In this issue of Blood, Sarzotti-Kelsoe and colleagues provide strong evidence that hematopoietic stem cell transplantation in primary T-cell deficiencies provides long-term maintenance of thymopoiesis and T-cell function.
Allogeneic hematopoietic stem cell transplantation (HSCT) has become the established therapy for primary T-cell immune deficiencies, but concerns have remained about the long-term fate of thymopoiesis, particularly in patients in whom donor cells predominate only in the T-cell compartment.1 In this definitive study of a large cohort of recipients followed for as long as 25 years, Sarzotti-Kelsoe et al provide evidence that these transplants maintain T-cell function, thymic output, and T cell–receptor repertoire diversity.2
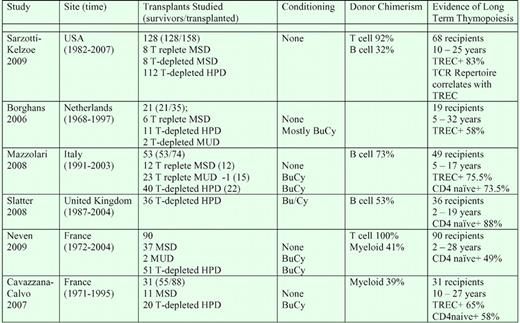
Severe combined immune deficiencies (SCIDs) are rare disorders that affect T-cell and, sometimes, B-cell and NK-cell production. They cause an inability to mount antigen- specific responses. SCID infants are highly susceptible to recurrent viral infections, and without treatment, rarely survive the first year of life. Marking 40 years since the first HSCT therapy in SCID children in 1968, a 2008 North American workshop noted that the key question was the extent and durability of T-, B-, and NK-cell reconstitution and function.1 Marking the same anniversary, several European centers have recently reviewed their experiences regarding long-term survival and T-cell function in SCID recipients.3-7 These reports complement the Sarzotti-Kelsoe report in demonstrating the broad success of hematopoietic cell transplantation (HCT) in treating SCID immune deficiency; the expansion of the donor pool to include HLA-matched–related, haploidentical-related, and HLA-matched unrelated donors; and the long-term stability of T-cell repopulation in SCID recipients (see table).
Evidence of long-term thymopoiesis after transplantation in SCID recipients. MSD indicates HLA-matched sibling donor; HPD, haploidentical parental donor; MUD, HLA-matched unrelated donor; and BuCy, myeloablative busulfan cytoxan conditioning.
Evidence of long-term thymopoiesis after transplantation in SCID recipients. MSD indicates HLA-matched sibling donor; HPD, haploidentical parental donor; MUD, HLA-matched unrelated donor; and BuCy, myeloablative busulfan cytoxan conditioning.
The Sarzotti-Kelsoe study is remarkable for its extensive, sophisticated analyses of thymic productivity and T-cell repertoire. The re-arrangement of T-cell receptor (TCR) genes during thymopoiesis was assessed. Rearrangement of V(D)J segments of the TCR α and β chains occurs by the excision of intervening sequences as episomal DNA. The resultant T-cell receptor rearrangement excision circles (TRECs) remain in T cells, but do not replicate. The new appearance of TREC-bearing cells in the periphery is therefore evidence of thymic maturation of T cells after HSCT. Because the frequency of TRECs is reduced by activation-induced expansion, the maintenance of a high frequency of TREC is evidence of continued thymopoiesis. Sarzotti-Kelsoe et al assessed TREC frequencies in serial patient samples collected from 1 to 25 years after transplantation, establishing the durable maintenance of high TREC levels after SCID transplantation.
The main source of diversity of TCR occurs during the rejoining of the V(D)J genes, by the random insertion of nucleotides coding for 6 to 14 amino acids. In newly generated naive T cells, the relative frequencies of insertions accordingly follow a Gaussian distribution, and expansion of activated T cells results in a skewing of the repertoire and a departure from the Gaussian pattern. By determining the divergence of a person's spectratype from that of an averaged normal donor standard, Sarzotti-Kelsoe et al quantified the diversity of the overall repertoire. Continuing thymopoietic productivity maintains a broad repertoire diversity in the peripheral T-cell pool. A gradual decline in peripheral T-cell repertoire diversity occurs with normal aging; a disproportionate expansion of oligoclonal effector T cells or a loss of production of naive cells would result in a loss of repertoire diversity. Sarzotti-Kelsoe et al report a maintenance of repertoire diversity in patients with high TREC levels for as long as 25 years.
A final concern is the long-term stability of donor chimerism limited mainly to T cells, a common outcome in nonmyeloablative SCID transplants (see table). Sarzotti-Kelsoe et al's breakdown of thymopoietic productivity by SCID subset is informative. In murine models, thymopoiesis is limited by the availability of engraftment “niches” available for early thymocyte progenitors (ETP). Dysfunctional early thymocytes that occupy these niches can limit thymopoietic productivity. In mice lacking the capacity to respond to IL-7 (IL-7Rα−/−, JAK3−/−, and γc−/− mice), host T cells die early in thymopoiesis, permitting full engraftment of normal donor ETP without conditioning regimens.8 In contrast, in Rag−/− and TCRβ−/− mice in which early thymocytes survive but fail to mature, engraftment of normal ETP is limited in intact hosts.8 Similar controls on thymic entry in humans may support more sustained thymopoiesis in unconditioned SCID recipients with mutations in γc, IL-7Rα, and JAK3 than in Rag mutations. Sarzotti-Kelsoe et al confirm that patients with these forms of SCID were able to sustain long-term thymopoiesis, independent of donor chimerism in non-T populations.1,4-6 Patients with Rag mutations, while showing donor engraftment in T cells, were less healthy and a lower percentage developed significant TREC frequencies.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal