Cdc42 is a member of the Rho GTPase family that has been implicated in several cell functions including proliferation and migration, but its physiologic role needs to be dissected in each cell type. We achieved B-cell and hematopoietic stem cell deletion of Cdc42 by conditional gene targeting in mice. Deletion of Cdc42 from proB/preB-cell stage significantly blocked B-cell development at T1 and later stages, resulting in reduced mature B-cell populations and reduced antigen-specific immunoglobulin M (IgM), IgG1, and IgG3 production. The Cdc42−/− B cells, themselves, were abnormal with impaired proliferation and survival. The mutant B cells were further characterized by a B-cell receptor (BCR) signaling defect with increased Erk and decreased Akt activation, as well as a defect in BCR-mediated B-cell–activating factor (BAFF) receptor up-regulation and subsequent BAFF receptor signaling in mature resting B cells. Surprisingly, Cdc42 was dispensable for stromal cell–derived factor-1α (SDF-1α)– or B-lymphocyte chemoattractant (BLC)–induced B-cell migration. Finally, loss of Cdc42 from hematopoietic stem cells did not alter common lymphoid progenitor production but severely reduced proB/preB- and immature B-cell populations, indicating that Cdc42 is also involved in B-cell precursor differentiation. These results reveal multifaceted roles of Cdc42 in B-cell development and activation.
Introduction
B lymphocytes develop from bone marrow hematopoietic stem cells through a series of differentiation stages.1,,–4 The most immature populations are B-cell progenitors termed proB cells. ProB cells undergo immunoglobulin heavy chain gene rearrangement and differentiate into preB cells. PreB cells are subjected to immunoglobulin light chain gene rearrangement and develop into immature B cells. Immature B cells emigrate from bone marrow to spleen, where they are identified as transitional type I (T1) B cells, and undergo further development into transitional type II (T2) B cells. Although a small proportion of T2 B cells mature into noncirculating marginal zone (MZ) B cells, most differentiate into follicular (FO) B cells that circulate to spleen follicles, lymph nodes, and bone marrow. MZ B cells are a major source of natural antibodies, and are thought to primarily mediate rapid immune responses through T cell–independent mechanisms. Upon encountering antigen and receiving T-cell help, FO B cells proliferate and differentiate in lymphoid follicles and germinal centers into antibody-producing plasma cells or memory B cells.
Cdc42 is an intracellular signal transducer of the Rho GTPase family that cycles between an inactive GDP-bound form and an active GTP-bound form under tight regulation.5,6 Cdc42 GTPase is a key signaling component governing actin cytoskeleton organization, adhesion, migration, proliferation, and survival in mammalian cells.7,,,,,–13 Using cell lines and constitutively active or dominant-negative mutants, Cdc42 has been shown to play a role in CD47-regulated B-cell motility and in filopodia formation in B cells.14,15 Cdc42 has also been shown to transduce signals from B-cell receptor (BCR).16 However, it is not known whether Cdc42 is essential for B-cell development—although 2 closely related members of Rho GTPases, Rac1 and Rac2, have been shown to be involved in B-cell differentiation.17 Most studies of Cdc42 GTPase used overexpression of constitutively active or dominant-negative mutants. Although this strategy has pioneered several key discoveries, including the critical involvement of Cdc42 GTPase in filopodia formation and cell adhesion.8 it is compromised by the existence of abundant cross talk between Cdc42 and other Rho GTPases.18,,,,–23 On the one hand, Cdc42 dominant-negative mutants work by sequestering upstream guanine nucleotide exchange factors—a family that includes more than 80 members in the human or mouse genome, many of which are capable of activating multiple Rho GTPases. Due to the noncatalytic nature of dominant-negative mutants, excessive expression is typically needed to effectively sequester the guanine nucleotide exchange factors and block endogenous Cdc42 activity, and this could impact function of other Rho GTPases. On the other hand, overexpression of constitutively GTP-bound Cdc42 mutants may activate several effectors shared between Cdc42 and other Rho GTPases (eg, PAKs, IQGAPs, and IRSp53), causing Cdc42-irrelevant functional outcomes. Because a balanced GTP-binding/GTP-hydrolysis cycle of Cdc42 is required for effective signal flow,24 overexpressed mutants may also introduce artifacts by locking the small GTPase in one conformation at a fixed intracellular location. It is therefore highly advantageous to use a gene-targeting strategy to assess Cdc42 functions and signaling requirements.
Several recent studies have reported the use of Cdc42 conditional gene-targeted mice to define Cdc42 functions.25,,,,,,,,–34 Deletion of Cdc42 in skin cells revealed that Cdc42 is required for differentiation of skin progenitor cells into hair follicles, and for β-catenin turnover. A study using loxP/Cre-mediated deletion of Cdc42 from an ES-derived fibroblastoid cell line indicated that Cdc42 is dispensable for actin filopodia induction, directed migration, cell polarization, and mitosis; this is contrary to other studies,7,,,,,–13 including our own work.27 We used Cdc42−/− primary mouse embryonic fibroblasts to indicate that Cdc42 is critical for filopodia formation, migration, and proliferation—thereby suggesting that Cdc42 plays cell type–specific signaling roles that differ in primary mouse embryonic fibroblasts and fibroblastoid cells. The Cdc42 gene-targeted mouse model has also been characterized in hematopoietic stem cells, liver cells, and nerve system.
To assess the physiologic role of Cdc42 in B-cell development, we generated mouse strains with a B cell–specific deletion of Cdc42 by crossing CD19-Cre transgenic or hematopoietic stem cell deletion by crossing Mx1-Cre transgenic and Cdc42flox/flox mice. Using these models, we demonstrate that Cdc42 is essential for B-cell development and signaling leading to proper proliferation, survival, and activation.
Methods
Mouse gene targeting
Conditional targeted Cdc42flox/flox mice were generated as described previously.27 The flox allele contains loxP sites flanking exon 2 of the Cdc42 alleles. To delete Cdc42 in vivo in the B-cell lineage, Cdc42flox/flox mice were mated with mice expressing Cre recombinase under the control of the CD19 proximal promoter (The Jackson Laboratory). Mice ranged in age from 6 to 12 weeks. To delete Cdc42 in vivo in hematopoietic stem cells, Mx-Cre+;Cdc42floxp/flox mice were generated by breeding Cdc42flox/flox mice to Mx-Cre+ transgenic mice carrying a bacteriophage Cre recombinase driven by an interferon-γ–inducible Mx1 promoter (gift of Dr Stuart H. Orkin, Dana-Farber Cancer Institute). Bone marrow cells from Mx-Cre+;Cdc42flox/flox mice were transplanted into lethally irradiated BoyJ mice. Two months after transplantation, the expression of Mx-Cre was induced by 3 to 4 intraperitoneal injections of 300 μg polyinosinic-polycytidylic acid (polyI:C; Amersham Pharmacia Biotech Inc) into recipient mice at 2-day intervals. Bone marrow cells from recipient mice were analyzed 14 to 17 days after polyI:C injection. Animals were housed under specific pathogen–free conditions in the animal facility at Cincinnati Children's Hospital Research Foundation, and all animal studies were approved by the institutional review board of the Cincinnati Children's Hospital.
Immunoblotting
B cells were purified from bone marrow and spleen using B220 or CD43 Microbeads (Miltenyi Biotec) and lysed, and protein content was normalized by the Bradford method. Lysates were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Expression or activation (phosphorylation) of Cdc42, WASP, Erk, JNK, p38, Akt, IκB, and BCR was blotted using the corresponding antibodies: anti-Cdc42 (Millipore), anti–phospho-WASP (Bethyl Laboratories Inc), anti-WASP (Santa Cruz Biotechnology), antibodies against phospho- or total ERK, JNK, p38, Akt, and IκB (Cell Signaling Technology).
Characterization of B-cell development by cell-surface staining
Single-cell suspensions were prepared from bone marrow, spleen, lymph nodes, blood, and peritoneal lavage fluid. Cells were stained with various combinations of cell-surface marker antibodies for 20 minutes at room temperature. Flow cytometric analysis was performed on a FACSCanto system using FACSDiVa software (BD Biosciences). Antibodies to cell-surface markers were anti-B220, –immunoglobulin M (IgM), -IgD, -CD21, -CD23, -CD5, -CD3, -CD4, -CD8, -Gr1, -CD11b, -TER119, –interleukin-7 receptor α (IL-7Rα), –c-kit, and -Sca1 (BD Biosciences), and anti-B cell–activating factor (BAFF) receptor (eBioscience).
Antibody ELISA assay
Serum antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) using isotype-specific goat anti–mouse antibodies coupled to horseradish peroxidase (HRP; Southern Biotechnology Associates Inc). For detection of antigen-specific antibodies, mice were injected intraperitoneally with 100 μg T-dependent antigen, nitrophenyl–keyhole limpet hemocyanin (NP-KLH; Biosearch Technologies) that was precipitated with alum (Pierce), or 10 μg T-independent type II antigen (TI-II), trinitrophenyl (TNP)–Ficoll (Biosearch Technologies). Serum was collected on days 0, 7, and 14 from NP-KLH–treated mice, or on days 0, 3, 7, and 10 from TNP-Ficoll–treated mice. Microtiter plates were coated with 20 μg/mL NP-BSA (Biosearch Technologies) for capturing NP-specific IgM and IgG1 in serum from NP-KLH–treated mice, or with 20 μg/mL TNP-BSA (Biosearch Technologies) for capturing TNP-specific IgM and IgG3 in serum from TNP-Ficoll–treated mice. Bound antigen-specific antibodies were detected using specific HRP-conjugated goat anti–mouse antibodies.
Cell apoptosis analysis
B cells were purified from spleen using magnetic-activated cell sorting beads. The cells were cultured for 72 hours on 96-well plates at 106 cells/mL in 200 μL B-cell culture medium (10% fetal calf serum; 10 mM Hepes, pH 7.3; l-glutamine; penicillin/streptomycin; nonessential amino acids; sodium pyruvate; and 5 × 10−5 M 2-mercaptoethanol, in RPMI-1640) in the presence or absence of 2 μg/mL anti-IgM F(ab′)2 antibody (Jackson Immunoresearch Laboratories), and/or 50 ng/mL recombinant human BAFF (PeproTech). Cells were then stained with annexin V (BD Biosciences) followed by flow cytometric analysis.
Cell proliferation assay
Splenic B cells were cultured for 48 hours on 96-well plates at 106 cells/mL in 200 μL B-cell culture medium in the presence or absence of 2 μg/mL either anti-IgM F(ab′)2 antibody or LPS. Cell growth rates were assayed by a nonradioactive cell proliferation assay kit (Promega)
Cell migration assay
Cell migration was measured using a transwell chamber. RPMI-1640 culture medium (containing 500 ng/mL stromal cell–derived factor-1α [SDF-1α; PeproTech] or B-lymphocyte chemoattractant [BLC; PeproTech]) was added to the exterior of the transwell chamber. Purified B cells (2 × 105) were suspended in RPMI-1640 media, added to the interior of the transwell chamber, and incubated for 4 hours. Cells that migrated through the polyester membrane were counted.
Gene expression analysis
RNA was isolated from purified splenic B cells using RNeasy Micro Kit (QIAGEN), and converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc). Real-time polymerase chain reaction (PCR) was performed with a Taqman system on a 7900HT Real-Time machine (Applied Biosystems Inc). The primer sequences for amplification of BAFF-R cDNA were CCTCCGCTCAAAGAAGATGCA (forward primer) and GTGGAGCCCAGTTCTGT (reverse primer). Data were analyzed using SDS 2.3 software (Applied Biosystems Inc), and normalized to glyceraldehyde-3-phosphate dehydrogenase.
Results
Generation of Cdc42-deficient B cells
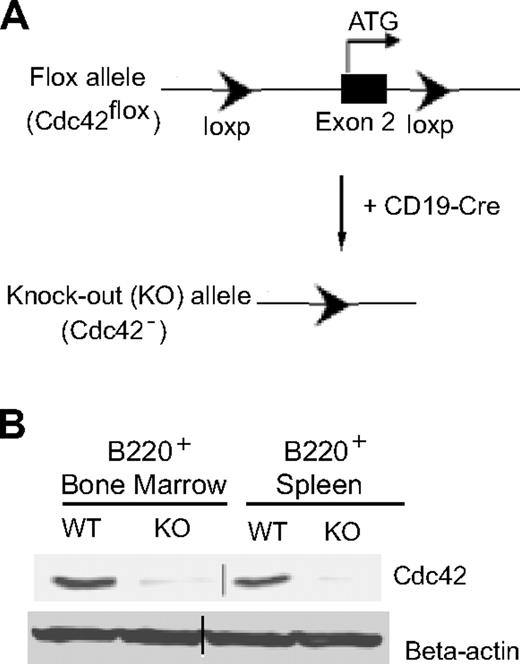
To study the role of Cdc42 in B-cell development, Cdc42flox/flox mice, generated as described previously,27 were crossbred with CD19-Cre transgenic mice (Figure 1A). Bone marrow and spleen were collected from the resulting CD19-Cre;Cdc42flox/flox mice, as well as control wild-type (WT) mice. B220+ B cells were purified from bone marrow and spleen using magnetic-activated cell sorting beads. Lack of Cdc42 expression in B cells from CD19-Cre;Cdc42flox/flox mice was confirmed by anti-Cdc42 Western blot (Figure 1B).
Generation of Cdc42−/− B cells by a loxP/Cre recombinase system. (A) The loxP/Cre-mediated gene-targeting strategy to generate Cdc42 gene–deleted (Cdc42−) allele in B cells. (B) Western blots showing Cdc42 expression in B220+ B cells, purified from bone marrow and spleen of WT and Cdc42−/− (KO) mice using B220 microbeads. Parallel blots of beta-actin serve as loading controls. Vertical line has been inserted to indicate the gel lanes being switched in position from the original blot.
Generation of Cdc42−/− B cells by a loxP/Cre recombinase system. (A) The loxP/Cre-mediated gene-targeting strategy to generate Cdc42 gene–deleted (Cdc42−) allele in B cells. (B) Western blots showing Cdc42 expression in B220+ B cells, purified from bone marrow and spleen of WT and Cdc42−/− (KO) mice using B220 microbeads. Parallel blots of beta-actin serve as loading controls. Vertical line has been inserted to indicate the gel lanes being switched in position from the original blot.
Effect of deletion of Cdc42 on B-cell development
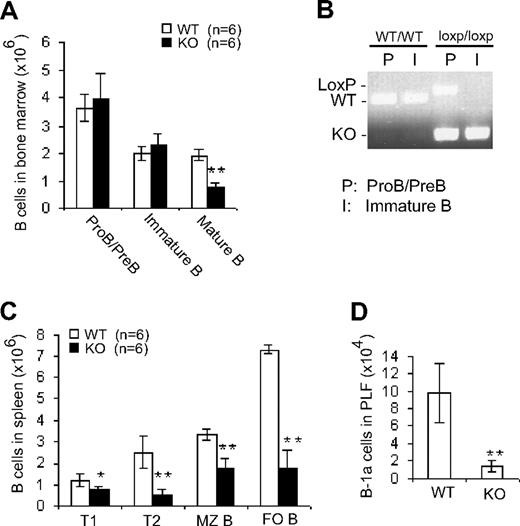
There was no significant difference between Cdc42−/− and WT mice in terms of the number of proB/preB cells (B220lowIgM−) and immature B cells (B220lowIgM+) in bone marrow (Figure 2A); this might be due to an incomplete deletion of the Cdc42 gene in proB/preB cells (Figure 2B). In contrast, Cdc42−/− mice exhibited an approximately 60% decrease in the number of recirculating B cells (B220HIGHIgM+) in bone marrow (Figure 2A), suggesting that late B-cell development in spleen is altered by disruption of Cdc42 expression.
B cell–specific deletion of Cdc42 has no effect on early B-cell development in bone marrow but impairs late B-cell development in spleen. (A) Bone marrow cells from WT and Cdc42−/− (KO) mice were counted and stained with antibody against B220 and IgM. Immunopositive cells were analyzed by flow cytometry. The number of B-cell subsets was calculated by multiplying the total number of bone marrow cells by the percentage of cells of each subset. (B) ProB/preB and immature B cells from bone marrow were collected by fluorescence-activated cell sorting (FACS) and subjected to PCR analysis of Cdc42 WT, floxed, and KO alleles. (C) Splenocytes were isolated, counted, and stained with antibodies against B220, IgM, IgD, CD21, and CD23. Immunopositive cells were analyzed by flow cytometry. The number of cells within each of the B-cell subsets was calculated as described in panel A. (D) Cells from peritoneal lavage fluid (PLF) were counted and stained with antibodies against IgM and CD5. Immunopositive cells were analyzed by flow cytometry. The number of B-1a cells was calculated by multiplying the total number of PLF cells times the percentage of B-1a cells (**P < .01; *P < .05). Data are means ± SD.
