Abstract
Abstract 1085
Poster Board I-107
Benign types of bone marrow failure such as aplastic anemia (AA) and refractory anemia (RA) of MDS defined by the FAB classification occasionally present various chromosomal abnormalities, although the clinical significance of each abnormality is unclear. Small populations of CD55-CD59- granulocytes and erythrocytes are detected in approximately 50% of patients with AA and 15% of patients with RA (Br J Haematol 2009). Analyzing the relationship between the presence of a certain chromosomal abnormality and PNH-type cells may be useful in deducing the clinical significance of the karyotype abnormalities because such PNH-type cells represent a good marker for the benign bone marrow failure with immune pathophysiology.
From 1995 through 2009, peripheral blood from 2487 patients with AA or RA was examined for the presence of PNH-type cells using high sensitivity flow cytometry. Chromosome data on bone marrow cells were available in 1513 patients. The proportion of patients possessing PNH-type cells was determined in a subset of AA or RA defined by various chromosomal abnormalities. The sorted PNH-type granulocytes from some patients with chromosomal abnormalities were subjected to FISH analysis to determine the origin of the cells.
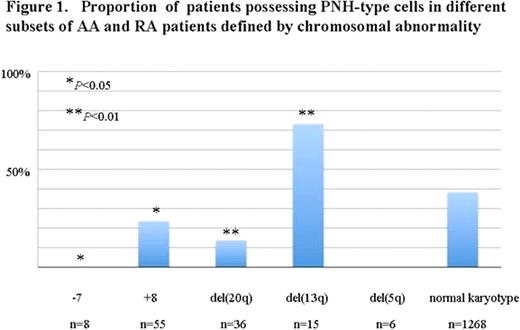
Chromosomal abnormalities were detected in 16% of the patient population, 11% (59/546) in patients possessing PNH-type cells (PNH+ patients) and 19% (186/967) in patients without increased PNH-type cells (PNH- patients). The most frequent chromosomal abnormalities were trisomy 8 (+8) (3.6%), deletion of 20q (del(20q)) (2.4%), deletion of 13q (del(13q)) (1.0%), monosomy 7 (-7) (0.5%), deletion of 5q (del(5q)) (0.3%) in order of descending prevalence. The proportion of PNH+ patients in each patient group defined by chromosomal abnormality was 24%, 14%, 73%, 0% and 0% respectively. The proportion of PNH+ patients in patients with normal karyotype was 35%. Notably, patients with del(13q) showed a remarkably high prevalence of PNH-type cells (73%), even significantly higher than the prevalence of the patients without chromosomal abnormalities (P<0.01). The high prevalence of PNH-type cells was compatible with the previous finding that patients with del(13q) were likely to respond to immunosuppressive therapy (Br J Haematol, 2002). On the other hand, the proportion of PNH+ in patients with +8 and del(20q) or -7 was significantly lower than that of patients without chromosomal abnormalities (P<0.05, P<0.01 and P<0.05). In particular, none of the patients with -7 and del(5q) showed PNH-type cells, indicating the pathophysiology of the bone marrow failure with these chromosomal abnormalities to be non-immune mediated. FISH analysis of sorted PNH-type granulocytes showed the normal karyotype, indicating the PNH-type cells were derived from normal karyotype cells.
The presence of del(13q) in AA or RA represents benign bone marrow failure associated with a good response to immunosuppressive therapy, while the presence of -7 and del(5q) is thus considered to be related to a non-immune pathophysiology. AA and RA with +8 or del(20q) comprise a small subset of immune-mediated bone marrow failure.
Proportion of patients possessing PNH-type cells in different subsets of AA and RA patients defined by chromosomal abnormality
Proportion of patients possessing PNH-type cells in different subsets of AA and RA patients defined by chromosomal abnormality
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal