Abstract
Despite the success of passive immunotherapy with monoclonal antibodies (mAbs), many lymphoma patients eventually relapse. Induction of an adaptive immune response may elicit active and long-lasting antitumor immunity, thereby preventing or delaying recurrence. Immunomodulating mAbs directed against immune cell targets can be used to enhance the immune response to achieve efficient antitumor immunity. Anti-CD137 agonistic mAb has demonstrated antitumor efficacy in various tumor models and has now entered clinical trials for the treatment of solid tumors. Here, we investigate the therapeutic potential of anti-CD137 mAb in lymphoma. We found that human primary lymphoma tumors are infiltrated with CD137+ T cells. We therefore hypothesized that lymphoma would be susceptible to treatment with anti-CD137 agonistic mAb. Using a mouse model, we demonstrate that anti-CD137 therapy has potent antilymphoma activity in vivo. The antitumor effect of anti-CD137 therapy was mediated by both natural killer (NK) and CD8 T cells and induced long-lasting immunity. Moreover, the antitumor activity of anti-CD137 mAb could be further enhanced by depletion of regulatory T cell (Tregs). These results support the evaluation of anti-CD137 therapy in clinical trials for patients with lymphoma.
Introduction
Lymphoma is responsive to immunotherapy.1 Despite the success of passive immunotherapy with monoclonal antibodies (mAbs) directed against tumor cells (eg, anti-CD20, rituximab), many lymphoma patients eventually relapse. Active immunotherapy for the treatment of lymphoma aims to induce an adaptive and long-lasting antitumor immune response to prevent or prolong time to recurrence. Although antitumor immune cells can be found in cancer patients, these cells may be rendered ineffective in eradicating cancer due to tumor-induced immunosuppression.2 Monoclonal antibodies that target and modulate the function of tumor-reactive immune cells may enhance antitumor immune responses to therapeutic levels.3 Targeting the immune environment of the tumor as opposed to the malignant cells presents unique advantages. Whereas targeting tumor cells with mAbs requires a tumor-specific antigen (Ag), stimulating or inhibiting nonmalignant immune cells is expected to be applicable across different patients and cancer types. Further, unlike tumor cells that may mutate into therapy-resistant clones under selective pressure of mAb treatment, normal immune cells are not expected to be clonally selected for such mutations. Finally, whereas tumor-directed mAbs are considered passive immunotherapy and therefore offer only transient efficacy, targeting the immune system with mAbs aims to induce and/or potentiate an active and long-lasting immune response against cancer.
Antibody-mediated T-cell modulation can be accomplished in several ways, including (1) enhancing costimulation on conventional T cells (Tconvs; eg, agonistic anti-CD137 and anti-OX40 mAbs); (2) blocking negative signals on Tconvs (eg, antagonistic anti-CTLA4 mAb); or (3) abrogating regulatory T cell (Treg)–mediated suppression (eg, agonistic anti-GITR mAb).3,4 Some of these mAbs are thought to have multiple effects. For example, anti-OX40 and anti-GITR mAbs trigger costimulatory molecules on Tconvs as well as block the suppressive function of Tregs.5-9
CD137 (4-1BB) is a surface glycoprotein that belongs to the tumor-necrosis factor receptor superfamily (TNFRSF). CD137 is broadly inducible on immune cells including activated CD4 and CD8 T cells, Tregs, natural killer (NK) cells, NK-T cells, monocytes, neutrophils, and dendritic cells.10 On T cells specifically, CD137 functions as a costimulatory receptor induced upon T-cell receptor (TCR) activation. Binding of CD137 to its ligand leads to increased T-cell proliferation, cytokine production, functional maturation, and prolonged CD8 T-cell survival.10 The effect of CD137 ligation on Tregs is not as clearly understood, with conflicting reports showing both stimulation and inhibition of the immunosuppressive functions of these cells.10-14 Consistent with the costimulatory function of CD137 on Tconvs, agonistic mAbs against this receptor have been shown to provoke powerful tumor-specific T-cell responses capable of eradicating tumor cells in a variety of murine tumor models including sarcoma, mastocytoma, glioma, colon carcinoma, and myeloma.3,15
Anti-CD137 mAb has now entered clinical trials for solid tumors (melanoma, renal cell carcinoma, lung cancer, and ovarian cancer) but little is known about its potential therapeutic effect in lymphoma. In this study, we investigated the potential relevance of anti-CD137 therapy in lymphoma. We found that bulk tumor specimens from lymphoma patients overexpressed CD137 mRNA compared with other tumor types. Single-cell analysis performed on primary lymphoma samples of various histologies demonstrated that CD137 was not expressed on the tumor cells but by tumor-infiltrating T cells. This suggested that the target of anti-CD137 mAb was present and selectively expressed on cells with potential antitumor activity in lymphoma patients. Using a mouse model, we next examined the antitumor effect of anti-CD137 agonistic mAb in vivo and investigated the contribution of various immune cell types to the therapy, including regulatory T cells. Our findings support the evaluation of anti-CD137 agonistic mAb in clinical trials for patients with lymphoma and suggest approaches to optimize this therapy.
Methods
Analysis of microarray gene expression data for CD137 mRNA expression
CD137 mRNA (TNFRSF9) expression data were analyzed across diverse histopathological tumor groups in 2 independent microarray datasets (Ramaswamy et al16 ; expO intgen, https://expo.intgen.org/geo17 ) obtained from Oncomine.18 Using a 2-tailed Student t test, log2 relative gene expression measurements were analyzed for tumor-specific patterns of expression in the primary dataset (n = 184, U03397_s_at, Ramaswamy et al16 ). After identification of biased overexpression of TNFRSF9 in follicular lymphoma (FL, n = 11) and diffuse large B-cell lymphoma (DLBCL, n = 11), overexpression of TNFRSF9 in a second cohort of lymphomas (n = 21) was independently validated in a separate microarray dataset (207536_s_at; National Center for Biotechnology Information [NCBI] Gene Expression Omnibus [GEO] GSE210919 ).
CD137 expression on human primary lymphoma specimens
Tumor biopsies were obtained from patients with FL or mantle cell lymphoma (MCL) at Stanford Hospital or from patients with de novo DLBCL or MCL at the Norwegian Radium Hospital. Biopsies were obtained with informed consent in accordance with the Declaration of Helsinki, and this study was approved by Stanford University's Administrative Panels on Human Subjects in Medical Research and the regional Committee for Medical Research Ethics, Region Eastern Norway, University of Oslo. Tumor specimens were obtained before patients had received any treatment and single-cell suspensions were prepared and frozen as previously described.20,21 Peripheral blood mononuclear cells (PBMCs) from healthy individuals were isolated using density gradient separation Ficoll-Paque Plus (Amersham Biosciences) and subsequently analyzed as described for FL specimens. For immunophenotyping, an individual cryotube was thawed, washed, and resuspended in RPMI + 10% fetal bovine serum. Thawed cells were allowed to rest at 37°C for 15 minutes in a 5% CO2 tissue culture incubator, before staining with mAbs and fluorescence-activated cell sorting (FACS) analysis.
Cell lines and mice
The A20 cell line, a BALB/c B-cell lymphoma expressing MHC class I and class II H-2d molecules, was obtained from the ATCC. Tumor cells were cultured in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% heat-inactivated FCS (HyClone Laboratories), 100 U/mL penicillin, 100 μg/mL streptomycin (both from Invitrogen Life Technologies), and 50 μM 2-ME (Sigma-Aldrich). Cells were grown in suspension culture at 37°C in 5% CO2.
Eight- to 10-week-old female BALB/c mice were purchased from Charles River Laboratories and were housed at the Laboratory Animal Facility at the Stanford University Medical Center. All experiments were conducted in accordance with Stanford University Animal Facility and National Institutes of Health guidelines.
Tumor transplantation and immunotherapy
A20 lymphoma cells were implanted into 8- to 10-week-old female BALB/c mice at a dose of 5 × 106 cells in 50 μL RPMI by the subcutaneous route into the abdomen. In vivo systemic administration of monoclonal antibodies against T-cell targets was performed by intraperitoneal injections. Anti-CD137 (4-1BB) mAb (rat IgG2a, clone 2A22 ), anti-OX40 (CD134) mAb (rat IgG1, clone OX86; European Collection of Cell Cultures), anti-CTLA4 (CD152) mAb (hamster IgG, clone 9H10; kind gift from Dr J. Allison, Howard Hughes Medical Institute, Memorial Sloan-Kettering Cancer Center, NY), anti-GITR mAb (rat IgG2b, clone DTA-1; kind gift from Dr S. Sakaguchi, Institute for Frontier Medical Sciences, Kyoto University, Kyoto, Japan), and anti-FR4 mAb (rat IgG2a, clone TH6; kind gift from Dr S. Sakaguchi) were produced from ascites in severe combined immunodeficient mice. None of the targeted molecules (CD137, OX40, CTLA4, GITR, FR4) was expressed on the surface of A20 tumor cells (data not shown). IgG from rat serum was used as control antibody for anti-CD137 mAb and obtained from Sigma-Aldrich. Antibodies used for T-cell modulation in Figure 2 were given intraperitoneally on days 5 and 10 after tumor inoculation at what is generally considered to be the optimal dose according to prior publications22-25 : 150 μg per injection for anti-CD137, 400 μg per injection for anti-OX40, 100 μg per injection for anti-CTLA4, and 500 μg per injection for anti-GITR. In all other experiments, anti-CD137 mAb was given as a single intraperitoneal injection on day 6 at a dose of 150 μg per mouse unless otherwise specified. The growth of tumor was monitored by a caliper twice a week, and expressed as length by width in square centimeters. Mice were killed when tumor size reached 4 cm2 or when tumor sites ulcerated.
Detection of tumor-reactive T cells
BALB/c mice that have been cured with anti-CD137 therapy for more than 100 days were rechallenged subcutaneously with A20 tumors. A week later, splenocytes from αCD137-cured mice or control splenocytes from naive mice were harvested and made into single-cell suspensions, and the red blood cells were lysed. A total of 5 × 105 splenocytes were cocultured with 5 × 105 irradiated A20 cells for 24 hours at 37°C and 5% CO2. Monensin was added during the last 8 hours of culture. Afterward, cells were washed and stained with anti–mouse mAbs as indicated.
Depletion of NK cells, CD4 T cells, CD8 T cells, and Tregs
Ascitic fluid was harvested from severe combined immunodeficient mice bearing hybridoma GK1.5, 2.43, and TH6, producing anti-CD4 (rat IgG2b), anti-CD8 (rat IgG2b), and anti-FR4 (rat IgG2a, clone TH6; kind gift from Dr S. Sakaguchi) mAbs, respectively. The ascites were diluted in sterile PBS. Anti–asialo GM1 antiserum was used to deplete NK cell activity in vivo and was purchased from Wako. Depleting antibodies were injected intraperitoneally on day −1, day 0 of tumor inoculation, and every 5 days (or every 4 days for anti-FR4 mAb) thereafter for 3 weeks at a dose of 50 μL per injection for anti–asialo GM1, 500 μg per injection for anti-CD4 and anti-CD8, and 100 μg per injection for anti-FR4. The depletion conditions were validated by flow cytometry of peripheral blood showing more than 95% depletion of CD4 and CD8 T cells. Treg depletion after anti-FR4 treatment was confirmed by flow cytometry of peripheral blood showing approximately 80% depletion, similar to a previous publication.26
Antibodies and FACS analysis
The following mAbs to human antigens were used for staining of human primary lymphoma specimens: CD4 Pacific Blue, CD8 FITC, CD20 APC-Cy7, CD25 PE, and CD45RO PE-Cy7 (all from Becton Dickinson Biosciences [BD]), CD3 QD605 (from Invitrogen), CD137 APC (clone 4B4-1; from Biosource), and FoxP3 (clone PCH101; from eBioscience). The following mAbs to mouse antigens were used: anti-CD8 FITC, anti-CD4 PerCP, and anti-CD44 APC mAb (BD). Intracellular IFN-γ expression was assessed using a BD Cytofix/Cytoperm kit per the instructions. To detect FoxP3, single-cell suspensions from spleens or tumors were stained with CD25, CD3, CD4, and CD8 (all from BD) in addition to CD137 (eBioscience). Cells were washed and then fixed, permeabilized, and stained with anti-FoxP3 (eBioscience, clone FJK-16s), using the eBioscience protocol. Stained cells were collected on a FACSCalibur or a LSRII 3-laser cytometer (BD) and data were analyzed using Cytobank (http://www.cytobank.org).
Statistical analysis
Prism software (GraphPad Software) was used to analyze tumor growth and to determine statistical significance of difference between groups by applying an unpaired Student t test. Kaplan-Meier plots were used to analyze survival. Comparison of survival curves was made by the log-rank test. P values less than .05 were considered significant. For tumor burdens, comparison of means was done by analysis of variance.
Results
Tumor-involved lymph nodes from lymphoma patients express high levels of CD137 mRNA and are infiltrated by CD137+ T cells
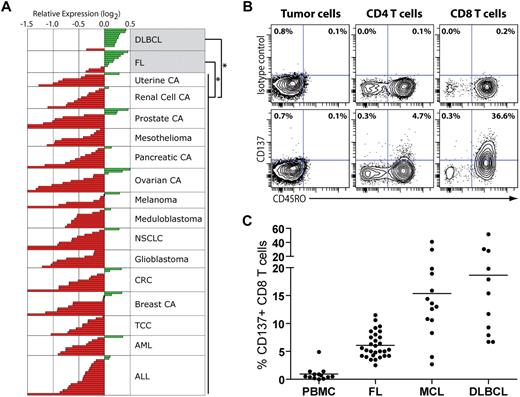
To investigate whether anti-CD137 mAb would be a clinically translatable therapy for patients with lymphoma, we first examined expression of CD137 mRNA (TNFRSF9) in bulk tumor specimens from lymphoma patients and compared it with a variety of other tumors. Using publicly available microarray gene expression data,16,18 we found that bulk tumor specimens from patients with follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) significantly overexpressed TNFRSF9 compared with nonlymphoma tumor specimens (Figure 1A, P < .001 for each comparison). Aside from FL and DLBCL, no other histopathological group significantly overexpressed TNFRSF9 compared with other tumor types. The overexpression of TNFRSF9 in non-Hodgkin lymphomas (n = 21) compared with a diverse group of tumors from 1898 patients was validated in an independent dataset27 (P < .001).
Tumor-involved lymph nodes from lymphoma patients express high levels of CD137 mRNA and are infiltrated by CD137+ T cells. (A) Publicly available microarray gene expression data16,18 from bulk tumor specimens of 184 patients were analyzed for the expression of CD137 across histopathological groups. DLBCL and FL specimens significantly overexpressed CD137 compared with nonlymphoma specimens (P < .001 for each comparison). Aside from FL (n = 11) and DLBCL (n = 11), no other histopathological group significantly overexpressed CD137 compared with other tumor types. CA indicates carcinoma; NSCLC, non–small cell lung adenocarcinoma; CRC, colorectal adenocarcinoma; TCC, transitional cell carcinoma; AML, acute myeloid leukemia; and ALL, acute lymphoblastic leukemia. (B-C) Tumor-involved lymph nodes from untreated lymphoma patients (FL, MCL, and DLBCL) and PBMCs from healthy donors were analyzed by flow cytometry for CD137 expression on B and T cells. (B) CD137 expression on tumor B cells, and CD4 and CD8 T cells for 1 representative lymphoma sample (DLBCL). (C) The percentage of CD137+ cells among CD8 T cells in healthy PBMCs and lymphoma samples from different histologies.
Tumor-involved lymph nodes from lymphoma patients express high levels of CD137 mRNA and are infiltrated by CD137+ T cells. (A) Publicly available microarray gene expression data16,18 from bulk tumor specimens of 184 patients were analyzed for the expression of CD137 across histopathological groups. DLBCL and FL specimens significantly overexpressed CD137 compared with nonlymphoma specimens (P < .001 for each comparison). Aside from FL (n = 11) and DLBCL (n = 11), no other histopathological group significantly overexpressed CD137 compared with other tumor types. CA indicates carcinoma; NSCLC, non–small cell lung adenocarcinoma; CRC, colorectal adenocarcinoma; TCC, transitional cell carcinoma; AML, acute myeloid leukemia; and ALL, acute lymphoblastic leukemia. (B-C) Tumor-involved lymph nodes from untreated lymphoma patients (FL, MCL, and DLBCL) and PBMCs from healthy donors were analyzed by flow cytometry for CD137 expression on B and T cells. (B) CD137 expression on tumor B cells, and CD4 and CD8 T cells for 1 representative lymphoma sample (DLBCL). (C) The percentage of CD137+ cells among CD8 T cells in healthy PBMCs and lymphoma samples from different histologies.
Lymphoma tumors contain significant numbers of tumor-infiltrating nonmalignant T cells.20 To identify which cells within the tumor specimens expressed CD137, we used flow cytometry to examine lymph node biopsies from untreated lymphoma patients. We did not observe any significant expression of CD137 on the tumor cells in any non-Hodgkin lymphoma (NHL) samples tested (Figure 1B and data not shown): FL (n = 35), mantle cell lymphoma (MCL, n = 29), and DLBCL (n = 18). In contrast, we found a significant percentage of CD137+ cells among tumor-infiltrating T cells in all lymphoma subtypes tested (Figure 1B-C and supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This percentage was significantly higher compared with T cells from peripheral blood of healthy individuals (P < .001 for all NHLs). This difference remained significant when CD4 and CD8 T-cell subsets were analyzed separately (P < .001 for both). However, the percentage of CD137+ tumor-infiltrating T cells was generally higher in the CD8 compartment than in the CD4 compartment (Figure 1B-C and supplemental Figure 1B). In the CD8 compartment, the mean percentage of CD137+ cells in healthy PBMCs, FL, MCL, and DLBCL was 0.9%, 6%, 15.4%, and 18.7%, respectively (Figure 1C). CD137+ tumor-infiltrating T cells were almost exclusively confined to the CD45RO+ memory compartment (Figure 1B). These results show that lymphoma tumor specimens overexpress CD137 compared with other tumor types, presumably not due to the tumor cells themselves but to the presence of CD137+ tumor-infiltrating T cells. Our observation suggests that lymphoma patients may therefore be sensitive to anti-CD137 therapy.
Anti-CD137 agonistic mAb has potent antilymphoma activity in vivo
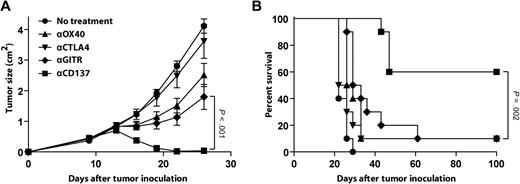
Based on our observations in human primary lymphoma samples, we next assessed the therapeutic potential of anti-CD137 agonistic mAb in vivo using the A20 mouse model of lymphoma. In this model, we have previously reported that anti-OX40, anti-CTLA4, and anti-GITR mAbs exhibit antitumor activity when administered in combination with a CpG-based vaccine.28 We compared these mAbs to anti-CD137 mAb as monotherapy for the treatment of established A20 tumors. A20 lymphoma cells (5 × 106 per mouse) were inoculated subcutaneously in BALB/c mice on day 0, and the corresponding mAb was injected intraperitoneally on days 5 and 10. At the time of treatment, the tumor was well established, with the largest diameter measuring approximately 5 mm. At 100 days after tumor inoculation, 60% of the mice treated with anti-CD137 mAb had no evidence of tumor, whereas only 10% of animals treated with anti-OX40, anti-CTLA4, or anti-GITR mAbs remained tumor free (Figure 2A-B). Antitumor effects were not due to direct targeting of the tumor, as the A20 tumor cells did not express CD137, OX40, CTLA4, or GITR (data not shown).
Anti-CD137 agonistic mAb has potent antilymphoma activity in vivo. BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Mice received either no treatment or 2 intraperitoneal injections of mAb anti-OX40 mAb, anti-CTLA4 mAb, anti-GITR mAb, or anti-CD137 mAb at days 5 and 10 after tumor inoculation as described in “Tumor transplantation and immunotherapy.” Mice (10 per group) were then monitored for tumor growth (A, mean ± SEM) and overall survival (B).
Anti-CD137 agonistic mAb has potent antilymphoma activity in vivo. BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Mice received either no treatment or 2 intraperitoneal injections of mAb anti-OX40 mAb, anti-CTLA4 mAb, anti-GITR mAb, or anti-CD137 mAb at days 5 and 10 after tumor inoculation as described in “Tumor transplantation and immunotherapy.” Mice (10 per group) were then monitored for tumor growth (A, mean ± SEM) and overall survival (B).
Anti-CD137 mAb efficacy is time dependent
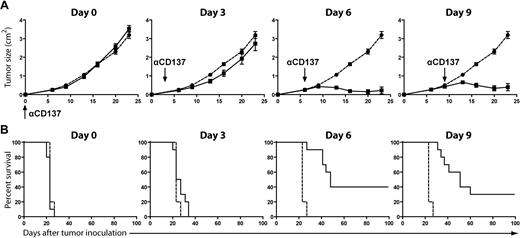
To determine the optimal schedule for administering anti-CD137 mAb, BALB/c mice were inoculated subcutaneously with A20 lymphoma cells and subsequently treated with a single injection of anti-CD137 mAb at various time points after tumor challenge. Surprisingly, treatment at early time points (days 0 and 3) resulted in no tumor rejection, whereas treatment at later time points (days 6 and 9) resulted in significant tumor regression and increased overall survival, curing 30% to 40% of the mice for greater than 100 days (Figure 3). Treatment at day 12 or later failed to cure the mice (data not shown), potentially due to excessive tumor burden.
Anti-CD137 mAb efficacy is time dependent. BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Mice received either no treatment (● and  ) or 1 intraperitoneal injection of anti-CD137 mAb (■ and —) at various time points after tumor inoculation: day 0, day 3, day 6, or day 9. Mice (10 per group) were monitored for tumor growth (A, mean ± SEM) and overall survival (B).
) or 1 intraperitoneal injection of anti-CD137 mAb (■ and —) at various time points after tumor inoculation: day 0, day 3, day 6, or day 9. Mice (10 per group) were monitored for tumor growth (A, mean ± SEM) and overall survival (B).
Anti-CD137 mAb efficacy is time dependent. BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Mice received either no treatment (● and  ) or 1 intraperitoneal injection of anti-CD137 mAb (■ and —) at various time points after tumor inoculation: day 0, day 3, day 6, or day 9. Mice (10 per group) were monitored for tumor growth (A, mean ± SEM) and overall survival (B).
) or 1 intraperitoneal injection of anti-CD137 mAb (■ and —) at various time points after tumor inoculation: day 0, day 3, day 6, or day 9. Mice (10 per group) were monitored for tumor growth (A, mean ± SEM) and overall survival (B).
Mice cured with anti-CD137 mAb develop long-lasting antitumor immunity
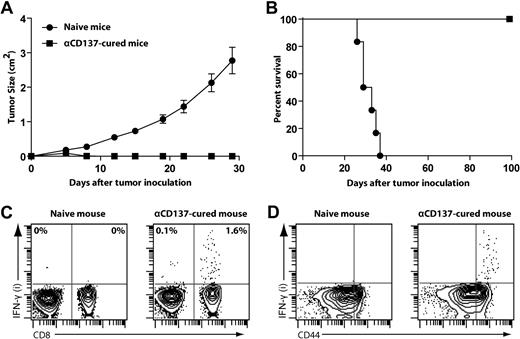
To determine whether anti-CD137 therapy elicits long-lasting antitumor immunity, mice that had been successfully treated with anti-CD137 mAb were rechallenged subcutaneously with A20 lymphoma cells (5 × 106 per mouse) at a different site (on the back) from original tumor challenge. One hundred days after successful treatment, mice rejected this tumor rechallenge, whereas naive mice all died from progressive tumor growth (Figure 4A-B).
Mice cured with anti-CD137 mAb develop long-lasting antitumor immunity. (A-B) BALB/c mice treated and cured by anti-CD137 mAb therapy for more than 100 days or naive mice were (re)challenged subcutaneously with 5 × 106 A20 tumors at a different site from original tumor challenge. Mice were then monitored for tumor growth (A, mean ± SEM) and overall survival (B). (C-D) After tumor rechallenge, splenocytes from αCD137-cured mice or control splenocytes from naive mice were harvested, restimulated in vitro with irradiated A20 tumor cells for 24 hours, and assessed for intracellular IFN-γ secretion by flow cytometry. Dot plots from FACS analysis show the proportion of IFN-γ–positive cells among all CD3+ T cells (C) and CD8 T cells (D).
Mice cured with anti-CD137 mAb develop long-lasting antitumor immunity. (A-B) BALB/c mice treated and cured by anti-CD137 mAb therapy for more than 100 days or naive mice were (re)challenged subcutaneously with 5 × 106 A20 tumors at a different site from original tumor challenge. Mice were then monitored for tumor growth (A, mean ± SEM) and overall survival (B). (C-D) After tumor rechallenge, splenocytes from αCD137-cured mice or control splenocytes from naive mice were harvested, restimulated in vitro with irradiated A20 tumor cells for 24 hours, and assessed for intracellular IFN-γ secretion by flow cytometry. Dot plots from FACS analysis show the proportion of IFN-γ–positive cells among all CD3+ T cells (C) and CD8 T cells (D).
We also observed that mice cured with anti-CD137 treatment contained antitumor IFN-γ–producing CD8 T cells. Splenocytes from cured mice and naive control mice were restimulated with irradiated A20 tumor cells for 24 hours. CD8 T cells from treated but not from naive spleens produced IFN-γ in response to restimulation (Figure 4C). IFN-γ–producing CD8 T cells were restricted to the CD44hi memory T-cell population (Figure 4D), consistent with the finding that these mice elicited long-lasting protection against the tumor.
Anti-CD137 therapy requires NK cells and CD8 T cells and is enhanced by CD4 T-cell depletion
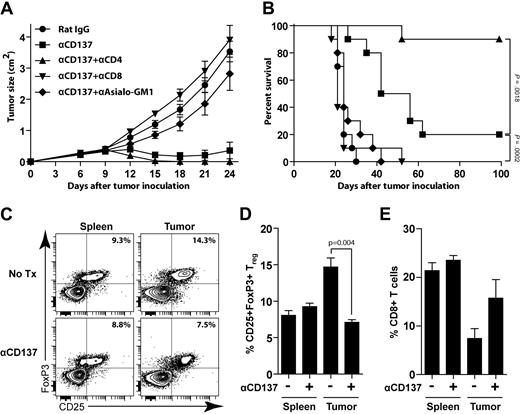
We next investigated the requirements for the antitumor response generated by anti-CD137 mAb. To identify the immune cells responsible for the antitumor activity of anti-CD137 mAb, we carried out in vivo leukocyte subset depletion before anti-CD137 treatment. Depletion of either NK cells or CD8 T cells significantly impaired the therapeutic effect of anti-CD137 treatment (Figure 5A-B). In contrast, CD4 T-cell depletion significantly improved efficacy of anti-CD137 therapy (90% vs 20% overall survival at day 100, P = .002).
Anti-CD137 therapy requires NK and CD8 T cells and is enhanced by CD4 T-cell depletion. (A-B) BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Depletion was initiated before tumor challenge as described in “Depletion of NK cells, CD4 T cells, CD8 T cells, and Tregs.” Mice received 1 intraperitoneal injection of either rat IgG or anti-CD137 mAb at day 6 after tumor inoculation. Groups include: rat IgG alone, anti-CD137 alone, CD4 depletion + anti-CD137, CD8 depletion + anti-CD137, or asialo GM1 depletion + anti-CD137. Mice were monitored for tumor growth (A, mean ± SEM) and overall survival (B). (C-E) BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells and received either no treatment (Tx) or 1 intraperitoneal injection of anti-CD137 mAb at day 8 after tumor inoculation. Five days after treatment, mice were killed and tumors and spleens were collected for analysis. (C) Representative data of the percentage of CD25+FoxP3+ Tregs among CD4 T cells in both spleen and tumor from untreated and treated groups. (D-E) The average percentage of CD25+FoxP3+ Tregs among CD4 T cells (D) and CD8 T cells (E) among total lymphocytes between the untreated and treated groups (3 mice per group).
Anti-CD137 therapy requires NK and CD8 T cells and is enhanced by CD4 T-cell depletion. (A-B) BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Depletion was initiated before tumor challenge as described in “Depletion of NK cells, CD4 T cells, CD8 T cells, and Tregs.” Mice received 1 intraperitoneal injection of either rat IgG or anti-CD137 mAb at day 6 after tumor inoculation. Groups include: rat IgG alone, anti-CD137 alone, CD4 depletion + anti-CD137, CD8 depletion + anti-CD137, or asialo GM1 depletion + anti-CD137. Mice were monitored for tumor growth (A, mean ± SEM) and overall survival (B). (C-E) BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells and received either no treatment (Tx) or 1 intraperitoneal injection of anti-CD137 mAb at day 8 after tumor inoculation. Five days after treatment, mice were killed and tumors and spleens were collected for analysis. (C) Representative data of the percentage of CD25+FoxP3+ Tregs among CD4 T cells in both spleen and tumor from untreated and treated groups. (D-E) The average percentage of CD25+FoxP3+ Tregs among CD4 T cells (D) and CD8 T cells (E) among total lymphocytes between the untreated and treated groups (3 mice per group).
To understand the changes induced by anti-CD137 therapy, we analyzed tumors and spleens from tumor-bearing mice 5 days after anti-CD137 treatment and compared them with untreated mice. We found that anti-CD137 therapy was accompanied by a significant reduction of Tregs (Figure 5C-D, P = .004) and an increase of CD8 T cells at the tumor site (Figure 5E). This result further confirmed the importance of CD8 T cells in mediating the antitumor effect of anti-CD137 mAb.
Selective depletion of Tregs enhances anti-CD137 therapy
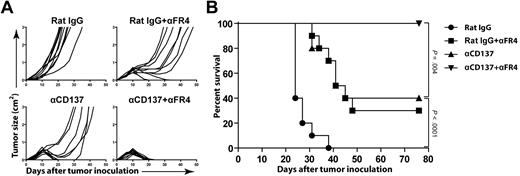
The finding that CD4 T-cell depletion enhanced anti-CD137 therapy led us to hypothesize that CD25+FoxP3+ Tregs contained in the CD4 compartment may impair the efficacy of anti-CD137 therapy. Anti–folate receptor 4 (FR4) mAb has been described to selectively deplete Tregs while sparing other cell populations.26 Using this mAb, we selectively depleted Tregs and determined whether this treatment improved anti-CD137 therapy. Treg depletion was measured in peripheral blood as described in the original publication.26 Anti-FR4 mAb depleted approximately 80% of the FoxP3+ Treg population from the blood and removed only a minority of FoxP3− CD4 Tconvs (< 25%). Anti-FR4 mAb also preserved CD8 T cells, as no CD8 depletion was observed (data not shown). After FR4 depletion, mice received a single dose of anti-CD137 mAb at day 6 or control rat IgG. Selective Treg depletion significantly enhanced the efficacy of anti-CD137 therapy, raising the overall survival from 40% to 100% (P = .004) after 70 days (Figure 6). Although CD137 could be expressed on Tregs in tumor-bearing mice, there was no increase in their suppressive capacity or their ability to proliferate in vitro after treatment with anti-CD137 mAb (data not shown). This result is consistent with the observation that Tregs were not increased (but instead decreased) at the tumor site after anti-CD137 therapy in vivo (Figure 5C-D). These results suggest that Tregs did not directly benefit from anti-CD137 therapy but impaired the efficacy of anti-CD137 therapy due to independent immune suppression.
Selective depletion of Tregs enhances anti-CD137 therapy. BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Mice then received 1 intraperitoneal injection of either rat IgG or anti-CD137 mAb at day 6 after tumor inoculation. FR4 depletion was initiated before tumor challenge as described in “Depletion of NK cells, CD4 T cells, CD8 T cells, and Tregs.” Groups include the following: rat IgG alone, rat IgG + anti-FR4, anti-CD137 mAb alone, and anti-CD137 mAb + anti-FR4. Mice were then monitored for tumor growth (A) and overall survival (B).
Selective depletion of Tregs enhances anti-CD137 therapy. BALB/c mice were inoculated subcutaneously with 5 × 106 A20 tumor cells. Mice then received 1 intraperitoneal injection of either rat IgG or anti-CD137 mAb at day 6 after tumor inoculation. FR4 depletion was initiated before tumor challenge as described in “Depletion of NK cells, CD4 T cells, CD8 T cells, and Tregs.” Groups include the following: rat IgG alone, rat IgG + anti-FR4, anti-CD137 mAb alone, and anti-CD137 mAb + anti-FR4. Mice were then monitored for tumor growth (A) and overall survival (B).
Discussion
Immunomodulation with anti-CD137 agonistic mAb has demonstrated efficacy in several tumor models and has been translated to phase 1 and 2 clinical trials for solid tumors (NCT00309023 and NCT00612664). Application of this novel therapy has not been previously tested in lymphoma. In the present study, we used human primary lymphoma samples as well as an in vivo mouse model to assess the potential relevance of anti-CD137 therapy in lymphoma.
We found that bulk tumor specimens from patients with lymphoma significantly overexpressed CD137 mRNA compared with nonlymphoma tumor specimens. Using a single-cell approach, we showed that the tumor B cells are uniformly negative for CD137, whereas there is a significant population of CD137+ tumor-infiltrating T cells (Figure 1). These findings have important consequences. First, the absence of CD137 expression on the tumor cells addresses the concern that anti-CD137 agonistic mAb might stimulate the malignant cells. Second, the presence of a significant amount of CD137+ T cells at the tumor site as seen in all 3 NHL subtypes tested (FL, MCL, and DLBCL) is suggestive of a therapeutic target for anti-CD137 mAb. Indeed, CD137+ tumor-infiltrating T cells are thought to be tumor-reactive cells that can potentially be targeted and stimulated by the agonistic mAb. More specifically, the number of CD137+ tumor-infiltrating T cells was particularly high in the CD8 compartment—an observation that is important as it has been shown that the therapeutic effect of anti-CD137 mAb is mediated through the engagement and stimulation of CD8 T cells in most, if not all, preclinical tumor models,3 including ours. We hypothesize that a correlation will exist between the amount of CD137-expressing T cells and the response to therapy in anti-CD137–treated cancer patients. Both ongoing and future clinical trials with anti-CD137 mAb should investigate this relationship. Third, overexpression of CD137 mRNA in lymphoma patients (presumably due to the presence of CD137+ tumor-infiltrating T cells) compared with other tumor types suggests that lymphoma may be particularly sensitive to anti-CD137 therapy.
Having shown that lymphoma patients harbor CD137+ tumor-infiltrating T cells that could be targets for anti-CD137 therapy, we next evaluated the therapeutic effect of anti-CD137 mAb in vivo. Using a mouse model of lymphoma, we found that anti-CD137 agonistic mAb had potent antitumor activity in vivo, curing large and established tumors (Figure 2). The antitumor effect was due not to direct targeting of malignant cells, as the murine tumor cells did not express CD137, but instead to immunomodulation of antitumor immune cells. In our model, anti-CD137 mAb appeared to be significantly more potent as a monotherapy than other immunomodulating mAbs previously studied (anti-OX40, anti-CTLA4, anti-GITR). However, this observation should not preclude the potential interest of these mAbs in lymphoma, as we have previously shown that they could be successfully used in combination strategies.28 We also found that anti-CD137 treatment was most effective when administered after tumors had become established (Figure 3). This result may have clinical relevance, as patients present with pre-existing and sometimes large tumor burden. This temporal dependency of anti-CD137 therapy may suggest that a critical tumor burden or “tumor antigenic load” must be present, likely for the purpose of immune cell activation upon which these cells up-regulate CD137 and become sensitive to stimulation with the mAb. Consistent with this hypothesis, we showed that anti-CD137 therapy required NK and CD8 T cells. In addition, it has been shown that administration of anti-CD137 mAb during early infection of lymphocytic choriomeningitis virus induces suppression and death of virus-specific T cells, whereas injection of anti-CD137 mAb in later stage enhances CD8 T cell–mediated antiviral immunity,29 suggesting that CD8+ T cells are programmed differently in the early and late responses in responding to anti-CD137 mAb. Anti-CD137 therapy also induced long-lasting immunologic memory as successfully treated mice harbored antitumor IFN-γ–producing memory CD8 T cells and were protected from tumor rechallenge more than 100 days later (Figure 4). This finding is of consequence, as an important aim of active immunotherapy is the induction of prolonged immune protection against cancer to prevent recurrence in patients. In addition, the crucial role of CD8 T cells in the antitumor response induced by anti-CD137 therapy in mice—both at the early (tumor regression) and late (tumor protection) stage—is encouraging, as we found that CD137 was expressed on a significant number of tumor-infiltrating CD8 T cells in patients with lymphoma.
Similar to the majority of other murine tumor models,10 we found that NK and CD8 T-cell depletion completely abrogated the effect of anti-CD137 therapy (Figure 5). The mechanism by which NK cells participate in tumor eradication after anti-CD137 therapy has been investigated in other studies.30,31 These studies suggested that NK cells promote T-cell help rather than mediating direct tumor cytotoxicity. Surprisingly, we found that CD4 depletion significantly improved the therapeutic effect of anti-CD137 mAb (Figure 5). Our laboratory32 and others have shown that CD4 T-cell help can support lymphoma growth of primary tumor cells. This phenomenon might account, at least in part, for increased efficacy of anti-CD137 therapy after CD4 T-cell depletion in our model. In this study, we hypothesized that Tregs might be responsible for this effect. Although others have reported similar enhancement of anti-CD137 therapy with CD4 depletion in a different tumor model,33 there has been no definitive proof that Tregs were responsible for this effect. In fact, some studies have reported that high numbers of Tregs correlate with better clinical outcome in patients with lymphoma,34,35 putatively due to direct suppression or killing of tumor B cells by Tregs.36,37 In this study, using a mAb (anti-FR4) capable of selectively depleting Tregs,26 we demonstrate a synergistic enhancement when combining Treg depletion with anti-CD137 therapy (Figure 6). This result suggests that Tregs interfere with anti-CD137 therapy, presumably through inhibition of antitumor immune cells. As suggested by our data, it may be necessary to remove Tregs for anti-CD137 therapy to function more effectively. However, clinical translation of this finding will require elucidation of the optimal time point for Treg depletion, as this has been shown to be important for lymphoma rejection,38 and identification of an anti–human CD137 mAb with similar biologic activity and therefore, hopefully, antitumor efficacy as observed in the mouse model.
In conclusion, lymphoma appears to be a promising candidate for anti-CD137 therapy. First, lymphoma has proven to be responsive to immunotherapy.1 Second, human primary lymphoma tumors overexpress CD137 mRNA compared with other tumors, presumably not due to the tumor cells themselves but to the presence of CD137+ tumor-infiltrating T cells. These CD137+ T cells are thought to be tumor-reactive cells whose function could be further stimulated with anti-CD137 agonistic mAb. Third, we showed that anti-CD137 agonistic mAb has potent antilymphoma activity in vivo. This effect can be further improved by Treg depletion, leading to spectacular tumor regression and prolonged remission in mice. In addition, lymphoma offers the possibility to combine anti-CD137 mAb with other lymphoma-specific therapies such as rituximab. Due to the presumed role of NK cells in both anti-CD137 (shown here in Figure 5) and anti-CD20 (through antibody-dependent cell cytotoxicity) mAb therapies, we hypothesize that the combination of a tumor-directed mAb (rituximab) and anti-CD137 mAb will be synergistic. This hypothesis is currently under investigation in our laboratory.
Overall, our study demonstrates the promise of anti-CD137 agonistic mAb for the treatment of lymphoma. Our results identify important insights into the therapeutic use of anti-CD137 mAb and support the testing of anti-CD137 agonistic mAb in clinical trials for patients with lymphoma.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank J. Allison for the 9H10 hybridoma, S. Sakaguchi for the TH6 and DTA-1 hybridomas, D. Czerwinski for excellent technical help, and O. Murillo for review of this paper.
This work was supported by the National Institutes of Health (CA34233 and CA33399) and a Leukemia & Lymphoma Society Specialized Centers of Research (SCOR) grant. M.J.G. is supported by the Howard Hughes Medical Institute Research Training Fellowship. J.H.M. is supported by the Norwegian Cancer Society and the Research Council of Norway.
National Institutes of Health
Authorship
Contribution: R.H. designed and performed experiments, analyzed data, and wrote the paper; M.J.G., H.E.K., J.H.M., A.A.A., J.T.L., J.M.I., and J.A.T. designed and performed experiments, analyzed data, and reviewed the paper; A.K. collected and provided the human lymphoma biopsies from Norway; L.C. provided the 2A hybridoma and reviewed data and the paper; and R.L. designed experiments and reviewed data and the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Professor of Medicine, Chief, Division of Oncology, 269 Campus Dr, CCSR 1105, Stanford University Medical Center, Stanford, CA 94305-5306; e-mail: levy@stanford.edu.