Toll-like receptors (TLRs) and complement are 2 major components of innate immunity that provide a first-line host defense and shape the adaptive immune responses. We show here that coincidental activation of complement and several TLRs in mice led to the synergistic production of serum factors that promoted T-helper cell 17 (Th17) differentiation from anti-CD3/CD28 or antigen-stimulated T cells. Although multiple TLR-triggered cytokines were regulated by complement, Th17 cell–promoting activity in the serum was correlated with interleukin (IL)–6 induction, and antibody neutralization of IL-6 abrogated the complement effect. By using both in vitro and in vivo approaches, we examined in more detail the mechanism and physiologic implication of complement/TLR4 interaction on Th17-cell differentiation. We found that the complement effect required C5a receptor, was evident at physiologically relevant levels of C5a, and could be demonstrated in cultured peritoneal macrophages as well as in the setting of antigen immunization. Importantly, despite an inhibitory effect of complement on IL-23 production, complement-promoted Th17 cells were functionally competent in causing autoimmunity in an adoptive transfer model of experimental autoimmune encephalomyelitis. Collectively, these data establish a link between complement/TLR interaction and Th17-cell differentiation and provide new insight into the mechanism of action of complement in autoimmunity.
Introduction
Innate immunity provides a first-line host defense against invading pathogens. Two of the best-characterized components of the innate immune system are toll-like receptors (TLRs) and complement.1,,–4 TLRs are transmembrane proteins most prominently expressed on antigen-presenting cells such as macrophages and dendritic cells. They recognize different microbial and, in some cases, endogenous ligands. For example, TLR4 can be activated by lipopolysaccharide (LPS) from Gram-negative bacteria, heat shock proteins,5,–7 and the anticancer drug taxol.8,9 TLR2 can be activated by the yeast cell-wall component zymosan and lipoteichoic acid from gram-positive bacteria.10 TLR3 is activated by double-stranded RNAs from viruses, and TLR9 recognizes CpG DNA motifs present in viruses and bacteria.11,12 Activation of TLRs initiates a cascade of intracellular signaling events, ultimately resulting in the production and release of inflammatory cytokines and up-regulation of adhesion and costimulatory molecules on the cell surface.1,2
Complement is a group of plasma proteins that can also be activated by microbial components such as LPS and zymosan. Complement activation is initiated via 3 different pathways, the classical (antibody dependent), alternative, and lectin pathway.3,4 Activation of the alternative pathway is considered a default process that occurs spontaneously at a low level, and distinction between self and nonself is achieved through complement regulatory proteins.13,14 Activated complement protects the host by opsonizing pathogens with activated C3 and C4 fragments, direct pathogen lysis with the membrane attack complex, and generation of inflammatory peptides C3a and C5a (anaphylatoxins).3,4 C3a and C5a are chemotactic factors for leukocytes and exert their biologic activities through C3aR and C5aR, which are G-protein–coupled membrane receptors expressed on target cells.
Apart from generating an immediate inflammatory reaction to invading pathogens, the activation of TLRs and complement also serves to prime the adaptive immune responses.1,2,15 For example, complement has long been recognized as a natural adjuvant for antibody production,16 and recent works17,,,–21 have suggested a role of complement in both CD4 and CD8 T-cell immunity. Likewise, the TLR system has been shown to play a key role in adaptive immunity.1,2 TLR-mediated dendritic-cell maturation, characterized by up-regulation of major histocompatibility complex II and costimulatory molecules, is critical for T-cell priming and activation. Furthermore, TLR-dependent cytokine production regulates the differentiation of naive T cells into different classes of effector T cells, T-helper (Th) cell type 1, 2, or 17.22,–24 Th1 cells produce interferon (IFN)-γ and play a key role in controlling intracellular pathogens such as viruses and certain bacteria. Th2 cells produce interleukin (IL)-4, IL-5, and IL-13 and mediate antibody-dependent clearance of extracellular pathogens and parasites.25,26 Th17 cells produce the hallmark cytokine IL-17 and are now recognized as contributing to many inflammatory and autoimmune diseases.24,27,,–30 Recent mechanistic studies have established that differentiation of Th17 cells from naive T cells is driven by transforming growth factor (TGF)-β and IL-6,31,–33 whereas IL-23 plays an important role in propagating and differentiating pathogenic Th17 cells from antigen-experienced T cells.34
Although the TLR and complement systems often are studied separately, many pathogen-associated molecular patterns such as LPS and zymosan activate both innate immune systems. How coincidental activation of these 2 systems in vivo affects the final outcomes of innate and adaptive immune responses in an organism has not been well studied. In the present study, we show that coincidental activation of complement and TLR4, TLR2, and TLR9 in mice can lead to synergistic production of inflammatory cytokines that drive Th17-cell differentiation.
Methods
Mice
C57BL/6 wild-type (WT) and IL-6−/− mice (B6.129S6-Il6tm1Kopf) were purchased from The Jackson Laboratory. The generation and source of C3−/−, C3aR−/−, C5aR−/−, decay-accelerating factor (DAF)−/−, and DAF−/−/C5aR−/− mice was described previously.19,35 Sex- and age-matched mice were used throughout this study. Mice were housed in a specific pathogen–free facility, and all experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Treatment of mice
Mice were treated by intraperitoneal injection of LPS (026:B6, Sigma; 2 mg/kg or 20 mg/kg), zymosan A (derived from Saccharomyces cerevisiae, Sigma; 1 g/kg), CPG1826 (5′-TCCATGACGTTCCTGACGTT-3′; 20 mg/kg), and poly I:C (15 mg/kg) alone or in combination with cobra venom factor (CVF, 15 U per mouse; Quidel Corporation) or recombinant mouse C5a (Hycult Biotechnology; dosage specified in text and figure legends). At 3 hours after treatment (6 hours in the case of poly I:C treatment), mice were killed, and blood was collected for serum preparation.
ELISA
Serum levels of IL-6, tumor necrosis factor (TNF)-α, IL-1β, IL-23, IL-10, IL-12, TGF-β1, and C5a were determined by enzyme-linked immunosorbent assay (ELISA) kits per manufacturer's instruction. ELISA kits for IL-23 and TGF-β1 were from eBioscience, the ELISA kit for TNF-α was from R&D Systems, and ELISA kits for other cytokines and antibodies for mouse C5a ELISA (purified: clone I52-I486; biotinylated: clone I52-278) were from BD Pharmingen.
Purification of naive CD4+ T cells
Pooled single-cell suspension from lymph nodes and spleens were treated with ACK buffer to remove red blood cells. CD4+ T cells were isolated with CD4 Microbeads and LS column (Miltenyi Biotec) on a MidiMACS Separator. Column-purified CD4+ T cells were then stained with PE-rat-anti–mouse CD25 (clone PC61.5; eBioscience), FITC-rat-anti–mouse CD62L (clone MEL-14; BD Pharmingen), and APC-rat-anti–mouse CD4 (clone L3T4; BD Pharmingen) and sorted by a high-speed cell sorter (FACSVantage SE, BD). CD4+CD25−CD62Lhigh-naive cells were collected into a tube containing cell-culture medium (Dulbecco modified Eagle medium containing 10% fetal bovine serum, 2 mmol/L glutamine, 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.1 mmol/L nonessential amino acids, 50 μmol/L 2-ME, 1 mmol/L sodium pyruvate, and 100 U/mL penicillin/streptomycin). The purity was consistently greater than 98%.
Elicitation and culture of peritoneal macrophages
Mouse peritoneal macrophages were elicited by injection (intraperitoneal) of 2 mL of 3% thioglycollate medium (Becton Dickinson Microbiology System). On day 5, peritoneal exudates cells were prepared by peritoneal lavage with ice-cold phosphate-buffered saline (PBS). Peritoneal exudates cells (2 × 105) in 0.5 mL of culture medium were seeded into each well of a 48-well plate. Two hours later, unattached cells were removed by gentle washing with warm PBS. Cells were then cultured in 0.8 mL of medium in the presence of recombinant human C5a (50 nmol/L, Sigma-Aldrich), LPS (10 ng/mL), or both. Triplicate wells were set up for each condition. In some experiments, C5a receptor antagonist AcF(OP(D)ChaWR (kindly provided by Dr John Lambris, University of Pennsylvania; 10 μmol/L final concentration)36 was added to the wells at 0 or 20 hours after macrophage culture setup.
Induction of Th17 cells in vitro
We used 2 protocols to induce Th17 cells from naive CD4+ T cells. In the first protocol, macrophages were cultured for 20 hours as described previously, and 2 × 105 purified CD4+ T cells in 100 μL of culture medium were added to each well of macrophages (ie, macrophage and T cells were at 1:1 ratio). CD4+ T cells were activated by anti-CD3 antibody (0.5 μg/mL; clone 145-2C11, BD Pharmingen) for 3 days in the presence of 1 ng/mL recombinant human TGF-β1 (PeproTech). In the second protocol, 2 × 105 purified CD4+ T cells per well were activated by plate-bound anti-CD3/CD28 for 3 days in 2 mL of culture medium containing 5% mouse serum. Wells were precoated overnight at 4°C with anti-CD3 (2 μg/mL) and anti-CD28 (5 μg/mL, clone 37.51; BD Pharmingen) in PBS. In some experiments, the following reagents were also added to the cell culture (individually or in combination as specified): LPS, recombinant mouse C5a, IL-6 (PeproTech), IL-23 (R&D Systems), human TGF-β1 (PeproTech), and neutralizing antibodies (all from eBioscience) for mouse IL-6 (rat, MP5-20F3; 20 μg/mL), IL-1β (hamster, B122; 10 μg/mL), TNF-α (hamster, TN3-19.12; 10 μg/mL), and isotopes controls for hamster IgG1 (clone eBio299Arm, 10 μg/mL) and rat IgG1 (cat. no. 16-4301; 20 μg/mL).
Intracellular staining
After 3 days of culture, cells were harvested and resuspended in fresh culture medium at a concentration of 5 × 106 cells/mL. Cell suspension (100 μL) was reseeded into each well of a 96-well U-bottom plate. Then, 100 μL of culture medium containing GolgiStop (1 to 750 dilution; BD Pharmingen), 100 ng/mL phorbol 12-myristate 13-acetate, and 1000 ng/mL ionomycin were added to each well. After 5 hours, cells were stained sequentially for surface CD4 (FITC-rat-anti–mouse CD4; clone L3T4; BD Pharmingen) and intracellular IL-17 (PE-rat-anti–mouse IL-17, clone TC11-18H10; BD Pharmingen), IFN-γ (APC-rat-anti–mouse IFN-γ, clone XMG1.2; BD Pharmingen), or IL-10 (APC-rat-anti–mouse IL-10, clone JES5-16E3; eBioscience). Cell processing for intracellular staining was described previously.19 Fluorescence-activated cell sorting (FACS) analysis was performed on a FACSCalibur instrument by use of the CellQuest software (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
Immunization of mice and passive induction of EAE
C57BL/6 male mice (7 to 8 weeks old) were used for immunization with myelin oligodendrocyte glycoprotein peptide (MOG38-50). Peptide (synthesized by Sigma-Aldrich) stock solution was prepared by dissolving the peptide in PBS at 5 mg/mL. Each mouse was immunized by subcutaneous injection on the back with 300 μg of MOG38-50 peptide emulsified in complete Freund adjuvant (CFA) containing 500 μg of heat-killed Mycobacterium tuberculosis H37 RA (Difco). Then, 10 days later, cells from draining lymph nodes were restimulated (5 × 106 cells per well in 2 mL of medium) for 4 days in the presence of MOG38-50 peptide (20 μg/mL) and 5% mouse serum. At the end of culture, aliquots of cells were analyzed by CD4 gating and intracellular staining of IFN-γ, IL-17, or IL-10. For experimental autoimmune encephalomyelitis (EAE) induction, 7- to 8-week-old male C57BL/6 mice were used as recipients. At 6 hours before cell transfer, mice were irradiated with 600 rad by the use of a γ-irradiator. Twenty-million unfractionated live cells from MOG38-50-restimulated lymphocytes were adoptively transferred into recipients by intraperitoneal injection (0.4 mL per mouse). Mice were checked daily for the development of EAE. The following criteria were used to score EAE disease: grade 0: no disease; grade 0.5: partially limp tail; grade 1: paralyzed tail; grade 2: hind limb paresis; grade 2.5: 1 hind limb paralyzed; grade 3: both hind limbs paralyzed; grade 3.5: hind limbs paralyzed and weakness in forelimbs; grade 4: forelimbs paralyzed; grade 5: moribund or dead.
In a second immunization experiment, WT, DAF−/−, C5aR−/−, and DAF−/−/C5aR−/− mice were immunized with 100 μg of MOG38-50 emulsified in incomplete Freund adjuvant (IFA) containing 100 μg of LPS. Another group of WT mice were immunized with the same mixture that contained also recombinant mouse C5a (1 μg per mouse). At 10 days later, mice were killed, and splenocytes were harvested and seeded at 1.5 × 106 cells/well in 0.2 mL of medium. They were restimulated with MOG38-50 for 48 hours in the presence of 20 ng/mL IL-23. The level of IL-17 in the cell culture supernatant was measured by ELISA (BD Pharmingen).
Statistical analysis
Data were expressed as mean plus or minus SEM. Groups were compared by 2-tailed, unpaired Student t test, and significance was defined at a P value less than .05.
Results
Coincidental TLR4 and complement activation in mice produced serum factors that promoted Th17-cell differentiation
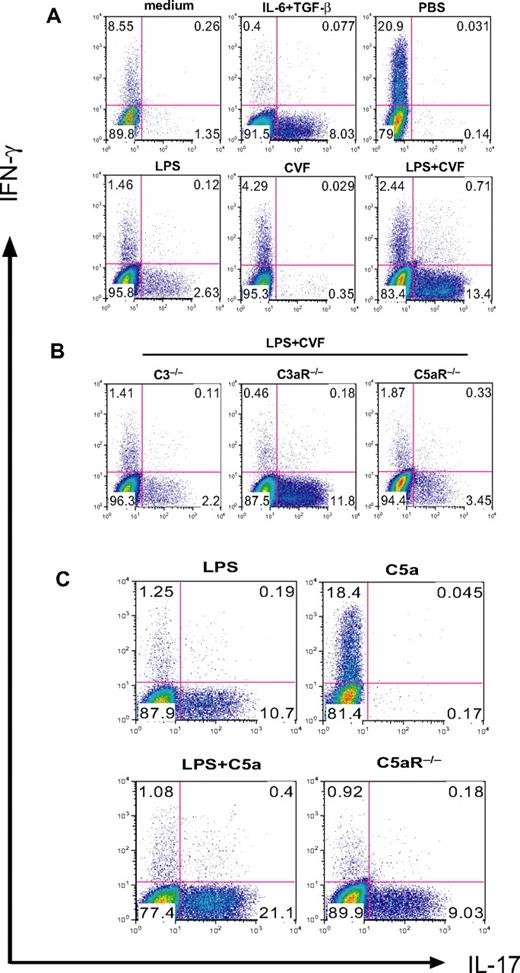
We previously showed that coincidental complement activation in mice augmented TLR4-dependent plasma IL-6, IL-1β, and TNF-α levels and suppressed plasma IL-12 levels.35 Because IL-12 and IL-6 are known to play a critical role in Th1- and Th17-cell differentiation, we evaluated what impact TLR4 and complement interaction might have on T-cell differentiation. We used LPS and CVF to activate TLR4 and complement, respectively, in mice and collected serum 3 hours after treatment. Purified naive mouse CD4+ T cells were stimulated by plate-bound anti-CD3/CD28 in the presence of 5% serum from PBS-, LPS-, CVF-, or LPS/CVF-treated mice. After 3 days, cells were analyzed by intracellular staining for IFN-γ and IL-17 expression. Figure 1A shows that serum from LPS-treated but not PBS- or CVF-treated mice stimulated Th17-cell differentiation. Notably, despite the lack of effect of CVF treatment on its own, serum from mice cotreated with LPS and CVF dramatically enhanced Th17-cell differentiation. This synergistic effect of CVF was dependent on complement C3 and C5aR but not C3aR because the effect was abolished in C3−/− and C5aR−/− but not C3aR−/− mice (Figure 1B).
Complement C5a synergizes with TLR4 to produce serum factors that drive Th17-cell differentiation. (A) Mouse CD4+ T cells were activated by plate-bound anti-CD3/CD28 in the presence of 5% serum from WT mice treated with PBS, LPS, CVF, or LPS+CVF. Culture medium and recombinant IL-6 + TGF-β were used as negative and positive controls, respectively, for Th17-cell differentiation. (B) CD4+ T cells were activated as in panel A in the presence of 5% serum from C3−/−, C3aR−/−, and C5aR−/− mice treated with LPS + CVF. (C) CD4+ T cells were activated as in panel A in the presence of 5% serum from WT mice treated with LPS, C5a, and LPS + C5a or from C5aR−/− mice treated with LPS + C5a. Cells were cultured for 3 days after activation, and IFN-γ and IL-17–producing cells were detected by flow cytometry after intracellular staining. Data in panels A and B were from the same experiment, whereas data in panel C were from a separate experiment.
Complement C5a synergizes with TLR4 to produce serum factors that drive Th17-cell differentiation. (A) Mouse CD4+ T cells were activated by plate-bound anti-CD3/CD28 in the presence of 5% serum from WT mice treated with PBS, LPS, CVF, or LPS+CVF. Culture medium and recombinant IL-6 + TGF-β were used as negative and positive controls, respectively, for Th17-cell differentiation. (B) CD4+ T cells were activated as in panel A in the presence of 5% serum from C3−/−, C3aR−/−, and C5aR−/− mice treated with LPS + CVF. (C) CD4+ T cells were activated as in panel A in the presence of 5% serum from WT mice treated with LPS, C5a, and LPS + C5a or from C5aR−/− mice treated with LPS + C5a. Cells were cultured for 3 days after activation, and IFN-γ and IL-17–producing cells were detected by flow cytometry after intracellular staining. Data in panels A and B were from the same experiment, whereas data in panel C were from a separate experiment.
In separate experiments, we confirmed that LPS or CVF itself, when added to the T-cell culture directly, had no effect on Th17-cell differentiation (data not shown). Furthermore, by substituting CVF with recombinant C5a (5 μg/mouse), we found that C5a could mimic CVF in synergizing with LPS to induce Th17 cell–promoting activity in the mouse serum, even though it had no effect on its own when used to treat mice (Figure 1C). These results indicated that coincidental TLR4 and complement signaling in mice produced serum factors that promoted Th17-cell polarization. It is notable that although sera from mice treated with PBS or C5a did not promote Th17-cell differentiation, they induced substantial Th1-cell polarization (Figure 1). This finding suggested the existence of serum factors in naive mice that favored Th1-cell polarization. Alternatively, it may imply that naive mouse serum supported more T-cell activation and differentiation of activated T cells into Th1 cells was a default outcome in the absence of Th17-driving cytokines, as has been suggested by others.31,37,38
Complement-mediated increase in serum IL-6 was critical for enhancing Th17-cell differentiation
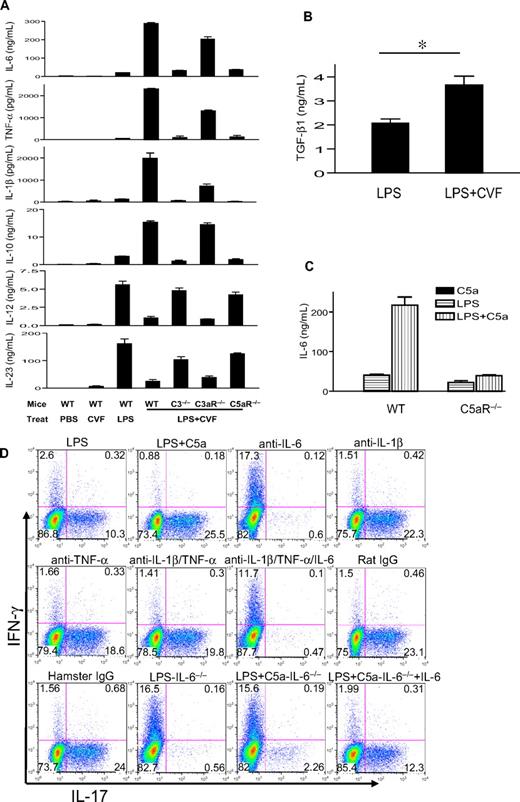
We measured serum levels of IL-6, TNF-α, IL-1β, IL-12, IL-23, and IL-10 in different groups of mice. Figure 2A shows that CVF treatment by itself did not induce an appreciable amount of any of the cytokines. Treatment with LPS induced moderate levels of serum IL-6, TNF-α, IL-β, and IL-10 and high levels of serum IL-12 and IL-23. Notably, LPS and CVF cotreatment dramatically enhanced serum IL-6, TNF-α, IL-1β, and IL-10 levels but markedly inhibited serum IL-23 and IL-12 levels (Figure 2A). The regulatory effect of CVF on LPS-induced serum cytokines was essentially abolished by C3 and C5aR deficiency but only moderately attenuated by C3aR deficiency (Figure 2A). We also measured the serum level of TGF-β1, a critical cytokine for Th17-cell differentiation,31,–33 and detected a similar synergistic increase by CVF cotreatment (Figure 2B). Finally, we established that the regulatory effect of CVF on LPS-dependent serum cytokine production could be mimicked by C5a (Figure 2C and data not shown).
IL-6 is critical for the increased Th17 cell–promoting activity in mouse serum after coincidental TLR4 and complement activation. (A) Serum levels of IL-6, TNF-α, IL-1β, IL-10, IL-12, and IL-23 in WT, C3−/−, C3aR−/−, and C5aR−/− mice, as measured by ELISA at 3 hours after intraperitoneal injection with PBS, CVF, LPS, or LPS + CVF. (B) Serum TGF-β levels in WT mice 3 hours after challenge with LPS or LPS + CVF. *P < .01, Student t test. (C) Serum IL-6 levels in WT or C5aR−/− mice 3 hours after challenge with LPS, recombinant mouse C5a, or LPS + C5a. n = 4 for each group of mice in panels A through C. Values shown are mean ± SEM. (D) Mouse CD4+ T cells were activated by plate-bound anti-CD3/CD28 in the presence of 5% serum from WT or IL-6−/− mice treated with LPS or LPS + C5a. Some sera from WT mice treated with LPS + C5a were depleted of 1 or more cytokines with neutralizing antibodies or isotype IgG controls, as indicated. Cells were cultured for 3 days after activation, and IFN-γ and IL-17–producing cells were detected by flow cytometry after intracellular staining. Plots are representative of 2 independent experiments.
IL-6 is critical for the increased Th17 cell–promoting activity in mouse serum after coincidental TLR4 and complement activation. (A) Serum levels of IL-6, TNF-α, IL-1β, IL-10, IL-12, and IL-23 in WT, C3−/−, C3aR−/−, and C5aR−/− mice, as measured by ELISA at 3 hours after intraperitoneal injection with PBS, CVF, LPS, or LPS + CVF. (B) Serum TGF-β levels in WT mice 3 hours after challenge with LPS or LPS + CVF. *P < .01, Student t test. (C) Serum IL-6 levels in WT or C5aR−/− mice 3 hours after challenge with LPS, recombinant mouse C5a, or LPS + C5a. n = 4 for each group of mice in panels A through C. Values shown are mean ± SEM. (D) Mouse CD4+ T cells were activated by plate-bound anti-CD3/CD28 in the presence of 5% serum from WT or IL-6−/− mice treated with LPS or LPS + C5a. Some sera from WT mice treated with LPS + C5a were depleted of 1 or more cytokines with neutralizing antibodies or isotype IgG controls, as indicated. Cells were cultured for 3 days after activation, and IFN-γ and IL-17–producing cells were detected by flow cytometry after intracellular staining. Plots are representative of 2 independent experiments.
To determine which serum factors produced by coincidental complement and TLR4 activation were responsible for promoting Th17-cell differentiation, we added neutralizing antibodies against IL-6, IL-1β, and TNF-α, either individually or in combination, to sera of LPS/C5a-treated mice. Figure 2D shows that neutralizing IL-6 but not IL-1β or TNF-α was effective at abolishing the Th17 cell–promoting activity of the mouse serum. To confirm the critical role of serum IL-6, we tested the sera of IL-6−/− mice after treatment with LPS or LPS/C5a. As shown in Figure 2D, unlike serum from LPS/C5a-treated WT mice, serum from LPS/C5a-treated IL-6−/− mice had minimal Th17 cell–promoting activity. However, supplementation of exogenous IL-6 to the latter serum substantially restored Th17-promoting activity (Figure 2D).
Coactivation of TLR4 and C5aR pathways on murine macrophages increased their Th17 cell–inducing activity
To assess whether coactivation of TLR4 and C5aR on antigen-presenting cells could influence their ability to prime and differentiate T cells, we studied murine peritoneal macrophages stimulated with LPS and C5a. Macrophages were stimulated with LPS, C5a, or LPS/C5a for 20 hours, and naive CD4+ T cells were then added and activated by anti-CD3 for 3 days. As shown in Figure 3A, treatment of macrophages with LPS led to considerable IL-6 production. Notably, although C5a treatment alone had no effect on IL-6 production, it significantly enhanced LPS-induced IL-6 production. When naive CD4+ T cells in the coculture were activated with anti-CD3 for 3 days, we found that macrophages that had been stimulated with LPS and C5a promoted a significantly greater number of Th17 cells than those stimulated with LPS or C5a alone (Figure 3B). The enhancing effect of C5a on Th17-cell development was blocked by a C5aR antagonist when it was added at the beginning of macrophage stimulation. However, the C5aR antagonist had no effect on Th17-cell differentiation if it was applied at the start of T-cell coculture and activation (Figure 3B), suggesting that C5a regulated TLR4 activation on macrophages rather than macrophage/T-cell interaction. We observed a similar synergistic effect of C5a on Th17-cell development when the CD4+ T cells were purified from C5aR−/− mice (Figure 3C), further supporting the conclusion that the observed effect of C5a did not originate from C5aR signaling on T cells.
TLR4/C5aR cosignaling on macrophages enhances their capacity to differentiate Th17 cells. (A) Thioglycollate-elicited peritoneal macrophages from WT mice were stimulated with C5a, LPS, LPS + C5a, or vehicle control (medium) for 20 hours, and IL-6 levels in the supernatant were measured by ELISA. (B) Macrophages were stimulated for 20 hours as in panel A, and naive CD4+ T cells from WT mice were then added to the culture and activated by anti-CD3 for 3 days in the presence of recombinant TGF-β1. Some wells were treated with a C5aR antagonist at the time of macrophage stimulation by LPS and C5a (Ant-0 hour) or at the time of T-cell addition and activation (Ant-20 hour). Th17-cell frequencies were determined by FACS after intracellular staining. (C) Macrophage and CD4+ T-cell cocultures were set up and analyzed as in panel B except that CD4+ T cells were from C5aR−/− mice. Data are representative of 2 independent experiments. Wells were set up in triplicates, and values shown are mean ± SEM. *P < .01 by Student t test.
TLR4/C5aR cosignaling on macrophages enhances their capacity to differentiate Th17 cells. (A) Thioglycollate-elicited peritoneal macrophages from WT mice were stimulated with C5a, LPS, LPS + C5a, or vehicle control (medium) for 20 hours, and IL-6 levels in the supernatant were measured by ELISA. (B) Macrophages were stimulated for 20 hours as in panel A, and naive CD4+ T cells from WT mice were then added to the culture and activated by anti-CD3 for 3 days in the presence of recombinant TGF-β1. Some wells were treated with a C5aR antagonist at the time of macrophage stimulation by LPS and C5a (Ant-0 hour) or at the time of T-cell addition and activation (Ant-20 hour). Th17-cell frequencies were determined by FACS after intracellular staining. (C) Macrophage and CD4+ T-cell cocultures were set up and analyzed as in panel B except that CD4+ T cells were from C5aR−/− mice. Data are representative of 2 independent experiments. Wells were set up in triplicates, and values shown are mean ± SEM. *P < .01 by Student t test.
Complement enhanced TLR4-dependent Th17-cell development from antigen-experienced CD4+ T cells
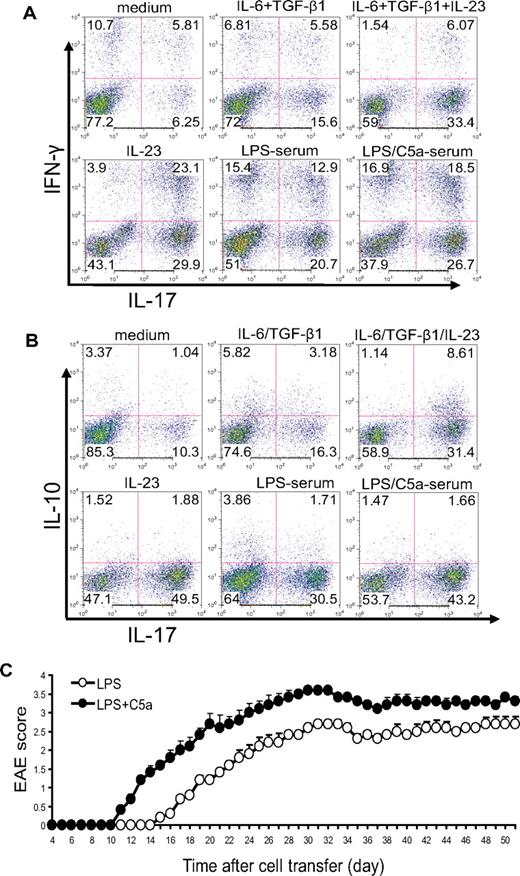
In the aforementioned experiments, we activated naive CD4+ T cells by anti-CD3/CD28 stimulation. To determine whether complement and TLR4 interaction could influence T-cell differentiation during antigen-dependent T-cell stimulation, we immunized WT mice with MOG38-50 in CFA and, after 10 days, stimulated lymph node cells in vitro with MOG38-50 in the presence of 5% mouse serum. We used IL-23 and IL-6/TGF-β treatments as controls in this experiment. Consistent with earlier findings,39 we observed that IL-23 was more effective than IL-6/TGF-β at differentiating Th17 cells (both IFN-γ positive and negative) from antigen-activated T cells (Figure 4A). Interestingly, although IL-23 level was significantly suppressed in the serum of mice with coincidental TLR4 and complement activation (Figure 2A), we found that serum from mice cotreated with LPS and C5a displayed significantly enhanced Th17 cell–promoting activity compared with serum from mice treated with LPS alone (Figure 4A).
Complement-TLR4 interaction promotes the development of pathogenic Th17 cells from antigen-experienced autoreactive CD4+ T cells. WT mice were immunized with MOG38-50 in CFA. After 10 days, cells from draining lymph nodes were isolated and restimulated with MOG38-50 peptide for 4 days in the presence of 5% serum from WT mice treated with LPS or LPS + C5a or in the presence of recombinant cytokines or vehicle control (medium). (A) Cells were analyzed by FACS for IFN-γ and IL-17 production after intracellular staining. (B) Cells were analyzed by FACS for IL-10 and IL-17 production after intracellular staining. (C) Twenty million CD4+ T cells propagated by in vitro restimulation in the presence of LPS- or LPS/C5a-treated mouse serum were adoptively transferred into naive mice (n = 6 for each group). Clinical EAE scores were determined daily. Data are representatives of 2 independent experiments.
Complement-TLR4 interaction promotes the development of pathogenic Th17 cells from antigen-experienced autoreactive CD4+ T cells. WT mice were immunized with MOG38-50 in CFA. After 10 days, cells from draining lymph nodes were isolated and restimulated with MOG38-50 peptide for 4 days in the presence of 5% serum from WT mice treated with LPS or LPS + C5a or in the presence of recombinant cytokines or vehicle control (medium). (A) Cells were analyzed by FACS for IFN-γ and IL-17 production after intracellular staining. (B) Cells were analyzed by FACS for IL-10 and IL-17 production after intracellular staining. (C) Twenty million CD4+ T cells propagated by in vitro restimulation in the presence of LPS- or LPS/C5a-treated mouse serum were adoptively transferred into naive mice (n = 6 for each group). Clinical EAE scores were determined daily. Data are representatives of 2 independent experiments.
Th17 cells promoted by TLR4 and complement synergy were pathogenic in vivo in an adoptive transfer model of EAE
Previous studies have shown that Th17 cells differentiated by IL-6/TGF-β produced IL-10, whereas those differentiated by IL-23 did not.39 This difference in IL-10 production was believed to be responsible for the difference observed in the pathogenic potential of the 2 types of Th17 cells.39 Given that IL-23 production was suppressed in the serum of mice cotreated with TLR4 and complement activators, we wondered whether the Th17 cells developed in the presence of such serum were more or less likely to produce IL-10 and whether they would be pathogenic in vivo. To address these issues, we restimulated total lymph node cells from mice immunized with MOG38-50 in the presence of IL-6/TGF-β, IL-23, or 5% mouse serum. As shown in Figure 4B, we confirmed that a greater percentage of Th17 cells differentiated by IL-6/TGF-β produced IL-10 compared with those differentiated by IL-23. On the other hand, we observed no difference in IL-10 production by Th17 cells differentiated in the presence of serum from mice treated with LPS/C5a or LPS alone (Figure 4B). Furthermore, when similar numbers of restimulated CD4+ T cells were transferred into naive mice, mice that received T cells restimulated in the presence of LPS/C5a mouse serum developed significantly more severe EAE disease compared with those of the LPS serum group (Figure 4C). The latter result suggested that Th17 cells promoted by TLR4 and complement synergy were functionally competent in causing autoimmune injury in vivo.
Physiologic relevance of complement/TLR4 interaction on Th17-cell development
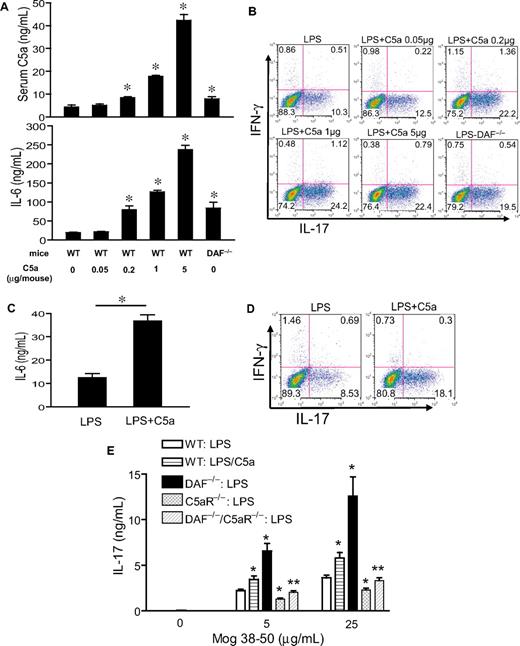
The experiments described previously used a potent systemic complement activator CVF and relatively high doses of C5a and LPS (5 μg per mouse and 20 mg/kg, respectively). To confirm our findings in more physiologically relevant settings, we first titrated the doses of C5a and LPS. Figure 5A shows a clear dose-dependent effect of C5a in stimulating LPS-dependent serum IL-6 production. Importantly, we found C5a to be fully effective in synergizing with LPS to induce serum Th17 cell–polarizing activity at 0.2 μg/mouse (Figure 5B), a dosage that produced a plasma level comparable with what has been detected in humans with severe bacterial infections (Figure 5A).40 Furthermore, significant synergy on serum IL-6 level and Th17 cell–promoting activity also was observed when mice were treated with this low dose of C5a and one tenth of the sublethal dosage of LPS (ie, 2 mg/kg instead of 20 mg/kg; Figure 5C-D). In separate experiments, we studied mice deficient in the membrane complement regulator DAF.41 Compared with WT mice, LPS treatment produced greater serum C5a and IL-6 levels in DAF−/− mice, and serum from these mice supported more Th17-cell development (Figure 5A-B). Thus, in the context of a complement regulator deficiency, LPS caused more endogenous C5a production, which synergized with TLR4 to enhance IL-6 level and Th17 cell–promoting activity.
Effect of TLR4 and complement synergy on Th17-cell development at low C5a and LPS dosages and in an antigen immunization model. (A) WT and DAF−/− mice were treated with LPS (20 mg/kg intraperitoneally) and the indicated dose of C5a (0-5 μg/mouse intraperitoneally). Serum C5a and IL-6 levels were measured at 1 hour and 3 hours, respectively. *P < .01, Student t test; all comparisons are with WT mice treated with LPS alone. (B) Th17 cell–promoting activity of mouse sera from panel A (collected at 3 hours after treatment). (C) Serum IL-6 levels in WT mice treated with 2 mg/kg LPS alone or in combination with 0.2 μg per mouse C5a. *P < .01, Student t test. (D) Th17-promoting activity of sera from mice in panel C. Experiments panels in B and D were performed with naive CD4+ T cells activated with plate-bound anti-CD3/CD28 and are representative of 2 independent experiments. Values in panels A and C are the mean ± SEM, n = 3 for each group. (E) ELISA of IL-17 levels in the cell culture medium of restimulated splenocytes from mice immunized with MOG38-50 in IFA containing LPS (100 μg per mouse) or LPS plus C5a (1 μg per mouse). Values are mean ± SEM, n = 4 for each group. *P < .01, Student t test, comparison with WT mice immunized with LPS alone; **P < .01, Student t test, comparison with DAF−/− mice.
Effect of TLR4 and complement synergy on Th17-cell development at low C5a and LPS dosages and in an antigen immunization model. (A) WT and DAF−/− mice were treated with LPS (20 mg/kg intraperitoneally) and the indicated dose of C5a (0-5 μg/mouse intraperitoneally). Serum C5a and IL-6 levels were measured at 1 hour and 3 hours, respectively. *P < .01, Student t test; all comparisons are with WT mice treated with LPS alone. (B) Th17 cell–promoting activity of mouse sera from panel A (collected at 3 hours after treatment). (C) Serum IL-6 levels in WT mice treated with 2 mg/kg LPS alone or in combination with 0.2 μg per mouse C5a. *P < .01, Student t test. (D) Th17-promoting activity of sera from mice in panel C. Experiments panels in B and D were performed with naive CD4+ T cells activated with plate-bound anti-CD3/CD28 and are representative of 2 independent experiments. Values in panels A and C are the mean ± SEM, n = 3 for each group. (E) ELISA of IL-17 levels in the cell culture medium of restimulated splenocytes from mice immunized with MOG38-50 in IFA containing LPS (100 μg per mouse) or LPS plus C5a (1 μg per mouse). Values are mean ± SEM, n = 4 for each group. *P < .01, Student t test, comparison with WT mice immunized with LPS alone; **P < .01, Student t test, comparison with DAF−/− mice.
Finally, we examined the effect of TLR4 and C5aR interaction on Th17-cell development in vivo in the setting of immunization. Groups of WT, DAF−/−, C5aR−/−, and DAF−/−/C5aR−/− mice were immunized with MOG38-50 emulsified in IFA with LPS or LPS/C5a. At 10 days, splenocytes were harvested and restimulated with MOG38-50 for 48 hours, and the level of IL-17 in the cell culture medium was determined by ELISA. Figure 5E shows that splenocytes from WT mice immunized with LPS/C5a generated significantly more IL-17 than cells from WT mice immunized with LPS alone. Furthermore, splenocytes from DAF−/− mice immunized with LPS generated markedly elevated levels of IL-17. Importantly, production of IL-17 was significantly impaired in splenocytes of mice with C5aR deficiency. The C5aR effect was especially striking when we compared IL-17 production by splenocytes from DAF−/− and DAF−/−/C5aR−/− mice (Figure 5E). These results collectively demonstrated that C5aR signaling synergized with TLR4 to promote the differentiation of Th17 cells in vivo.
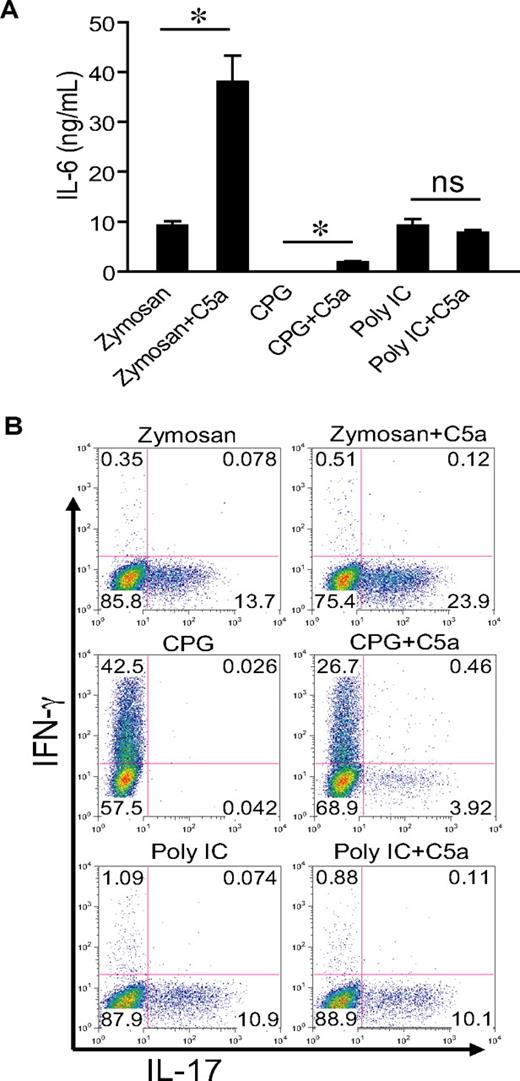
C5a enhanced TLR2- and TLR9-dependent serum IL-6 production and Th17 cell–promoting activity
To evaluate whether our findings could be extended to other TLRs, we cotreated WT mice with C5a (0.2 μg/mouse) and zymosan, CpG, and polyI:C, the respective ligand for TLR2, TLR9, and TLR3.42 Figure 6 shows that C5a markedly enhanced zymosan-induced serum IL-6 levels and Th17 cell–promoting activity. It had a much smaller but detectible effect on CpG-induced serum IL-6 level but did not influence serum IL-6 in mice cotreated with polyI:C (Figure 6A). A similar pattern of C5a effect was observed for the Th17 cell–promoting activity in the sera of these mice. Thus, complement could interact with multiple TLRs to promote Th17-cell development, and this activity correlated with IL-6 production.
C5a enhances TLR2- and TLR9-dependent serum IL-6 production and Th17 cell–promoting activity. (A) WT mice were treated intraperitoneally with zymosan (1 g/kg), CPG1826 (20 mg/kg), and polyI:C (15 mg/kg) alone or in combination with C5a (0.2 μg per mouse). Sera were collected after 3 hours (6 hours for polyI:C), and IL-6 levels were determined by ELISA. Values shown are the mean ± SEM, n = 3 for each group, *P < .01 by Student t test; ns indicates not statistically significant. (B) Naive mouse CD4+ T cells were activated by plate-bound anti-CD3/CD28 for 3 days in the presence of 5% serum from mice in panel A, and production of IFN-γ and IL-17 by the activated T cells was assessed by intracellular staining and flow cytometry. Plots are representative of 2 independent experiments.
C5a enhances TLR2- and TLR9-dependent serum IL-6 production and Th17 cell–promoting activity. (A) WT mice were treated intraperitoneally with zymosan (1 g/kg), CPG1826 (20 mg/kg), and polyI:C (15 mg/kg) alone or in combination with C5a (0.2 μg per mouse). Sera were collected after 3 hours (6 hours for polyI:C), and IL-6 levels were determined by ELISA. Values shown are the mean ± SEM, n = 3 for each group, *P < .01 by Student t test; ns indicates not statistically significant. (B) Naive mouse CD4+ T cells were activated by plate-bound anti-CD3/CD28 for 3 days in the presence of 5% serum from mice in panel A, and production of IFN-γ and IL-17 by the activated T cells was assessed by intracellular staining and flow cytometry. Plots are representative of 2 independent experiments.
Discussion
Complement is a major part of innate immunity that protects the host from pathogen infection. Activated complement eliminates pathogens by opsonization with activated C3 and C4, direct lysis with membrane attack complex, and elicitation and activation of innate immune cells through the generation of anaphylatoxins.3,4 Apart from these direct activities, complement also plays a facilitating role in the development of adaptive immunity. It is well known that complement functions as a natural adjuvant for B-cell response and antibody production, and recent studies15,,,,,–21 have provided evidence for the involvement of complement mediators in T-cell immunity.
Activation of naive T cells and their subsequent differentiation into specific types of effector T cells are dependent on TLR-mediated major histocompatibility complex and costimulatory molecule induction, as well as on cytokine production by antigen-presenting cells.1,2 The cytokine IL-12 is known to drive IFN-γ–producing Th1 cells, whereas IL-6 and TGF-β have been shown to promote Th17 cells.31,–33 We previously have demonstrated a strong regulation by complement of TLR-induced inflammatory cytokine production.35 In the present study, we have extended the earlier finding and shown that complement, through its regulation of cytokine production, could strongly augment TLR-mediated Th17-cell differentiation. We demonstrated this effect by using 2 different experimental systems. First, we showed that serum from mice with coincidental complement and TLR4 activation, but not complement activation alone, strongly promoted Th17-cell differentiation. A similar interaction also was observed between complement and TLR2 or TLR9, and the Th17 cell–promoting activity in the mouse sera was correlated with IL-6 levels. Second, we recapitulated the synergy between TLR4 and complement in Th17-cell induction by using cultured peritoneal macrophages. We further demonstrated that the complement effect is independent of the mode of T-cell activation: both anti-CD3–activated naive CD4+ T cells and restimulated antigen-experienced CD4+ T cells responded to the complement-dependent cytokine regulation.
Our current study corroborated and extended the earlier finding that complement regulated the production of a broad spectrum of cytokines. Coincidental complement activation in mice greatly augmented TLR4-induced IL-6, TNF-α, IL-1β, IL-10, and TGF-β production but markedly suppressed IL-12 and IL-23 production. We have shown that the complement effect was primarily mediated by the C5aR pathway. The precise mechanism(s) responsible for this regulation remains to be defined, but there is experimental evidence to suggest TLR4 and complement interactions at the step of MAPK kinase activation.35,43 Of the cytokine changes, we established, through the use of neutralizing antibodies and IL-6−/− mice, that the increase in IL-6 production was most critical for the observed effect on Th17-cell development. The observation of increased differentiation of pathogenic Th17 cells in the context of reduced IL-23 level was notable. Although it has been well established that IL-6 and TGF-β are required for driving Th17-cell differentiation from naive CD4+ T cells,31,–33 a previous study39 has found that autoreactive Th17 cells differentiated in the presence of IL-23, but not IL-6/TGF-β, were pathogenic when transferred into naive mice. This functional difference was attributed to the production of IL-10, considered as an anti-inflammatory cytokine, by the IL-6/TGF-β–differentiated but not IL-23–differentiated Th17 cells.39
We found no difference in IL-10 production by Th17 cells differentiated in the presence of LPS- or LPS/C5a-treated mouse serum, and autoreactive Th17 cells differentiated in the presence of LPS/C5a serum caused severe EAE when tested in vivo. Thus, the marked reduction in IL-23 level had no impact on the differentiation of pathogenic Th17 cells under our experimental setting. It is possible that there is a certain threshold requirement of IL-23 stimulation to inhibit IL-10 production in Th17 cells, and this threshold was still maintained in the LPS/C5a mouse serum despite the marked reduction in IL-23 level. Alternatively, potential effect of reduced IL-23 could have been compensated by increases in other inflammatory cytokines such as TNF-α and IL-1β.
A role for complement in modulating T-cell immunity has been suggested by several recent studies.17,–19,21,44 Some of these studies involved in vivo models in which C5a receptor signaling was shown to be required, but its precise mechanism of action remains to be defined.18,19 In other studies,44,45 isolated antigen-presenting cells and T-cell receptor transgenic T cells were used, and models implicating C3a receptor- and C5a receptor-mediated events within the immunologic synapse have been proposed. Under the current experimental setting, we found that the effect of complement on Th17-cell differentiation also was mediated by C5a receptor signaling. It was clear, however, that the effect of C5a was indirect, involved TLR4 signaling, and was mediated by IL-6. The addition of a C5a receptor antagonist at the beginning of macrophage stimulation with LPS/C5a, but not at the time of T-cell activation, blocked the Th17 cell–promoting effect of C5a (Figure 3B). Furthermore, similar results were obtained regardless of whether CD4+ T cells from WT or C5aR−/− mice were used in the assay. These results suggested that C5a receptor signaling exerted its regulatory activity on TLR4 activation and cytokine production by macrophages and not at the immunologic synapse between T cells and macrophages during T-cell activation.
Many pathogen-associated molecular patterns activate multiple innate immune systems. The synergistic interaction demonstrated here between TLRs and complement in cytokine and Th17-cell induction may typify a general relationship between complement and other innate immune sensors. Although the phenomenon was initially demonstrated with CVF and relatively high doses of C5a and LPS, we have recapitulated the interaction between C5a and TLR4 on IL-6 level and/or Th17 cell–promoting activity in several physiologically relevant settings. With regard to Th17-cell induction, the interaction and synergy between complement and TLRs is likely to benefit host defense. Th17 cells are known to play a role in controlling the infection of certain pathogens, including the bacteria Klebsiella pneumonia and Porphyromonas gingivalis, the yeast Candida albicans, and the parasite Toxoplasma gondii.46,,–49 On the other hand, given the potential detrimental role of Th17 cells in autoimmune diseases, it is also possible that complement causes autoimmune and inflammatory tissue injury through interaction with TLRs and promotion of Th17 cells, particularly in the context of pathogen infection. Indeed, the mechanism(s) of many infection-associated autoimmune disorders remain poorly defined, and it is conceivable that in the setting of microbial infection, cross talks and amplification between complement and TLRs could help to create a condition that is conducive to the priming and propagation of autoreactive Th17 cells through molecular mimicry. The occurrence of such consequential interactions between TLRs and complement, however, may be context specific, as illustrated by a previous study50 showing the lack of effect of C5a receptor deficiency in a common experimental model of murine EAE. Nevertheless, our demonstration of the potential of complement to interact with TLRs to enhance Th17-cell development identifies a novel mechanism by which complement causes autoimmunity, a conclusion that may bear relevance to the pathogenesis and treatment of certain autoimmune conditions in humans.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr John Lambris (University of Pennsylvania) for C5aR antagonist, Dr Craig Gerard (Children's Hospital of Boston) for the use of C5aR−/− mice, and Dr Rick Wetsel (University of Texas at Houston) for the use of C3aR−/− mice.
This work was supported by National Institutes of Health grants AI-49344, AI-44970, and AI-62388.
National Institutes of Health
Authorship
Contributions: C.F. performed research, analyzed data, and contributed to the writing of the paper; X.Z. and T.M. performed research and analyzed data; and W.-C.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Wen-Chao Song, Institute for Translational Medicine and Therapeutics and Department of Pharmacology, University of Pennsylvania School of Medicine, 1254 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: songwe@upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal