorylation in trans by associated Jak2 and initiates intracellular signal transduction. This model is largely based on the structural determination of a soluble ternary complex containing the extracellular domains of the GM-CSF receptor bound to GM-CSF, and requires further testing by examining the assembly of receptor complex on the cell surface. Nevertheless, this model raises 2 important questions: (1) Do intermediate forms of receptor assembly exhibit different biological activities? In particular, the tantalizing possibility that the hexameric form of the GM-CSF receptor could mediate distinct functions deserves further studies. (2) Whereas the IL-3R and IL-5R may be predicted to be activated by similar dodecamer assemblies, will their activation be qualitatively the same? The recent finding that IL-4 and IL-13 induce different signals despite forming the same IL-4Rα:IL-13RαI complex69 suggests that important functional and structural surprises lay ahead.
Model of receptor activation. The low-affinity binary complex consists of GM-CSF (magenta) bound to GMRα (red). Interaction with free βc (blue and green) forms the high-affinity hexamer complex. Dodecamer (or higher order) complexes form by lateral aggregation of hexamer complexes to form a fully competent signaling complex. Jak2 associated with βc (data not shown) is able to dimerize and transphosphorylate in the dodecamer complex, but not in the hexamer complex.
Model of receptor activation. The low-affinity binary complex consists of GM-CSF (magenta) bound to GMRα (red). Interaction with free βc (blue and green) forms the high-affinity hexamer complex. Dodecamer (or higher order) complexes form by lateral aggregation of hexamer complexes to form a fully competent signaling complex. Jak2 associated with βc (data not shown) is able to dimerize and transphosphorylate in the dodecamer complex, but not in the hexamer complex.
How does the GM-CSF receptor signal multiple biologic activities?
The new structural model of GM-CSF receptor and Jak2 activation needs to be put in the context of our current understanding of GM-CSF receptor intracellular signaling. As a consequence of Jak2 activation and tyrosine phosphorylation of the cytoplasmic tail of βc, Src homology 2 and phosphotyrosine binding domain proteins are recruited to the active receptor and initiate the major tyrosine phosphorylation-dependent signaling pathways, including the Jak/signal transducer and activator of transcription, Ras/mitogen-activated protein kinase, and phosphatidylinositol 3 (PI-3) kinase pathways.80 The cell biological outcomes of signaling include proliferation and cell survival. However, it is not clear how these signaling pathways are segregated or integrated within the cell and how GM-CSF may control these from outside the cell in an analogous manner to IL-4R/IL-13R.69 Early attempts to answer these questions in the cytokine receptor family at large invoked their activation in quantitatively or temporally different manners to mediate specific biologic responses;81 however, the underlying molecular mechanisms have remained elusive.
One such mechanism is the segregation of function through the differential activation of serine phosphorylation versus tyrosine phosphorylation pathways that emanate from the GM-CSF receptor. For example, whereas low pM concentrations of GM-CSF cause phosphorylation of βc Ser585 and initiate a cell survival pathway in the absence of tyrosine phosphorylation of βc and cell proliferation, at higher concentrations of GM-CSF tyrosine phosphorylation of βc is observed, βc Ser585 phosphorylation is turned off, and the integration of survival and proliferation functions occurs.82,83 This explains previous reports that GM-CSF and IL-3 can selectively promote cell survival by suppressing apoptosis and that this can occur in response to very low cytokine concentrations (fM)84 and in the absence of proliferation.85 Interestingly, βc Ser585 is embedded in a 14-3-3–binding motif also present in other cytokine receptors, suggesting a widespread use for this mechanism22,86-88 (Figure 6).
Evolutionary conservation of the GM-CSF receptor binary switch and analogous sequences in other hemopoietic growth factor receptors. Sequence alignment of the binary switch in βc from different species is shown (top) as well as analogous sequences in receptors for other human hemopoietic regulators (bottom). The conserved Ser and Tyr residues subject to phosphorylation are highlighted.
Evolutionary conservation of the GM-CSF receptor binary switch and analogous sequences in other hemopoietic growth factor receptors. Sequence alignment of the binary switch in βc from different species is shown (top) as well as analogous sequences in receptors for other human hemopoietic regulators (bottom). The conserved Ser and Tyr residues subject to phosphorylation are highlighted.
Importantly, the biochemical effects of GM-CSF signaling at different concentrations have been determined and have also been shown to be separable (Figure 7). Low pM concentrations of GM-CSF mediate βc Ser585 phosphorylation, leading to 14-3-3 binding, PI-3 kinase activation, and hemopoietic cell survival, whereas at concentrations of 10 pM or more, GM-CSF mediates βc Tyr577 phosphorylation, Shc recruitment, and PI-3 kinase activation, thereby promoting both survival and proliferation.22 Biochemically, Ser585 and Tyr577 phosphorylation are mutually exclusive (Ser585 phosphorylation is extinguished as Tyr phosphorylation comes on) and unidirectional (Ser585 phosphorylation occurs first as GM-CSF concentrations are increased),22 which raises interesting questions as to whether the conformation of the receptor at low pM concentrations of GM-CSF is the same as at higher cytokine concentrations. The lack of tyrosine phosphorylation at low pM GM-CSF concentrations suggests that the βc-bound Jak2 molecules remain sufficiently separated to prevent transphosphorylation, which in turn may permit sustained Ser585 phosphorylation and activation of the survival-only pathway.
Hemopoietic cell survival is regulated by exogenous concentrations of GM-CSF. The selective activation of the Ser585/14-3-3/PI-3 kinase survival-only pathway is triggered by low pM concentrations of GM-CSF (left). The alternative survival and proliferation pathway triggered by higher concentrations of GM-CSF is dependent on the dodecamer assembly and involves the Jak/STAT, Ras/mitogen-activated protein kinase, and PI-3 kinase pathways (right). The key receptor-proximal events that initiate these signaling pathways are the mutually exclusive phosphorylation of Ser585 and of Tyr577 of βc.
Hemopoietic cell survival is regulated by exogenous concentrations of GM-CSF. The selective activation of the Ser585/14-3-3/PI-3 kinase survival-only pathway is triggered by low pM concentrations of GM-CSF (left). The alternative survival and proliferation pathway triggered by higher concentrations of GM-CSF is dependent on the dodecamer assembly and involves the Jak/STAT, Ras/mitogen-activated protein kinase, and PI-3 kinase pathways (right). The key receptor-proximal events that initiate these signaling pathways are the mutually exclusive phosphorylation of Ser585 and of Tyr577 of βc.
The 14-3-3–binding motif centered around Ser585 and the phosphotyrosine-binding motif around Tyr577 (that binds Shc to βc) are adjacent to each other in the primary βc structure and together constitute a bidentate motif (Figure 6). Substitutions of both Ser585 and Tyr577 inactivate the GM-CSF receptor. Significantly, this bidentate motif behaves functionally as a phospho-Tyr/phospho-Ser binary switch that confers the ability to respond in distinct biochemical and biological ways to increasing concentrations of cytokine (Figure 7). This mechanism appears to be conserved in this family of receptors across species and in other hemopoietic receptor systems (Figure 6),22 and indeed other signaling proteins.22,88 Perhaps one reason that such a switch is conserved is that very low cytokine concentrations would be sufficient to support a steady state, in which slowly dividing or quiescent stem cells are kept viable in the absence of proliferation and differentiation. The switch to a proliferative and differentiation response, and to mature cell priming would occur at times of emergency with increased cytokine concentrations, such as during an inflammatory response or after neutropenia caused by chemotherapy.
Understanding and defining the organization of these phospho-Tyr/phospho-Ser binary switches also has implications for cellular transformation and leukemogenesis because constitutive Ser585 phosphorylation of the βc in certain myeloid leukemias may confer a survival advantage on these cells.22 The clinical significance of constitutive phosphorylation of Ser585 or its failure to be switched off remains to be determined, and the kinase and phosphatases involved are yet to be identified.22 The composition of the signosome assembled around the Ser585:14-3-3 interaction, the mechanism of PI-3 kinase recruitment and activation, and the biochemical and genetic programs by which survival is promoted remain to be answered. The hope is that this serine kinase survival-only pathway may offer new diagnostic and therapeutic opportunities because this may be the very reason that tyrosine kinase inhibitor (TKI)–based therapies many times fail their curative promise. Similarly, the composition of the integration signosome that builds on Tyr577 is of interest because it contains negative and positive regulatory elements. On the one hand, the recruitment of Src homology 2-containing inositol 5′ phosphatase to βc via Shc (bound to βc through Tyr577) acts as a negative regulator,89 whereas on the other hand the recruitment of 14-3-3 to βc through Ser585 couples the receptor to PI-3 kinase via a Shc interaction with 14-3-3 (bound to 14-3-3 through Tyr179), ensuring the maintenance of a survival signal.90 Ultimately, these signaling pathways must be able to control the Bcl-2 family that tightly regulates apoptosis.
Role of the Bcl-2 family in regulating GM-CSF–mediated cell survival
The Bcl-2 family consists of proteins that inhibit or promote apoptosis. Proteins that inhibit apoptosis induced by cytokine deprivation include Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and A1. GM-CSF can induce Bcl-2 and Mcl-1 expression.83,91 Importantly, deletion of 2 other proapoptotic family members, Bax and Bak, completely inhibits apoptosis after IL-3 deprivation,92,93 highlighting the central role of the activation of these 2 proteins. When bound by antiapoptotic Bcl-2 proteins, Bax and Bak are held in an inactive conformation. Released from this inhibitory interaction by a third proapoptotic subset of the Bcl-2 family, the BH3-only proteins, which bind and repress the function of Bcl-2–like family members (reviewed in Fletcher et al94 ), Bax and Bak undergo a conformational change, which initiates the apoptosis cascade. BH3-only proteins play a key role in sensing the loss of survival signals, and are themselves activated, either by increased transcription or by posttranslational modification. Although there are 10 mammalian BH3-only proteins, evidence that signaling through the βc directly regulates the activity of these BH3-only proteins is strongest for 3 family members, Puma, Bim, and Bad.93,95-99
Signal transduction molecules that link GM-CSF or IL-3 signaling to the transcriptional and posttranslational modification of BH3-only proteins include the following: protein kinase B/Akt98,99 mitogen-activated kinase 3 (extracellular signal-regulated kinase 1; reviewed in Ley et al100 ), and the Forkhead transcription factor FoxO3a.101,102 In addition, other GM-CSF–mediated pathways are involved in maintenance of the expression of the antiapoptotic family member Mcl-1.91,103,104 The challenge now is to fill in the details of receptor activation, uncover the composition of the survival-only receptor complex, and characterize the signal transduction pathways that trigger Bax and Bak activation to produce a comprehensive pathway describing how βc signaling leads to suppression of apoptosis.
The GM-CSF receptor in human disease: opportunities for targeted therapy
The elucidation of the structure of the GM-CSF receptor and its mechanism of activation has revealed distinct sites (sites 1-4) that may be targeted with mAb, modified cytokines, or small molecules. As GM-CSF, IL-3, and IL-5 are being found more and more to play a role in the pathology of certain hematologic malignancies and inflammatory disorders (see “Introduction”), it is important to develop blockers or antagonists. However, the selection of the preferred site will depend ultimately on the clinical condition, one that may involve only GM-CSF or another one that may involve not only GM-CSF, but also IL-3 and IL-5 as the contributors or drivers of disease. Antagonists that only bind at site 1 would be expected to block all the functions of GM-CSF, an example being the GM-CSF antagonist E21R.60 New therapies targeting site 1 may be useful in strictly GM-CSF–dependent hematological malignancies such as JMML or CMML. Clonal progenitor cells from patients with JMML, PV, and CMML show enhanced survival, proliferation, and colony formation in response to GM-CSF, and evidence for an autocrine loop involving GM-CSF has been demonstrated in JMML and CMML in vitro and in vivo.19,105,106 In addition, GM-CSF plays a nonredundant role in the production and maintenance of specific tumor-associated macrophages in solid tumors, a subclass of marrow-derived macrophages present in surrounding tumor tissue that have unique metabolic and immune-regulatory properties, and appear to correlate with prognosis and sensitivity to chemotherapy.107-109
The effects of blocking site 1 would be equivalent to blocking mAb directed against GM-CSF itself, many examples of which are currently in phase I and phase II clinical trials in autoimmune disease. Based largely on data from GM-CSF knockout animal models, site I antagonists would be expected to be of clinical value in certain inflammatory conditions and autoimmune diseases such as rheumatoid arthritis,26-28,110 multiple sclerosis,111-113 type I diabetes, and autoimmune glomerulonephritis.114,115
Equivalent sites 1 in the IL-3R and IL-5R are also likely to be excellent targets. Substantial evidence connects IL-3Rα (CD123) to acute myeloid leukemia. IL-3Rα is overexpressed in CD34+CD38− acute myeloid leukemia progenitor cells, with higher expression of CD123 being considered a marker for leukemic stem cells and associated with a poorer prognosis.116-118 The blocking mAb 7G3 to IL-3Rα prevents engraftment of leukemic cells in a nonobese diabetic–severe combined immunodeficiency mouse model119 and is currently under clinical development120 as a potential drug to eradicate human acute myeloid leukemia stem cells. In terms of IL-5, mAb to site 1 may be useful in clinical conditions in which only IL-5 is involved in the production of eosinophils, such as the hypereosinophilic syndrome,121 or in a subgroup of patients with severe, corticosteroid-resistant asthma.122,123
Targeting sites 2, 3, and 4 has the potential to simultaneously block GM-CSF as well as IL-3 and IL-5 activities. The utility of blocking site 2 in βc has been demonstrated in vitro with the mAb BION-1 that blocks the binding and function of GM-CSF as well as IL-3 and IL-5 on eosinophils.42 This may be desirable in some forms of asthma in which all 3 cytokines appear to be involved.124-126 Targeting βc for the treatment of asthma has recently been validated in a mouse model that showed in βc−/− animals a remarkable inhibition of eosinophil accumulation in the lung of allergic-challenged mice, and more widely, inhibition of all the hallmarks of allergic asthma, including airway hypersensitivity, mucus hypersecretion, and antigen-specific immunoglobulin E production.127 Antibodies to site 4 would also be expected to have similar therapeutic potential, as shown by a polyclonal antibody to a βc sequence in site 4, which was able to block GM-CSF function in primary cells.39
Not only the extracellular receptor component, but also the GM-CSF receptor intracellular signaling machinery may be amenable to therapeutic intervention. Although GM-CSF receptor mutations in leukemia or lymphoma have not been described, the signaling mechanisms downstream of the receptor are often dysregulated. Activating mutations do occur in the signaling pathways activated by βc. For example, tyrosine kinase-activating Jak2 mutations (V617F) are frequent in myeloproliferative disorders, and more recently have been identified in B-lymphoid and myeloid malignancies in children with Down syndrome.128 The V617F mutation occurs in the pseudokinase domain of the Jak2 protein, which normally exerts a negative control on the kinase domain. However, the FERM (band 4.1, ezrin, radixin, moesin) domain that interacts with cytokine receptors, including the GM-CSF receptor, remains intact and is crucial for the activity of Jak2V617F.129 Studies have shown it also requires dimeric receptor subunits to be present on the cell surface for full activation,130,131 and we eagerly await the clinical testing of high specificity Jak2 inhibitors in this disease. Progenitor cells from patients with PV exhibit extreme (> 48-fold) hypersensitivity to GM-CSF in colony-forming assays compared with normal progenitors.132 Based on the crystal structure of the GM-CSF receptor complex, it may be that Jak2V617F stabilizes the formation of dodecameric GM-CSF receptor oligomers to achieve this enhanced signaling output. Likewise in JMML, activating Ras mutations or inactivation of tumor suppressor NF1 renders cells hypersensitive to GM-CSF, and the inhibition of Ras activation by farnesyltransferase inhibitors in this condition is a promising approach to therapeutic intervention.133
Nonetheless, the interaction between tyrosine kinase inhibitors or Ras inhibitors and the GM-CSF receptor signaling pathway in some diseases is unlikely to be straightforward and predictable. For instance, in chronic myeloid leukemia, CD34+ Ph-positive cells treated with GM-CSF in the presence of TKIs such as imatinib and dasatinib exhibit increased survival of the quiescent population,134 and βc has been shown to be required for oncogenic transformation of fibroblasts by Bcr-Abl,135 suggesting that GM-CSF–mediated survival signaling is distinct from Abl kinase signaling and may contribute to disease relapse or TKI resistance in vivo. Similarly, although activating mutations in PI-3 kinase/Akt are rare in haematologic cancers, constitutive activation of PI-3 kinase/Akt signaling, even in the absence of mutations, is not.136-138 The development and use of a specific inhibitor have shown that PI-3 kinase activation contributes to proliferation and survival of leukemic cells, and inhibition of this pathway is a promising therapeutic approach. An intriguing, but speculative possibility is the potential connection between constitutive phosphorylation of βc Ser585 in acute myeloid leukemia and constitutive PI-3 kinase/Akt,22 because this is a potential route to specifically design inhibitors of survival in myeloid leukemic cells. Whereas these findings illustrate the variety of lesions and the several proteins that are involved in haematologic malignancies, it is clear that many of them are involved in survival pathways, hence the need to fully characterize these and develop a survival pathway-oriented perspective.139
Future directions
Although much knowledge has been gained to date, many questions remain. What is the survival conformation of the receptor? Is it different to the survival and proliferation (dodecameric) conformation? Which signaling pathways are activated when the receptor is in the survival conformation? How do these signaling pathways regulate the activity of Bcl-2 family members known to be involved in maintaining survival in the presence of cytokine or activating death when cytokine is removed? No doubt there will be some surprising answers as these gaps are filled. The evasion of cell death regulated by Bcl-2 family members directly contributes to oncogenic transformation, for example, in follicular lymphoma140 and in experimental models of lymphoma and leukemia.141,142 Small molecules that inhibit the activity of the antiapoptotic Bcl-2 family members, by mimicking BH3-only proteins, have been developed and their role as chemotherapeutic drugs is now being established (reviewed in Vogler et al143 ). Piecing together GM-CSF receptor assembly, signaling, and molecularly defining pleiotropy may provide new therapeutic approaches for the management of disease, such as lymphoma and leukemia.
Acknowledgments
We acknowledge Dr Guido Hansen's pivotal contributions to the structure determination of the receptor, and the secretarial assistance of Anna Nitschke.
This work was supported by the National Health and Medical Research Council of Australia (Canberra, Australia), National Institutes of Health (NIH, Bethesda, MD; RO1-AI50744-02), the Australian Cancer Research Foundation (Sydney, Australia), the Cancer Council South Australia (Adelaide, Australia), the Leukemia Research Fund (Melbourne, Australia), the Children's Cancer Center Foundation (Melbourne, Australia), Australian Research Council Federation Fellowship (Canberra, Australia), and Sylvia and Charles Viertel Senior Medical Fellowship (Melbourne, Australia).
National Institutes of Health
Authorship
Contribution: T.R.H., D.T., M.A.G., P.G.E., J.K.-S., M.W.P., and A.F.L. contributed to this paper by writing parts of the manuscript and reviewing the complete manuscript.
Conflict-of-interest disclosure: M.W.P. and A.F.L. are consultants for CSL Limited in the field of GM-CSF therapeutics and currently receive research support from CSL Limited. The remaining authors declare no competing financial interests.
Correspondence: Angel F. Lopez, Division of Human Immunology, Centre for Cancer Biology, SA Pathology, Frome Rd, Adelaide SA 5000, Australia; e-mail: Angel.Lopez@health.sa.gov.au.