B cell–specific deletion of Cdc42 has no effect on early B-cell development in bone marrow but impairs late B-cell development in spleen. (A) Bone marrow cells from WT and Cdc42−/− (KO) mice were counted and stained with antibody against B220 and IgM. Immunopositive cells were analyzed by flow cytometry. The number of B-cell subsets was calculated by multiplying the total number of bone marrow cells by the percentage of cells of each subset. (B) ProB/preB and immature B cells from bone marrow were collected by fluorescence-activated cell sorting (FACS) and subjected to PCR analysis of Cdc42 WT, floxed, and KO alleles. (C) Splenocytes were isolated, counted, and stained with antibodies against B220, IgM, IgD, CD21, and CD23. Immunopositive cells were analyzed by flow cytometry. The number of cells within each of the B-cell subsets was calculated as described in panel A. (D) Cells from peritoneal lavage fluid (PLF) were counted and stained with antibodies against IgM and CD5. Immunopositive cells were analyzed by flow cytometry. The number of B-1a cells was calculated by multiplying the total number of PLF cells times the percentage of B-1a cells (**P < .01; *P < .05). Data are means ± SD.
We next examined B-cell distribution in spleen from Cdc42−/− and WT mice (Figure 2C). Cdc42−/− mice exhibited reduced numbers of all subsets of B cells—including T1 (B220+IgMhighIgDlow), T2 (B220+IgMhighIgDhigh), MZ (B220+CD21+CD23−), and FO (B220+CD21+/−CD23+) cells. Similar reductions in B cells were also observed in lymph nodes and blood (data not shown). These results suggest that Cdc42 is critical for late B-cell development.
B-1 cells represent a distinct type of B cell whose development is believed to occur predominantly during fetal and perinatal life.35 Similar to MZ B cells in spleen, CD5+ B-1a cells are one of the major sources of natural antibodies, and mediate T-independent immune responses.36 Analysis of peritoneal lavage fluid revealed a substantial reduction in number of B-1a cells (CD5+IgM+) in Cdc42−/− mice (Figure 2D), indicating that Cdc42 plays an important role in B-1–cell development.
Effect of deletion of Cdc42 on antibody production
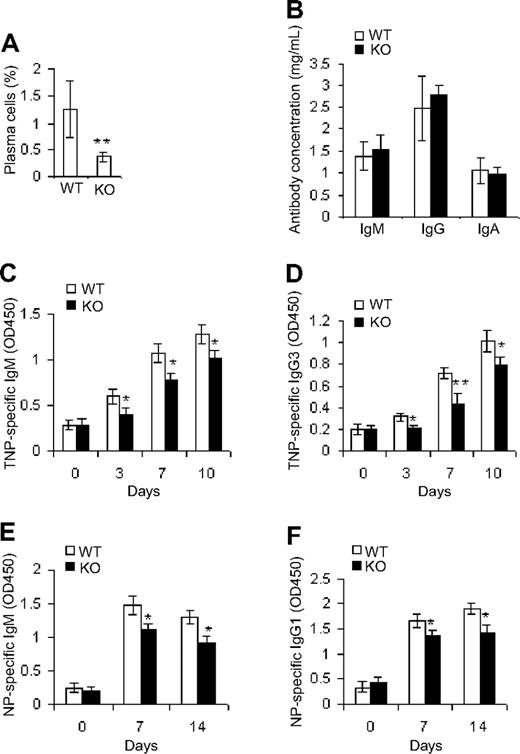
Given that Cdc42 deficiency led to reductions in mature B cells, we investigated whether antibody-producing plasma cells and antibody production were affected as well. Although the frequency of plasma cells (CD93−CD38+CD138+) was diminished in Cdc42-deficient mice (Figure 3A), measurement of serum natural antibody levels did not reveal any significant differences in IgM, IgG, or IgA production between Cdc42−/− and WT mice (Figure 3B), suggesting that the residual MZ B and B-1a cells in mutant mice are sufficient to sustain physiologic levels of natural antibodies.
Deletion of Cdc42 results in decreased production of antigen-specific antibodies. (A) Splenocytes from WT and Cdc42−/− (KO) mice were immunolabeled with antibodies against CD93, CD38, and CD138, and analyzed by flow cytometry for CD93−CD38+CD138+ plasma cells. (B) Serum from WT and KO mice was subjected to ELISA analysis for IgM, IgG, and IgA. (C-F) Mice were injected intraperitoneally with 100 μg NP-KLH precipitated with alum (E-F), or with 10 μg TNP-Ficoll (C-D). Serum was collected at different time points. Microtiter plates were coated with either 20 μg/mL NP-BSA for capturing NP-specific IgM and IgG1 in serum of NP-KLH–treated mice (E-F), or with 20 μg/mL TNP-BSA for capturing TNP-specific IgM and IgG3 in serum of TNP-Ficoll–treated mice (C-D). Bound antigen-specific antibodies were detected using specific HRP-conjugated goat anti–mouse antibodies (**P < .01; *P < .05). Data represent means ± SD.
Deletion of Cdc42 results in decreased production of antigen-specific antibodies. (A) Splenocytes from WT and Cdc42−/− (KO) mice were immunolabeled with antibodies against CD93, CD38, and CD138, and analyzed by flow cytometry for CD93−CD38+CD138+ plasma cells. (B) Serum from WT and KO mice was subjected to ELISA analysis for IgM, IgG, and IgA. (C-F) Mice were injected intraperitoneally with 100 μg NP-KLH precipitated with alum (E-F), or with 10 μg TNP-Ficoll (C-D). Serum was collected at different time points. Microtiter plates were coated with either 20 μg/mL NP-BSA for capturing NP-specific IgM and IgG1 in serum of NP-KLH–treated mice (E-F), or with 20 μg/mL TNP-BSA for capturing TNP-specific IgM and IgG3 in serum of TNP-Ficoll–treated mice (C-D). Bound antigen-specific antibodies were detected using specific HRP-conjugated goat anti–mouse antibodies (**P < .01; *P < .05). Data represent means ± SD.
To determine whether antigen-specific antibody production in Cdc42-deficient B cells is compromised, mutant and control mice were immunized with T-independent antigen (TI-II), TNP-Ficoll, or T-dependent antigen (NP-KLH). Serum TNP-specific IgM (Figure 3C), IgG3 (Figure 3D), NP-specific IgM (Figure 3E), and IgG1 (Figure 3F) were modestly, yet significantly, reduced in Cdc42-deficient mice at all time points—except day 0, which reflects the background level of IgM. The decrease in MZ and FO B cells in Cdc42-deficient mice might contribute to the defect in antibody production in response to antigen inoculation.
Effects of deletion of Cdc42 on B-cell proliferation and survival
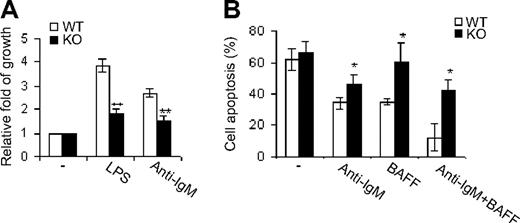
Cell proliferation and survival play crucial roles in B-cell development.17 To explore the mechanisms underlying impaired B-cell development in Cdc42-deficient mice, we first examined the proliferative properties of Cdc42-deficient B cells in response to BCR cross-linking by anti-IgM F(ab′)2 antibody or to LPS. Although either treatment induced growth of WT B cells, this induction was significantly impaired in Cdc42-null mice (Figure 4A). This finding suggests that Cdc42 plays a role in B-cell growth mediated by BCR, as well as Toll-like receptor.
Cdc42 deficiency causes decreased B-cell growth and increased apoptosis. (A) Splenic B cells were purified from WT and Cdc42−/− (KO) mice using CD43 microbeads, and cultured for 48 hours on 96-well plates (2 × 105 cells/well) with no stimulation, or with 2 μg/mL anti-IgM F(ab′)2 antibody or LPS. Cell growth rates were analyzed using a nonradioactive cell proliferation assay kit. Data were expressed as fold of growth relative to the growth rate of unstimulated cells. (B) Purified B cells were cultured for 72 hours on 96-well plates (2 × 105 cells/well) with no stimulation or with 2 μg/mL anti-IgM F(ab′)2 antibody and/or 50 ng/mL BAFF. Cells were then stained with annexin V followed by flow cytometric analysis (**P < .01; *P < .05). Data are means ± SD.
Cdc42 deficiency causes decreased B-cell growth and increased apoptosis. (A) Splenic B cells were purified from WT and Cdc42−/− (KO) mice using CD43 microbeads, and cultured for 48 hours on 96-well plates (2 × 105 cells/well) with no stimulation, or with 2 μg/mL anti-IgM F(ab′)2 antibody or LPS. Cell growth rates were analyzed using a nonradioactive cell proliferation assay kit. Data were expressed as fold of growth relative to the growth rate of unstimulated cells. (B) Purified B cells were cultured for 72 hours on 96-well plates (2 × 105 cells/well) with no stimulation or with 2 μg/mL anti-IgM F(ab′)2 antibody and/or 50 ng/mL BAFF. Cells were then stained with annexin V followed by flow cytometric analysis (**P < .01; *P < .05). Data are means ± SD.
To study survival of Cdc42-deficient B cells, we analyzed the response of these cells to BCR ligation and/or treatment with BAFF, a ligand of tumor necrosis factor family that promotes B-cell survival.17 Either BCR activation or BAFF treatment rescued WT B cells from apoptosis; moreover, these 2 treatments synergistically inhibited apoptosis of WT cells (Figure 4B). However, Cdc42-deficient B cells were resistant to inhibition of apoptosis by BCR engagement and/or BAFF (Figure 4B), indicating that Cdc42 regulates BCR- and BAFF-mediated B-cell survival.
Effect of deletion of Cdc42 on B-cell signaling
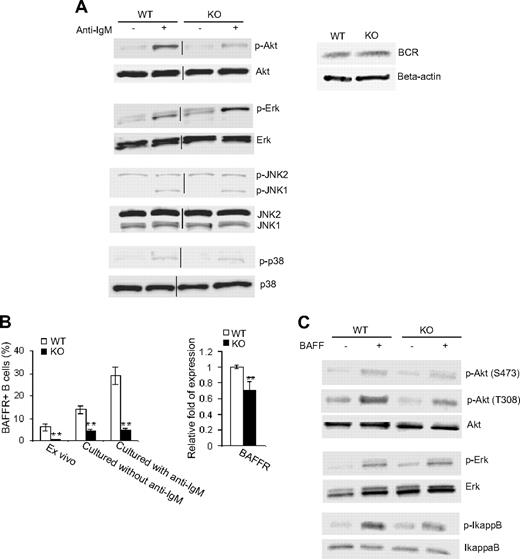
In view of the data that we have shown above, Cdc42 appears to transduce BCR signals in the regulation of B-cell proliferation and survival and thus B-cell development. To determine molecular pathways emanated from BCR that underlie Cdc42-mediated B-cell proliferation and survival, we measured activation (ie, phosphorylation) of Akt, Erk, JNK, and p38 in response to anti-IgM stimulation of Cdc42-deficient B cells and control cells. Western blot analysis showed that BCR cross-linking–activated Akt was reduced by approximately 2-fold in Cdc42-deficient B cells (Figure 5A). In contrast, BCR-induced activation of Erk, specifically Erk2, was further up-regulated in the absence of Cdc42 (Figure 5A). We did not detect changes in BCR expression in Cdc42-deficient B cells (Figure 5A), suggesting that alterations in signaling activities are not due to an alteration in BCR expression. Furthermore, BCR cross-linking activated JNK1 and p38 mitogen-activated protein kinases in Cdc42-deficient B cells to a similar extent to that in WT B cells (Figure 5A). Together, these data suggest that Cdc42 regulates BCR-mediated cell survival and proliferation through modulation of Akt and Erk, but not JNK and p38, activities.
Deletion of Cdc42 impairs BCR and BAFF-R signaling. Splenic B cells were purified from WT and Cdc42−/− (KO) mice using CD43 microbeads. (A) Freshly isolated cells were stimulated with or without 33 μg/mL anti-IgM F(ab′)2 antibody for 60 minutes and then subjected to Western blot (left). Vertical lines have been inserted to indicate the gel lanes being switched in position from the original blots. Western blotting for BCR was carried out in parallel (right) or (B) purified B cells were either stained immediately with BAFF-R antibody or cultured overnight in the presence or absence of 2 μg/mL anti-IgM F(ab′)2 antibody before the antibody labeling. Stained cells were analyzed by flow cytometry (left). Purified cells were also examined for BAFF-R mRNA expression by quantitative reverse transcription–PCR analysis (right; **P < .01). Data are means ± SD. (C) Purified B cells were stimulated with or without 100 ng/mL BAFF for 60 minutes and then subjected to Western blotting.
Deletion of Cdc42 impairs BCR and BAFF-R signaling. Splenic B cells were purified from WT and Cdc42−/− (KO) mice using CD43 microbeads. (A) Freshly isolated cells were stimulated with or without 33 μg/mL anti-IgM F(ab′)2 antibody for 60 minutes and then subjected to Western blot (left). Vertical lines have been inserted to indicate the gel lanes being switched in position from the original blots. Western blotting for BCR was carried out in parallel (right) or (B) purified B cells were either stained immediately with BAFF-R antibody or cultured overnight in the presence or absence of 2 μg/mL anti-IgM F(ab′)2 antibody before the antibody labeling. Stained cells were analyzed by flow cytometry (left). Purified cells were also examined for BAFF-R mRNA expression by quantitative reverse transcription–PCR analysis (right; **P < .01). Data are means ± SD. (C) Purified B cells were stimulated with or without 100 ng/mL BAFF for 60 minutes and then subjected to Western blotting.
In addition to BCR signaling, signaling induced by BAFF, through binding to one of its receptors, BAFF-R, is critical for B-cell development and survival.37 We have found that Cdc42 is required for BAFF-mediated B-cell survival (Figure 4B). To determine the mechanism of this effect, we examined BAFF-R levels in Cdc42-deficient and WT B cells. As shown in Figure 5B, basal BAFF-R was dramatically decreased in freshly isolated mutant cells and in mutant cells cultured in the absence of BCR cross-linking. Although BAFF-R was significantly up-regulated in WT cells upon BCR engagement, no such up-regulation was observed in mutant cells, suggesting that Cdc42 is required in BCR signaling to up-regulate BAFF-R. In fact, BAFF-R mRNA level was found significantly reduced in freshly isolated Cdc42-deficient B cells (Figure 5B). These data suggest that Cdc42 can in part regulate BAFF-R signaling by controlling its gene transcription.
We further analyzed activities of Akt and IκB in WT and Cdc42-deficient B cells upon stimulation with BAFF. Although WT cells showed significant increases in phosphorylation of Akt, at both 473 and 308, and IκB in response to BAFF, the increased phosphorylation of Akt at 308 and IκB was diminished in mutant cells (Figure 5C). Data from these experiments suggest that Cdc42 is required to transduce both BCR and BAFF/BAFF-R signals to regulate B-cell proliferation, survival, and development.
Effect of deletion of Cdc42 on B-cell migration
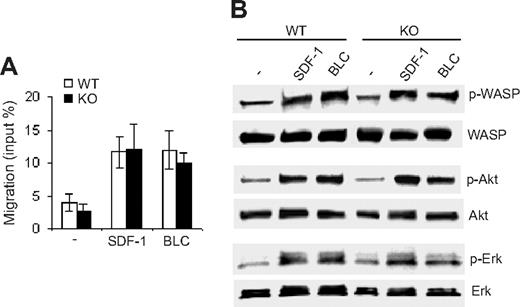
Cdc42 has been shown to regulate B-cell migration.14 The decrease in B cells in spleen, lymph nodes, blood, and bone marrow of Cdc42-deficient mice (Figure 2 and data not shown) suggests that migration of Cdc42-deficient B cells is compromised. To address this possibility, we examined chemotaxis of Cdc42-deficient B cells toward either SDF-1α or BLC. To our surprise, Cdc42 deficiency did not perturb B-cell migratory ability (Figure 6A). Moreover, SDF-1α and BLC-induced activation of WASP, Akt, and Erk in WT and mutant cells was indistinguishable (Figure 6B). These results indicate that Cdc42 is not required for B-cell migration.
Deletion of Cdc42 has no effect on B-cell migration or migration-related signaling pathways. (A) The migration of B cells from WT and Cdc42−/− (KO) mice toward SDF-1 and BLC was examined in a transwell assay. The number of cells that migrated to the exterior of the transwell chamber was normalized to the total number of cells added to the interior of the chamber. Data are means ± SD. (B) Purified B cells were incubated in B-cell culture medium at 37°C for 2 hours, and then stimulated for 3 minutes with 100 ng/mL SDF-1 or 200 nM BLC. Stimulation was stopped by adding cold phosphate-buffered saline, and the cells were subjected to Western blot analysis.
Deletion of Cdc42 has no effect on B-cell migration or migration-related signaling pathways. (A) The migration of B cells from WT and Cdc42−/− (KO) mice toward SDF-1 and BLC was examined in a transwell assay. The number of cells that migrated to the exterior of the transwell chamber was normalized to the total number of cells added to the interior of the chamber. Data are means ± SD. (B) Purified B cells were incubated in B-cell culture medium at 37°C for 2 hours, and then stimulated for 3 minutes with 100 ng/mL SDF-1 or 200 nM BLC. Stimulation was stopped by adding cold phosphate-buffered saline, and the cells were subjected to Western blot analysis.
Effect of deletion of Cdc42 in hematopoietic stem cells on B-cell precursor development in bone marrow
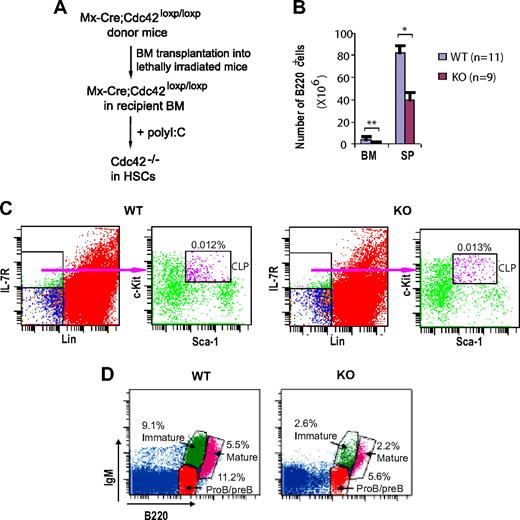
B cell–specific CD19 promoter-mediated Cre deletion of Cdc42 was incomplete in proB and preB cells (Figure 2B), precluding study of the role of Cdc42 in early B-cell development in bone marrow. To overcome this methodology limitation, we used previously generated Mx-Cre;Cdc42flox/flox mice. Bone marrow cells from Mx-Cre;Cdc42flox/flox mice were transplanted into lethally irradiated BoyJ host mice; the Cdc42 gene in the transplanted hematopoietic stem cells was then deleted by polyI:C injection into the host mice (Figure 7A). B220+ B cells in bone marrow and spleen were markedly reduced in Cdc42-deficient mice. Whereas common lymphoid progenitors (CLPs) in WT and mutant mice were comparable (Figure 7C), proB/preB, immature, and mature recirculating B cells in bone marrow were all drastically decreased in the absence of Cdc42 (Figure 7D). These results suggest that Cdc42 is critical for early B-cell development in bone marrow.
Hematopoietic stem celldeletion of Cdc42 causes defective B-cell development in bone marrow. (A) Bone marrow cells from Mx-Cre;Cdc42loxp/loxp donor mice were transplanted by intravenous injection into lethally irradiated BoyJ mice. Recipient mice were injected with polyI:C to delete Cdc42 in hematopoietic stem cells. (B) Bone marrow cells and splenocytes were isolated from WT and Cdc42−/− (KO) mice, counted, stained with anti-B220 antibody, and then analyzed by flow cytometry. The number of B220+ cells was calculated by multiplying the total number of bone marrow cells and splenocytes by the percentage of B220+ cells (**P < .01; *P < .05). The data are means ± SD. (C) Bone marrow cells were stained for IL-7R, lineage (Lin) markers (ie, B220, CD3, CD4, CD8, Gr1, CD11b, and TER119), Sca-1, and c-Kit followed by flow cytometric analysis. Common lymphoid progenitor (CLP) cells (Sca1medc-kitmed-high) were gated from Lin−IL-7R+ cell population. Data are representative of 4 mice. (D) Bone marrow cells were stained with antibodies against B220 and IgM and, using flow cytometry, analyzed for proB/preB cells ((B220lowIgM−), immature B cells (B220lowIgM+), and mature recirculating B cells (B220highIgM+). Data are representative of 4 mice.
Hematopoietic stem celldeletion of Cdc42 causes defective B-cell development in bone marrow. (A) Bone marrow cells from Mx-Cre;Cdc42loxp/loxp donor mice were transplanted by intravenous injection into lethally irradiated BoyJ mice. Recipient mice were injected with polyI:C to delete Cdc42 in hematopoietic stem cells. (B) Bone marrow cells and splenocytes were isolated from WT and Cdc42−/− (KO) mice, counted, stained with anti-B220 antibody, and then analyzed by flow cytometry. The number of B220+ cells was calculated by multiplying the total number of bone marrow cells and splenocytes by the percentage of B220+ cells (**P < .01; *P < .05). The data are means ± SD. (C) Bone marrow cells were stained for IL-7R, lineage (Lin) markers (ie, B220, CD3, CD4, CD8, Gr1, CD11b, and TER119), Sca-1, and c-Kit followed by flow cytometric analysis. Common lymphoid progenitor (CLP) cells (Sca1medc-kitmed-high) were gated from Lin−IL-7R+ cell population. Data are representative of 4 mice. (D) Bone marrow cells were stained with antibodies against B220 and IgM and, using flow cytometry, analyzed for proB/preB cells ((B220lowIgM−), immature B cells (B220lowIgM+), and mature recirculating B cells (B220highIgM+). Data are representative of 4 mice.
Discussion
In this study, we have shown that B cell–specific deletion of Cdc42 causes a defect in late B-cell development in spleen, resulting in a reduction in mature MZ and FO B cells. Consistent with the altered B-cell development, antibody-producing abilities of Cdc42-deficient B cells in response to antigen challenge are impaired. Also consistent are our findings that cell proliferation and survival—as well as BCR- and BAFF-R–induced signaling—are aberrant in Cdc42-deficient B cells. Finally, using a hematopoietic stem cell–specific Cdc42 gene-targeted mouse model, we are able to shown that Cdc42 deficiency also results in a defect in early B-cell development in bone marrow. Collectively, these data suggest that Cdc42 plays a critical role in early and late B-cell development, B-cell survival, proliferation, and B-cell signaling transduction.
Cdc42-deficient mice have reduced numbers of MZ and B-1a B cells. These 2 B-cell subsets are thought to play important roles in natural antibody production and T cell–independent antigen responses.3,35,36 However, levels of natural antibodies—such as IgM, IgG, and IgA—in Cdc42-deficient mice were normal; presumably the residual MZ and B-1 B cells are sufficient to produce normal levels of natural antibodies. In contrast, mice lacking Rac2 display abnormal production of most natural antibodies.38 On the other hand, we did observe defective production of IgM and IgG3 when Cdc42-null mice were immunized with a T cell–independent antigen, TNP-Ficoll; this suggests a malfunction of Cdc42-deficient MZ and/or B-1a cells in response to antigen challenge. Disruption of Cdc42 also leads to reduced number of FO B cells, which principally mediate T-dependent immune responses.1,2,4 Consistent with this finding, T-dependent antigen-specific IgM and IgG1 were decreased in Cdc42-deficient mice.
Cdc42-deficient B cells proliferate poorly in response to BCR activation. The proliferative response of Cdc42-deficient B cells to LPS is also attenuated, suggesting that Cdc42 transduces mitogenic signals emanating from Toll-like receptor 4. This feature of Cdc42-deficient B cells is difficult to compare with Rac2-deficient B cells. In response to LPS stimulation, Rac2-deficient B cells proliferate normally,38 whereas another paper demonstrates a proliferative defect.17
Cdc42 has been shown to be essential for cell survival in various cell types.6 Consistently, we found that Cdc42 is required for BCR- and BAFF-mediated B-cell survival, and that Cdc42 regulates B-cell survival—and proliferation—via BCR and BAFF-R signaling. Specifically, Cdc42-deficient B cells failed to appreciably activate Akt in response to either BCR activation or BAFF stimulation. Further, BAFF-stimulated activation of nuclear factor κB (NF-κB) is also impaired as manifested by decreased phosphorylation of NF-κB activation inhibitor, IκB. Given the important role of Akt and NF-κB in B-cell proliferation and survival,39,40 it is relevant that Cdc42 regulates BCR- and BAFF-mediated cell proliferation and survival, at least in part, through the Akt and NF-κB signaling pathways. It will be interesting to determine whether signals downstream of Akt, such as Bcl-xL and Mcl-1, are affected in the absence of Cdc42. Indeed, Bcl-xL is down-regulated in Rac2−/− B cells.17
MAP kinase family members—including Erk, JNK, and p38—have been reported to transduce BCR signaling leading to cell growth and survival.39,,,–43 However, the role of MAP kinases in cell growth and survival are controversial.42,44,,,–48 For example, MAP kinases have been shown to promote both activation and death of B cells. Other studies suggest that B-cell apoptosis depends on activation of Erk, but not JNK, or vice versa. We show that after BCR stimulation, phosphorylation of Erk is markedly increased in both WT and Cdc42−/− B cells. Because BCR stimulation decreases apoptosis in WT and Cdc42-deficient B cells, these results suggest that Erk activation may protect cells from death. On the other hand, Erk activity is higher in BCR cross-linking–activated Cdc42-deficient B cells than that in activated WT cells. Because activated Cdc42-deficient B cells are more susceptible to apoptosis that activated WT cells, these data suggest that Erk promotes cell death. Collectively, our findings lend support to the hypothesis that although modest Erk activation stimulates cell apoptosis, strong Erk activation is protective.49 Furthermore, it appears that Cdc42 regulates BCR-mediated B-cell proliferation and survival independent of JNK or p38 activation.
Consistent with previous reports,50 we found that BCR cross-linking up-regulates BAFF-R expression. We show that this up-regulation is mediated by Cdc42—a finding that is reminiscent of the role of Rac1 and Rac2 in up-regulating BAFF-R.17 The up-regulation of BAFF-R by BCR activation might account for the synergistic effect of BCR cross-linking and BAFF on B-cell survival. Our data also suggest that BCR signals might converge with BAFF-R signals in the regulation of B-cell proliferation and survival.
As discussed above, the B-cell developmental phenotypes of Cdc42−/− mice show similarities as well as differences from Rac1−/−/Rac2−/− mice. Similar to Cdc42 knockout, depletion of Rac1 and Rac2 in B cells leads to reductions in all splenic B-cell subpopulations and B-1a B cells, as well as defects in B-cell proliferation and survival.17 Distinct from Cdc42 knockout that has no effect on B-cell migration, disruption of Rac2 results in impaired B-cell migration.38 It appears then that both Cdc42 and Rac play an important role in late B-cell development in spleen, and in B-cell survival and proliferation. Whereas Rac is essential for B-cell migration, Cdc42 is not required. In contrast, a study using dominant-negative mutant suggested a role of Cdc42 in B-cell cytoskeleton regulation and migration14,15 ; however, the nonspecificity of the dominant-negative mutant raises concerns about the legitimacy of these findings.
Cdc42-deficient mice and mice lacking components of the BCR signaling pathway (ie, Btk, PKCβ, BLNK/SLP-65, PLC-γ2, PI 3-kinase subunit p85α, p110δ, Vav1/Vav2, and Rac1/Rac2) share X-linked immunodeficiency-like (xid-like) defects in B-cell development, proliferation, and survival.51,,,,,–57 Clearly, further effort is needed to delineate the functional relationships between Cdc42 and these BCR signaling elements. Future investigations will also be directed toward analyzing the mechanisms underlying impaired antigen-specific antibody production, by examining terminal differentiation of mature B cells into antibody-secreting plasma cells.
Finally, B cell–specific targeting of Cdc42 did not reveal differences in early B-cell development in bone marrow—perhaps as the result of incomplete deletion of Cdc42 in early B-cell progenitors. On the other hand, our hematopoietic stem cell–specific Cdc42 gene-targeted mouse model revealed a crucial role for Cdc42 in B-cell progenitor differentiation. The mechanisms underlying this defective early B-cell development are under investigation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants R01 HL085362 and R01 CA105117.
National Institutes of Health
Authorship
Contribution: F.G. designed and performed research, contributed vital new reagents or analytical tools, analyzed data, and wrote the paper; C.S.V. performed research, contributed analytical tools, and analyzed data; H.L.G. contributed vital new reagents or analytical tools and analyzed data; and Y.Z. designed research, contributed vital new reagents or analytical tools, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zheng, Division of Experimental Hematology, Children's Hospital Research Foundation, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: yi.zheng@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal