Abstract
Procoagulant platelets exhibit hallmark features of apoptotic cells, including membrane blebbing, microvesiculation, and phosphatidylserine (PS) exposure. Although platelets possess many well-known apoptotic regulators, their role in regulating the procoagulant function of platelets is unclear. To clarify this, we investigated the consequence of removing the essential mediators of apoptosis, Bak and Bax, or directly inducing apoptosis with the BH3 mimetic compound ABT-737. Treatment of platelets with ABT-737 triggered PS exposure and a marked increase in thrombin generation in vitro. This increase in procoagulant function was Bak/Bax- and caspase-dependent, but it was unaffected by inhibitors of platelet activation or by chelating extracellular calcium. In contrast, agonist-induced platelet procoagulant function was unchanged in Bak−/−Bax−/− or caspase inhibitor–treated platelets, but it was completely eliminated by extracellular calcium chelators or inhibitors of platelet activation. These studies show the existence of 2 distinct pathways regulating the procoagulant function of platelets.
Introduction
The hemostatic and prothrombotic function of platelets is critically dependent on 2 key properties: (1) their ability to adhere and aggregate at sites of vascular injury and (2) their ability to support blood coagulation, necessary for thrombin generation and fibrin formation (platelet procoagulant function, referring to the ability of platelets to support thrombin generation). The conversion of activated platelets to a procoagulant state is associated with specific biochemical and morphologic changes, some of which are similar to those occurring in apoptotic cells,1,2 including caspase activation, proteolytic processing of cytoskeletal elements, surface exposure of phosphatidylserine (PS), and membrane contraction, blebbing, and microvesiculation.
Platelets contain many well-known apoptotic regulators, including members of the Bcl-2 protein family, and downstream effectors such as caspases. It was recently shown that prosurvival Bcl-xL maintains platelet viability, primarily by restraining the proapoptotic function of Bak.3 Ex vivo treatment of platelets with the BH3 mimetic compound ABT-737, a potent inhibitor of Bcl-xL,4 triggers mitochondrial damage, caspase activation, and membrane externalization of PS.3,4 The relationship between platelet apoptosis and the activation-dependent pathways that drive platelet procoagulant activity is unclear. Several groups have suggested that Bcl-2 family proteins can be posttranscriptionally regulated in agonist-stimulated platelets.5-9 It has also been reported that Bcl-xL levels decline in stored platelets10,11 and that this coincides with an increase in PS exposure and increased platelet procoagulant activity.5,6,12,13 Taken together, these findings suggest that the apoptotic machinery may regulate the procoagulant function of platelets.
In this study, we have investigated whether direct induction of apoptosis stimulates platelet procoagulant activity, independent of platelet activation, and whether the apoptotic machinery in platelets plays an important role in regulating agonist-induced platelet procoagulant function.
Methods
The materials and methods used in this study are described in supplemental material (available on the Blood website; see the Supplemental Materials link at the top of the online article). All animal studies were approved by the animal ethics committee at the Alfred Medical Research and Education Precinct (Melbourne, Australia). All procedures involving collection of human blood were approved by the Monash Committee on Ethics in Research, and informed consent was obtained in accordance with the Declaration of Helsinki.
Results and discussion
Caspases are activated in apoptotic platelets independent of platelet activation
Membrane fragmentation (referring to blebbing and loss of membrane integrity, as observed through differential interference contrast [DIC] microscopy and scanning electron microscopy), microvesiculation, and the exposure of PS on the surface of agonist-stimulated platelets requires calcium influx and is associated with the proteolytic cleavage of a range of cellular substrates.7 Prominent among these are the cytoskeletal proteins, filamin-1 and gelsolin, that are cleaved by the calcium-activated thiol protease, calpain.8,9 Induction of apoptosis in washed human platelets with the BH3 mimetic compound ABT-73710 resulted in the time-dependent cleavage of both gelsolin and filamin that paralleled PS exposure (Supplemental Figure 1Ai,B) and platelet membrane blebbing (data not shown). However, in contrast to agonist-stimulated platelets (Supplemental Figure 1Aii filamin-1 and gelsolin) cleavage induced by ABT-737 was not inhibited by chelators of extracellular calcium (Figure 1A EGTA), calpain inhibitors (Supplemental Figure 1A, 737 + CP), or by global inhibitors of platelet activation (PGE1 and theophylline; Figure 1B), but it was completely blocked by the caspase inhibitor Q-VD-Oph (Supplemental Figure 1A, 737 + QVD).
ABT-737 induces caspase activation and promotes thrombin generation independent of platelet activation. Washed human platelets (3.0 × 108/mL) or C57BL/6 (C57) or PAR4-deficient mouse platelets (PAR4−/−; 0.5 × 108/mL) resuspended in Tyrode buffer in the absence (A,B) or presence (C-F) of BSA were incubated with vehicle (DMSO), CRP (10 μg/mL; 20 minutes), calcium ionophore A23187 (A23187; 1 μM; 20 minutes), or ABT-737 (737; 1 μM; 90-180 minutes). In some experiments, platelets were preincubated with PGE1 (2 μg/mL) and theophylline (20 mM; PGE1/Theo), before ABT-737 treatment. (A,B) Human platelets were lysed for Western blot analysis of procaspase-3, gelsolin, and filamin cleavage, as described in “Methods.” Immunoblots are representative of 3 independent experiments. (C-F) The ability of human (C-E) or PAR4−/− mouse platelets (F) to promote thrombin generation was assessed with the use of a Thrombinoscope (Thromboscope BV), as described in “Methods.” Line graphs (C,E) are taken from 1 representative of 3 independent experiments. Histograms (D,F) represent the means ± SEM (n = 3; nsP > .05; **P < .005).
ABT-737 induces caspase activation and promotes thrombin generation independent of platelet activation. Washed human platelets (3.0 × 108/mL) or C57BL/6 (C57) or PAR4-deficient mouse platelets (PAR4−/−; 0.5 × 108/mL) resuspended in Tyrode buffer in the absence (A,B) or presence (C-F) of BSA were incubated with vehicle (DMSO), CRP (10 μg/mL; 20 minutes), calcium ionophore A23187 (A23187; 1 μM; 20 minutes), or ABT-737 (737; 1 μM; 90-180 minutes). In some experiments, platelets were preincubated with PGE1 (2 μg/mL) and theophylline (20 mM; PGE1/Theo), before ABT-737 treatment. (A,B) Human platelets were lysed for Western blot analysis of procaspase-3, gelsolin, and filamin cleavage, as described in “Methods.” Immunoblots are representative of 3 independent experiments. (C-F) The ability of human (C-E) or PAR4−/− mouse platelets (F) to promote thrombin generation was assessed with the use of a Thrombinoscope (Thromboscope BV), as described in “Methods.” Line graphs (C,E) are taken from 1 representative of 3 independent experiments. Histograms (D,F) represent the means ± SEM (n = 3; nsP > .05; **P < .005).
Agonist-induced PS exposure and membrane fragmentation is associated with high sustained cytosolic calcium flux,2 collapse of the inner mitochondrial membrane potential (Δψm),11,14 and caspase activation.15 To examine the effect of ABT-737 on Δψm, platelets were loaded with tetramethylrhodamine-methyl ester (TMRM), a fluorescent dye retained in functional, intact mitochondria. The number of TMRM-positive platelets did not alter significantly after incubation of platelets with ABT-737 over a 3-hour time period (Supplemental Figure 1C). Dual labeling of ABT-737–treated platelets with TMRM and Alexa 488–labeled annexin V showed a high percentage of platelets staining positive for both fluorescent probes. In contrast, agonist stimulation of platelets resulted in an approximate 50% reduction in TMRM-positive cells, with a shift in the population from single-positive TMRM to single-positive annexin V staining, consistent with previous findings.11,14 Furthermore, inhibitors of the mitochondrial permeability transition pore (MPTP), cyclosporine and coenzyme Q, had minimal effect on ABT-737–induced PS exposure, while significantly reducing PS exposure induced by CRP/thrombin (Supplemental Figure 1D). These findings suggest that the mitochondrial changes in PS-positive apoptotic platelets are distinct from those occurring in agonist-stimulated procoagulant platelets.
Apoptotic platelets exhibit procoagulant activity
PS exposure on the surface of activated platelets is essential for the membrane assembly of coagulation factor complexes, including the tenase and prothrombinase complexes, necessary for thrombin generation.16 Whether PS exposure alone is sufficient to support blood coagulation, independent of other events linked to platelet activation, has not been defined. To investigate this, ABT-737–treated platelets were incubated with recalcified platelet-poor plasma, and thrombin generation was quantified in real time by monitoring cleavage of the fluorogenic thrombin substrate, Fluo-Substrate (Thromboscope BV). As shown in Figure 1 and Supplemental Figure 2, ABT-737–treated platelets supported robust thrombin generation, similar in rate and extent to that induced by calcium ionophore A23187 or CRP (Figure 1C-E; Supplemental Figure 2), in the absence of any apparent signs of platelet activation, as assessed by P-selectin expression, integrin αIIbβ3 activation (Supplemental Figure 3), and increases in cytosolic calcium (data not shown). This increase in procoagulant activity was independent of platelet costimulation by enzymatically generated thrombin, because PAR4−/− platelets, which are completely unresponsive to thrombin, were equally effective at supporting thrombin generation after ABT-737 treatment (Figure 1F). Furthermore, the potent platelet activation inhibitors, PGE1 and theophylline (PGE1/Theo) abolished CRP-induced thrombin generation but had no significant inhibitory effect on ABT-737–induced thrombin generation (Figure 1C-E). In contrast, Q-VD-Oph abolished ABT-737–induced thrombin generation but had no effect on CRP-stimulated thrombin generation (Figure 2C-D).
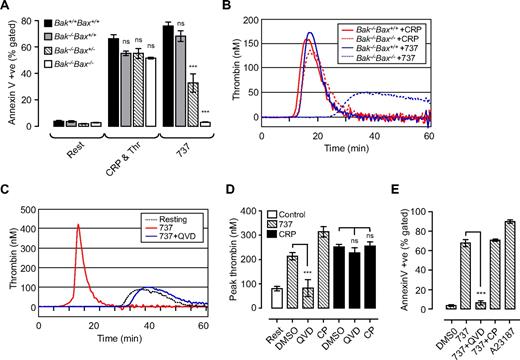
Two distinct pathways regulate phosphatidylserine exposure and platelet procoagulant activity. Washed Bak/Bax-deficient mouse platelets (A,B) or human platelets (C-E; 3.0 × 108/mL) were resuspended in Tyrode buffer in the presence of BSA (5 mg/mL), then incubated with vehicle (DMSO), ABT-737 (737, 1 μM; 90-180 minutes), calcium ionophore A23187 (A23187; 1 μM; 20 minutes), or CRP (10 μg/mL) and thrombin (1 U/mL; CRP & Thr; 20 minutes). In some experiments, platelets were preincubated with Q-VD-Oph (QVD; 50 μM) or calpeptin (CP; 100 μg/mL) before treatment with ABT-737/agonist. (A,E) The level of phosphatidylserine exposure (PS) in Bak/Bax-deficient mouse platelets (A) and human platelets (E), as measured by Alexa 488–conjugated annexin V binding, quantified by flow cytometry as described in “Methods.” Histograms depict the means ± SEM (n = 3). (B-D) The ability of platelets to promote thrombin generation in Bak/Bax-deficient mouse platelets (B) or human platelets (C,D) was assessed as described in Figure 1. Line graphs are taken from 1 representative of 3 independent experiments. The histogram (D) represents the means ± SEM (n = 3; nsP > .05; ***P < .001) of 3 independent experiments.
Two distinct pathways regulate phosphatidylserine exposure and platelet procoagulant activity. Washed Bak/Bax-deficient mouse platelets (A,B) or human platelets (C-E; 3.0 × 108/mL) were resuspended in Tyrode buffer in the presence of BSA (5 mg/mL), then incubated with vehicle (DMSO), ABT-737 (737, 1 μM; 90-180 minutes), calcium ionophore A23187 (A23187; 1 μM; 20 minutes), or CRP (10 μg/mL) and thrombin (1 U/mL; CRP & Thr; 20 minutes). In some experiments, platelets were preincubated with Q-VD-Oph (QVD; 50 μM) or calpeptin (CP; 100 μg/mL) before treatment with ABT-737/agonist. (A,E) The level of phosphatidylserine exposure (PS) in Bak/Bax-deficient mouse platelets (A) and human platelets (E), as measured by Alexa 488–conjugated annexin V binding, quantified by flow cytometry as described in “Methods.” Histograms depict the means ± SEM (n = 3). (B-D) The ability of platelets to promote thrombin generation in Bak/Bax-deficient mouse platelets (B) or human platelets (C,D) was assessed as described in Figure 1. Line graphs are taken from 1 representative of 3 independent experiments. The histogram (D) represents the means ± SEM (n = 3; nsP > .05; ***P < .001) of 3 independent experiments.
PS exposure by apoptotic platelets requires Bak and Bax
Because Bak and Bax are the central mediators of the intrinsic apoptotic pathway, we investigated their role in mediating platelet PS exposure and thrombin generation induced by ABT-737 or agonist. In mouse platelets, loss of Bak alone (Bak−/−Bax+/+) resulted in a 10% decrease in ABT-737–induced PS exposure, whereas the additional loss of one Bax allele (Bak−/−Bax+/−) inhibited ABT-737–induced PS exposure by approximately 60%. Bak−/−/Bax−/− platelets were completely resistant to the effects of ABT-737 (Figure 2A). In contrast, stimulation of Bak−/−Bax+/+, Bak−/−Bax+/−, or Bak−/−/Bax−/− platelets with either calcium ionophore A23187 (data not shown) or a combination of CRP and thrombin was associated with normal levels of PS expression and thrombin generation (Figure 2A-B). The caspase inhibitor Q-VD-Oph prevented ABT-737–induced PS exposure, consistent with a reduction in thrombin generation (Figure 2C-E), but had no effect on the procoagulant activity of platelets stimulated with calcium ionophore A23187 or physiologic agonists (data not shown; Figure 2D). In both agonist-stimulated and apoptotic platelets thrombin genration was dependent on surface exposure of PS, because it was completely inhibited by the addition of purified annexin V (Supplemental Figure 2C).
Conclusions
Our findings show that 2 distinct pathways can regulate PS exposure and the procoagulant activity of platelets in vitro: (1) a calcium-dependent, caspase-independent pathway induced by physiologic agonists and (2) a Bak/Bax-caspase–mediated pathway independent of platelet activation. Moreover, we have shown that, although agonist-induced procoagulant (PS+) platelets exhibit characteristics of apoptotic cells, the processes regulating their formation are distinct from those regulating platelet apoptosis. Whether PS exposure on the surface of apoptotic platelets has any relevance to thrombin generation in vivo, or whether it represents a signal for the clearance of platelets at steady state, remains to be established. Notably, in preliminary studies we have shown that ABT-737–treated platelets have reduced adhesive function and are rapidly cleared from the circulation after infusion into healthy mice, features that may limit any prothrombotic potential in vivo. Nonetheless, our demonstration that platelet procoagulant function can occur independently of platelet activation is of particular interest as BH3 mimetic compounds enter clinical trials for the treatment of malignant diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Sturgeon, J. Corbin, J. Blyth, A. Carmagnac, K. Lowes, and the WEHI Bioservices staff for outstanding technical assistance.
This work was supported by the National Heart Foundation of Australia (Melbourne, Australia; project grant G 06M 2617 to S.M.S.), the National Health and Medical Research Council of Australia (Canberra, Australia; project grant 516725; program grants 461221, 461219, IRIISS 361646; and fellowships to S.P.J., B.T.K., L.A.O., A.W.R., and D.C.S.H.), the Australian Research Council (Canberra, Australia; QEII fellowship to B.T.K.), the Victorian Cancer Agency (Carlton, Australia; fellowship to K.D.M.), a Victorian State Government OIS grant, the Cancer Council of Victoria (Carlton, Australia), the Sylvia and Charles Viertel Charitable Foundation (Melbourne, Australia; fellowship to B.T.K.), the Swedish Research Council (Stockholm, Sweden; fellowship to E.C.J.), and the Leukemia & Lymphoma Society (White Plains, NY; fellowship to E.C.J.; SCOR 7015-02).
Authorship
Contribution: S.M.S. designed and performed the research, analyzed data, and wrote the paper; Y. Yuan, E.C.J., and M.J.W. performed research and analyzed data; Y. Yao, K.D.M., L.A.O., K.J.H., A.O., S.H., and A.W. performed research; A.W.R. and H.H.S. designed research and analyzed data; D.C.S.H. designed research, analyzed data, and contributed vital new reagents; and B.T.K. and S.P.J. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaun P. Jackson, Australian Centre for Blood Diseases, Monash University, 6th Level Burnet Bldg, 89 Commercial Rd, Melbourne, Victoria 3004, Australia, e-mail: shaun.jackson@med.monash.edu.au; or Benjamin T. Kile, Division of Molecular Medicine, The Walter or Eliza Hall Institute of Medical Research, 1 G Royal Parade, Parkville, Victoria 3052, Australia; e-mail: kile@wehi.edu.au.
References
Author notes
*S.M.S. and Y. Yuan are equal first authors.
†B.T.K. and S.P.J. are equal senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal