It is widely accepted that destruction of CD4+ T cells and viral load are the primary markers for immunodeficiency in HIV-1–infected humans and in simian immunodeficiency virus (SIV)–infected macaques. However, monocyte/macrophages are also important targets of HIV/SIV infection and a critical link between innate and adaptive immunity. We therefore examined whether changes in cells of the monocyte/macrophage lineage could be linked to the pathogenesis of AIDS in the rhesus macaque model. Here, we show that massive turnover of peripheral monocytes associated with death of tissue macrophages correlates with AIDS progression in macaques. More importantly, the level of monocyte turnover was not linked to the CD4+ T-cell count and was a better predictive marker for AIDS progression than was viral load or lymphocyte activation. Our results show the importance of monocyte/macrophages in the pathogenesis of AIDS and suggest the dynamic changes of the monocyte/macrophages as a new marker for AIDS progression.
Introduction
Destruction of CD4+ T cells is considered to be the main cause of immunodeficiency resulting from HIV infection in humans as well as in the simian immunodeficiency virus (SIV)–infected macaque model of AIDS. However, the degree of CD4+ T-cell depletion does not always correlate with AIDS progression.1 It has also been shown that chronic immune activation predicts disease progression better than viral load.2 Recently, microbial translocation from a damaged intestine has been suggested as a major driver of this immune activation.3
In addition to a direct effect on the adaptive immune system through destruction of CD4+ T cells, the innate immune system may also be negatively affected by HIV infection. The presence of certain opportunistic infections in AIDS such as Pneumocystis carinii (P carinii) and Cryptococcus neoformans is supportive of such a hypothesis. Both of these agents are present in the environment, and humans are continuously exposed to them without any sign of infection. The fact that greater than 55% of infants seroconvert to P carinii by 20 months of age but 16% to 45.5% of healthy adults are still seronegative4,5 suggests that innate immunity may be sufficient to preclude the development of specific acquired immunity. The fact that opportunistic infections such as P carinii frequently occur in AIDS suggests that HIV infection may be doing more than destroying CD4+ T cells and crippling adaptive immune responses.
Macrophages, an important component of the innate immune system and link between innate and acquired immunity, are also important targets of HIV/SIV infection. Thus, this study focuses on the importance of monocyte/macrophages in the pathogenesis of simian AIDS. Tissue macrophages originate from either the intravascular pool of monocytes6 or from precursors in the bone marrow.7 In the bone marrow, monocytes originate from stem cells that undergo at least 3 stages of differentiation (monoblast, promonocyte, and monocyte) before they are released into the circulation. The circulating half-life of monocytes in normal circumstances has been shown to be approximately 71 hours in humans,8 approximately 42 hours in rats,9 and 17.4 hours in mice.10 The increase in tissue macrophages at sites of infection is accompanied by an increase in monocyte turnover in the circulation,7,10 and most of these tissue macrophages are derived from the circulating pool of monocytes. Most studies of monocyte kinetics have been performed with the use of radioisotopes.7,9,,–12 However, recently, the thymidine analog BrdU was successfully used to monitor monocyte kinetics by flow cytometry.13 In the study described here, we used in vivo BrdU labeling to monitor and compare dynamic changes of monocyte/macrophages from bone marrow to peripheral tissues in SIV-infected monkeys to assess the importance of monocyte/macrophages in determining the tempo of AIDS progression.
Methods
Monkeys, infection, and BrdU inoculation
Seventeen uninfected and 29 SIV- or simian human immunodeficiency virus (SHIV)–infected adult rhesus macaques (Macaca mulatta) were used in this study (Table 1). Four of 17 uninfected adult rhesus monkeys were intravenously infected with SIVmac239 for the longitudinal monocyte turnover study. All monkeys were housed at the Tulane National Primate Research Center in accordance with the standards of the Center's Animal Care and Use Committee and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. For in vivo BrdU pulse labeling, 30 mg/mL filter-sterilized BrdU, pH 7.2 (Sigma-Aldrich) was prepared in PBS (Ca/Mg-free; Mediatech) and administered by intraperitoneal or intravenous inoculation at 60 mg/kg body weight. Blood and bone marrow samples were collected at 24 hours or at different time points after BrdU injection as indicated in the figures.
SIV- or SHIV-infected rhesus macaques used in this study
| Animal no. . | Virus strain . | dpi* when inoculated with BrdU . | dpi* when euthanized . | Cause of death† . |
|---|---|---|---|---|
| CN77 | SIVmac251 | 78 | 79 | AIDS |
| BE86 | SIVmac251 | 826 | 827 | AIDS |
| EL95 | SIVmac251 | 54 | 55 | AIDS |
| EB54 | SIVmac251 | 497 | 498 | AIDS |
| BH25 | SIVmac251 | 1016 | 1017 | AIDS |
| BT51 | SIVmac251 | 812 | 1044 | AIDS‡ |
| DN85 | SIVmac251 | 89 | 90 | Non-AIDS |
| DR55 | SIVmac251 | 90 | 91 | Non-AIDS |
| EB31 | SIVmac251 | 89 | 90 | Non-AIDS |
| ED13 | SIVmac251 | 89 | 90 | Non-AIDS |
| EB17 | SIVmac251 | 111 | 112 | Non-AIDS |
| CE45 | SIVmac239 | 182 | 512 | AIDS‡ |
| FA97 | SIVmac239 | 182 | 316 | AIDS‡ |
| FB04 | SIVmac239 | 182 | 904§ | Still alive‡ |
| V754 | SIVmac239 | 182 | 792 | AIDS‡ |
| CT83 | SIVmac239 | 798 | 1062 | Non-AIDS |
| DD19 | SIVmac239 | 385 | 811 | Non-AIDS‡ |
| DR58 | SIVmac239 | 385 | 558 | Non-AIDS |
| EC40 | SIVmac239 | 385 | 597 | Non-AIDS |
| EN51 | SIVmac239 | 167 | 552 | Non-AIDS |
| EN84 | SIVmac239 | 385 | 569 | AIDS‡ |
| N708 | SIV B670 | 1191 | 1192 | Non-AIDS |
| BE63 | SHIV Ku1 | 135 | 387 | AIDS‡ |
| BV98 | SHIV Ku1 | 135 | 1198§ | Still alive‡ |
| CT81 | SHIV Ku1 | 119 | 122 | Non-AIDS |
| CR80 | SHIV 162p4 | 135 | 407 | Non-AIDS |
| CA50 | SHIV 162p4 | 149 | 407 | AIDS‡ |
| CL85 | SHIV 162p4 | 119 | 122 | Non-AIDS |
| Animal no. . | Virus strain . | dpi* when inoculated with BrdU . | dpi* when euthanized . | Cause of death† . |
|---|---|---|---|---|
| CN77 | SIVmac251 | 78 | 79 | AIDS |
| BE86 | SIVmac251 | 826 | 827 | AIDS |
| EL95 | SIVmac251 | 54 | 55 | AIDS |
| EB54 | SIVmac251 | 497 | 498 | AIDS |
| BH25 | SIVmac251 | 1016 | 1017 | AIDS |
| BT51 | SIVmac251 | 812 | 1044 | AIDS‡ |
| DN85 | SIVmac251 | 89 | 90 | Non-AIDS |
| DR55 | SIVmac251 | 90 | 91 | Non-AIDS |
| EB31 | SIVmac251 | 89 | 90 | Non-AIDS |
| ED13 | SIVmac251 | 89 | 90 | Non-AIDS |
| EB17 | SIVmac251 | 111 | 112 | Non-AIDS |
| CE45 | SIVmac239 | 182 | 512 | AIDS‡ |
| FA97 | SIVmac239 | 182 | 316 | AIDS‡ |
| FB04 | SIVmac239 | 182 | 904§ | Still alive‡ |
| V754 | SIVmac239 | 182 | 792 | AIDS‡ |
| CT83 | SIVmac239 | 798 | 1062 | Non-AIDS |
| DD19 | SIVmac239 | 385 | 811 | Non-AIDS‡ |
| DR58 | SIVmac239 | 385 | 558 | Non-AIDS |
| EC40 | SIVmac239 | 385 | 597 | Non-AIDS |
| EN51 | SIVmac239 | 167 | 552 | Non-AIDS |
| EN84 | SIVmac239 | 385 | 569 | AIDS‡ |
| N708 | SIV B670 | 1191 | 1192 | Non-AIDS |
| BE63 | SHIV Ku1 | 135 | 387 | AIDS‡ |
| BV98 | SHIV Ku1 | 135 | 1198§ | Still alive‡ |
| CT81 | SHIV Ku1 | 119 | 122 | Non-AIDS |
| CR80 | SHIV 162p4 | 135 | 407 | Non-AIDS |
| CA50 | SHIV 162p4 | 149 | 407 | AIDS‡ |
| CL85 | SHIV 162p4 | 119 | 122 | Non-AIDS |
dpi indicates days postinfection.
AIDS was defined when AIDS was diagnosed by pathology at necropsy. Non-AIDS was defined when no classic sign of AIDS was detected by pathology at necropsy.
Animals used for Figure 5D.
Animals still alive at indicated date.
White blood cell and monocyte counts
The counts of white blood cells (WBCs) and monocytes at various time points were analyzed on the ADVIA 120 Hematology System (Bayer Diagnostics).
Flow cytometry
Whole-blood and bone marrow samples were obtained and stained for flow cytometric analysis as described previously.14 Eight-parameter flow cytometric analysis was performed on a 2-laser FACSAria (Becton Dickinson). The following monoclonal antibodies (mAbs) were used in this study: anti–human CD3 (SP34-2; BD Biosciences), CD20 (B9E9; Beckman Coulter), CD8 (SK1; BD Biosciences), HLA-DR (L243; BD Biosciences), CD16 (3G8; BD Biosciences), CD14 (M5E2; BD Biosciences), anti–nonhuman primate CD45 (MB4-6D6; Miltenyi Biotec), anti-BrdU mAb, and anti–Ki-67 mAb (BD Biosciences). Data analysis was performed with the use of the FlowJo Version 6.0 software (TreeStar).
Histopathology and multilabel confocal microscopy
Formalin-fixed, paraffin-embedded mesenteric lymph nodes from uninfected and SIV-infected monkeys were sectioned at 6 μm and used for immunofluorescence confocal microscopy studies. Tissues were incubated for 1 hour at room temperature with mouse anti–human CD163 mAb (IgG1; 10D6; 1:50; Novocastra) and rat anti-BrdU mAb (IgG2a; BU1/75; 1:50; Novus Biologicals) followed by Alexa 568–conjugated goat anti–rat IgG (1:1000; Invitrogen) and Alexa 633–conjugated goat anti–mouse IgG1 (1:1000; Invitrogen) for 30 minutes. Apoptotic cells were detected with the ApopTag Plus Fluorescein In Situ Apoptosis Detection kit (Chemicon International) for the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems). Individual optical slices represent 0.2 μm, and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (Version 1.62; NIH) and Adobe Photoshop (Version 7.0; Adobe Systems) were used to assign colors to 3 channels collected (3 fluorochromes: Alexa 633 [blue], Alexa 568 [red], and fluorescein [green]). An oil objective 40× fluotar/NA 1.0 was used to image the slides. The number of positive cells was expressed as the mean plus or minus SD of 5 different fields each (375 μm × 375 μm/field) from follicle and cortical sinus areas.
Plasma RNA levels
Plasma viral RNA levels were measured by an ultrasensitive branched DNA amplification assay with detection limit of 125 copies/mL (Bayer Diagnostics).
Mathematical model to estimate monocyte turnover
To achieve a systemwide perspective of the kinetic capacity and turnover of the monocyte population, the short-term increase and disappearance of the labeled monocyte population after a single BrdU injection was calculated. Representative data of the percentage of labeled monocytes from 0 to 6 days after BrdU administration for the 4 animals exhibiting either a high or low percentage of labeled monocytes at 24 hours are given in Table 2. The production or rate of emigration of monocytes from bone marrow, s, and the loss rate by distribution into tissue, δ, were mathematically calculated with the experimental data in Table 2 and compared as shown in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Such analysis was implemented with the assumption that the bolus administration of BrdU resulted in instantaneous labeling and established a sufficient concentration of BrdU such that every dividing monocyte progeny in bone marrow was labeled during the same period of time.
Calculated export/emigration rates of monocytes from bone marrow, s, and loss rates, δ for the 4 rhesus macaques
| Calculated rate parameters . | ||
|---|---|---|
| Animal no. (% BrdU+ monocytes at 24 h) . | Parameter . | |
| s . | δ . | |
| CA50 (38.1) | 6.790 | 0.163 |
| BT51 (21.6) | 5.692 | 0.135 |
| R842 (9.89) | 4.325 | 0.111 |
| N195 (2.67) | 4.149 | 0.093 |
| Calculated rate parameters . | ||
|---|---|---|
| Animal no. (% BrdU+ monocytes at 24 h) . | Parameter . | |
| s . | δ . | |
| CA50 (38.1) | 6.790 | 0.163 |
| BT51 (21.6) | 5.692 | 0.135 |
| R842 (9.89) | 4.325 | 0.111 |
| N195 (2.67) | 4.149 | 0.093 |
Statistical analysis
The Mann-Whitney U test, the Wilcoxon matched pairs test, the unpaired t test, the Spearman rank correlation test, and a repeated measure ANOVA, were performed with the use of Graphpad Prism software (GraphPad Software). In all cases, 2-tailed P values less than .05 were considered significant.
Results
Kinetics of BrdU-labeled monocytes in rhesus macaques
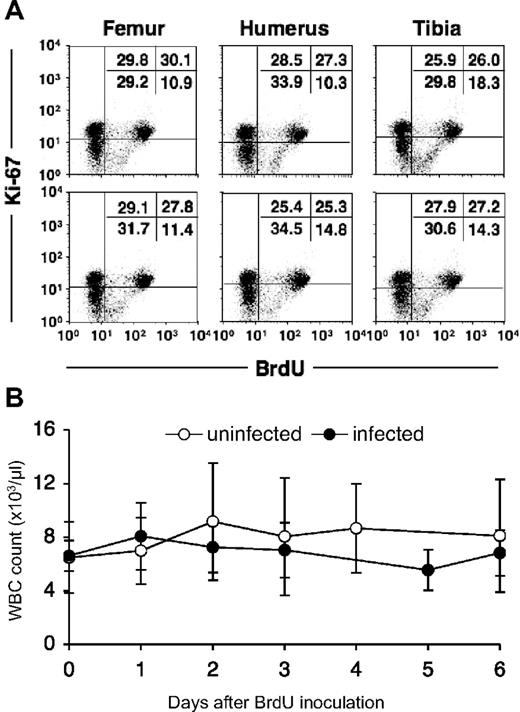
BrdU is a thymidine analog that is incorporated into cell DNA during DNA synthesis at the S-phase of the cell cycle. Therefore, it is considered a specific and reliable marker for dividing cells. Monocytes are derived from progenitor cells in bone marrow, circulate in the blood, and then enter tissues and further differentiate into macrophages. Cell division in this lineage occurs at the myelo/monoblast, promonocyte stage in bone marrow,7,12,15,–17 and monocytes are released into the circulation after completion of the S-phase.11 Cell-cycle studies have shown that newly formed monocytes cannot be induced to synthesize DNA or to undergo mitosis18,19 ; therefore, BrdU-labeled monocytes can be defined as having recently emigrated from bone marrow. To confirm uniform BrdU incorporation of dividing cells, bone marrow was collected from 6 different sites (right and left humeri; right and left femurs; right and left tibias) at 24 hours after intravenous injection of a single dose (60 mg/kg) of BrdU. Percentages of BrdU-labeled bone marrow cells from the different sites were nearly identical, showing efficient and uniform labeling by a single intravenous dose of BrdU (Figure 1A).
In vivo BrdU labeling of bone marrow cells in a rhesus macaque. (A) Bone marrow cells obtained from 6 distant bones (right and left humeri, femurs, and tibias) 24 hours after a single dose (60 mg/kg) of BrdU. The cells were stained for the presence of BrdU and Ki-67. Ki-67 was used to better distinguish BrdU+ cells. Numbers in the top right quadrants indicate the percentages of BrdU+ Ki67 bright bone marrow cells. (B) In vivo BrdU labeling does not affect total white blood cell counts. The total white blood cell (WBC) number in blood was monitored after BrdU injection in 7 uninfected (○) and 9 SIV/SHIV-infected (●) rhesus monkeys. Data show means ± SDs of each group per time point. Statistical analysis was performed by a repeated measure ANOVA. Data at each time point were also compared with data at time point 0 by a Dunnett test.
In vivo BrdU labeling of bone marrow cells in a rhesus macaque. (A) Bone marrow cells obtained from 6 distant bones (right and left humeri, femurs, and tibias) 24 hours after a single dose (60 mg/kg) of BrdU. The cells were stained for the presence of BrdU and Ki-67. Ki-67 was used to better distinguish BrdU+ cells. Numbers in the top right quadrants indicate the percentages of BrdU+ Ki67 bright bone marrow cells. (B) In vivo BrdU labeling does not affect total white blood cell counts. The total white blood cell (WBC) number in blood was monitored after BrdU injection in 7 uninfected (○) and 9 SIV/SHIV-infected (●) rhesus monkeys. Data show means ± SDs of each group per time point. Statistical analysis was performed by a repeated measure ANOVA. Data at each time point were also compared with data at time point 0 by a Dunnett test.
To examine possible confounding effects of BrdU on leukocyte homeostasis, absolute white blood cell (WBC) counts in blood were monitored after BrdU injection in 7 uninfected and 9 SIV/SHIV-infected rhesus monkeys (Figure 1B); no significant change in the number of WBCs after BrdU injection was observed in either group of animals.
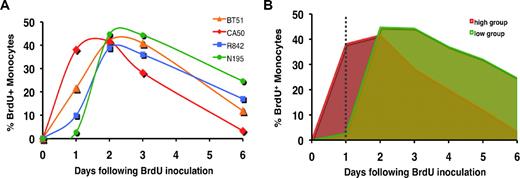
To accurately investigate the dynamics of the BrdU-labeled monocytes, we first established a standardized method for data collection. The appearance and disappearance of BrdU+ monocytes were compared at different time points through 6 days after BrdU administration (Figure 2). Among 4 macaques, considerable variation in the percentage of BrdU+ monocytes was observed before or after the 48-hour sampling time point. Of particular note was that distinct kinetic patterns of BrdU labeling of monocytes were observed. Specifically, animal CA50 with the highest percentage of BrdU+ monocytes at 24 hours after BrdU injection had the lowest percentage at 6 days and thus the greatest rate of disappearance of these cells from circulation. In contrast, animal N195 with the lowest percentage of BrdU+ monocytes at 24 hours had the highest percentage at 6 days (Figure 2A). In fact, the rank ordering of the percentage of BrdU+ monocytes in these animals at 24 hours was reversed at 6 days. These results suggest that the kinetic pattern of very rapid appearance and disappearance of large percentages of BrdU+ monocytes is a result of rapid transit of such cells through the blood from bone marrow to tissues.
Percentage of BrdU-labeled monocytes at 24 hours after BrdU administration as a reliable marker of monocyte turnover. (A) Kinetics of BrdU-labeled monocytes of 4 rhesus macaques after BrdU injection. Blood monocytes were defined as CD14+ cells after gating through CD45 and side scatter plots. Any T lymphocytes, B cells, and NK cells were further excluded by gating on CD3−CD8−CD20−HLA-DR+CD45+ population. Data shown is the percentage of BrdU+ monocytes at indicated time points after BrdU injection. Note the inverse relation in the rank order of the percentage of BrdU labeling at 1 day versus 6 days. (B) Percentage of BrdU-labeled monocytes observed at 24 hours after BrdU administration inversely correlates with the time those cells spend in circulation. In other words, animals with the greatest percentage of CD14+BrdU+ cells at 1 day have the lowest percentage of CD14+ cells by 6 days after labeling. Data show the kinetics of BrdU-labeled monocytes from animal CA50 (red) and N195 (green) that exhibit a high and low percentage of labeled monocytes 24 hours after BrdU administration, respectively. The dotted line highlights the differences in the dynamic behavior of the 2 representative animals within 24 hours of BrdU injection that correlate with the decline of the BrdU+ monocytes in circulation.
Percentage of BrdU-labeled monocytes at 24 hours after BrdU administration as a reliable marker of monocyte turnover. (A) Kinetics of BrdU-labeled monocytes of 4 rhesus macaques after BrdU injection. Blood monocytes were defined as CD14+ cells after gating through CD45 and side scatter plots. Any T lymphocytes, B cells, and NK cells were further excluded by gating on CD3−CD8−CD20−HLA-DR+CD45+ population. Data shown is the percentage of BrdU+ monocytes at indicated time points after BrdU injection. Note the inverse relation in the rank order of the percentage of BrdU labeling at 1 day versus 6 days. (B) Percentage of BrdU-labeled monocytes observed at 24 hours after BrdU administration inversely correlates with the time those cells spend in circulation. In other words, animals with the greatest percentage of CD14+BrdU+ cells at 1 day have the lowest percentage of CD14+ cells by 6 days after labeling. Data show the kinetics of BrdU-labeled monocytes from animal CA50 (red) and N195 (green) that exhibit a high and low percentage of labeled monocytes 24 hours after BrdU administration, respectively. The dotted line highlights the differences in the dynamic behavior of the 2 representative animals within 24 hours of BrdU injection that correlate with the decline of the BrdU+ monocytes in circulation.
To further assess this possibility we used mathematical modeling to help interpret the results. Similar use of mathematical modeling from in vivo labeling studies has contributed significantly to our understanding of T-lymphocyte turnover, specifically in the immune response to HIV infection.20,,–23 As such, to better interpret the kinetic patterns of monocyte labeling observed in the 4 animals after a single bolus infusion of BrdU, we elected to implement a simple mathematical model governed by the following equation: dM/dt = s − δM.
The use of this model has been validated and applied successfully in the analysis of monocyte labeling data in HIV infection.22,24 For our implementation, the parameter, s, is defined as the source or rate of export/emigration of BrdU-labeled monocytes, M(t), from the bone marrow into peripheral circulation, and therefore corresponds to a daily production of monocytes. We assume that proliferation of monocytes occurs during maturation in the bone marrow and that they appear in the periphery from this source already labeled. The parameter, δ, is the loss rate or rate of distribution of these cells from blood into tissue. Thus, δ accounts for the 2 cellular fate processes of death and emigration from the periphery as a combined loss. Analysis of this model showed that the observed differences in the pattern of BrdU labeling on monocytes at 24 hours are mostly due to differences in monocyte production, s. However, the observation that the peaks of labeling were similar (Figure 2A) among these animals indicates that the loss rates (distribution to tissue), δ, are also different and proportional to the production, s. Such behavior in the kinetic pattern exists because the peak of labeling is given by s/δ. Moreover, these differences in loss rate among the animals are further confirmed by the de-labeling kinetics or disappearance of monocytes, in which we found that the animals with a larger production or output of monocytes from bone marrow, s, were also shown to lose labeled cells faster (Figure 2A-B). This difference in loss rate among the 4 animals is statistically significant (P < .01, when analyzing the data from day 2 to day 6 with a linear mixed effects model; supplemental Table 1, Table 2). The focus of the remaining analysis is on the labeling at 24 hours, because it was determined to be a good proxy for the production of monocytes and emigration into the blood from bone marrow and also a good predictor of the eventual distribution of monocytes into tissue.
Increased monocyte turnover in SIV/SHIV-infected rhesus macaques
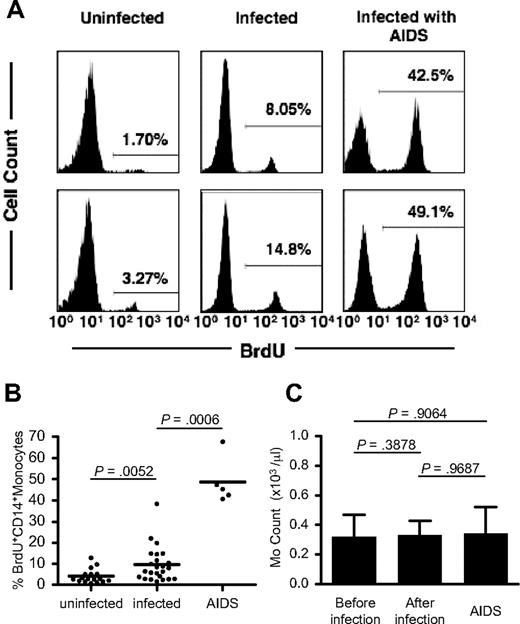
We next compared the percentage of BrdU-labeled peripheral blood monocytes in uninfected macaques, chronically SIV- or SHIV-infected macaques, and macaques with AIDS at 24 hours after BrdU administration (Figure 3A-B). Figure 3B clearly shows that the average percentage of BrdU-labeled monocytes from 24 chronically infected animals (9.5%; range, 1.63%-38.3%) is significantly higher than that from uninfected animals (n = 17; 4.1%; range, 0.75%-12.9%; P < .01). The highest percentage of BrdU+ monocytes (n = 5; 48.7%; range, 40.7%-67.8%) was observed in animals with clinical symptoms of AIDS (Figure 3B).
Increased monocyte turnover in SIV/SHIV-infected rhesus macaques. (A) Histograms of BrdU-labeled blood monocytes in uninfected and SIV/SHIV-infected animals and in animals with AIDS at 24 hours after BrdU treatment. Representative data from 2 animals per group are shown. Percentage values in histograms indicate BrdU+ CD14+ monocytes. (B) The percentage of BrdU-labeled monocytes in uninfected (n = 17) and chronically SIV- or SHIV-infected monkeys (n = 24) and in monkeys with AIDS (n = 5). P values were calculated by the Mann-Whitney U test. More information on the infected monkeys used in this study is provided in Table 1. Horizontal bars indicate the average percentage of BrdU+ CD14+ monocytes in each group. (C) Blood monocyte counts in monkeys before (n = 17; total 155 time points) and after infection (n = 17; total 156 time points) and in other monkeys with AIDS (n = 5; total 5 time points). Each bar indicates the average of monocyte counts in an individual monkey. P values were calculated by the Wilcoxon matched pairs test for the comparison between before and after infection and by the Mann-Whitney U test for the comparison between before infection and AIDS group. Error bars indicate SD of monocyte counts.
Increased monocyte turnover in SIV/SHIV-infected rhesus macaques. (A) Histograms of BrdU-labeled blood monocytes in uninfected and SIV/SHIV-infected animals and in animals with AIDS at 24 hours after BrdU treatment. Representative data from 2 animals per group are shown. Percentage values in histograms indicate BrdU+ CD14+ monocytes. (B) The percentage of BrdU-labeled monocytes in uninfected (n = 17) and chronically SIV- or SHIV-infected monkeys (n = 24) and in monkeys with AIDS (n = 5). P values were calculated by the Mann-Whitney U test. More information on the infected monkeys used in this study is provided in Table 1. Horizontal bars indicate the average percentage of BrdU+ CD14+ monocytes in each group. (C) Blood monocyte counts in monkeys before (n = 17; total 155 time points) and after infection (n = 17; total 156 time points) and in other monkeys with AIDS (n = 5; total 5 time points). Each bar indicates the average of monocyte counts in an individual monkey. P values were calculated by the Wilcoxon matched pairs test for the comparison between before and after infection and by the Mann-Whitney U test for the comparison between before infection and AIDS group. Error bars indicate SD of monocyte counts.
These data show that higher percentages of blood monocytes are released from bone marrow in SIV-infected macaques with or without symptoms of AIDS compared with uninfected controls. To determine whether the higher output of newly released monocytes in infected animals was due to increased turnover or simply increased production of monocytes, we determined the absolute number of blood monocytes from 17 animals before (155 different time points) and after (156 different time points) SIV or SHIV infection (Figure 3C). No significant difference in the peripheral blood monocyte count before or after SIV or SHIV infection was observed (P = .388). Most importantly, the group of SIV-infected animals with AIDS that had the highest percentage of BrdU+ monocytes maintained absolute monocyte counts that were no different from the other groups. Together, these data indicate that circulating monocyte turnover is higher in SIV- or SHIV-infected monkeys compared with uninfected controls and that this difference is particularly dramatic in SIV-infected macaques with AIDS.
Increased macrophage turnover in mesenteric lymph node from SIV-infected (AIDS) macaques
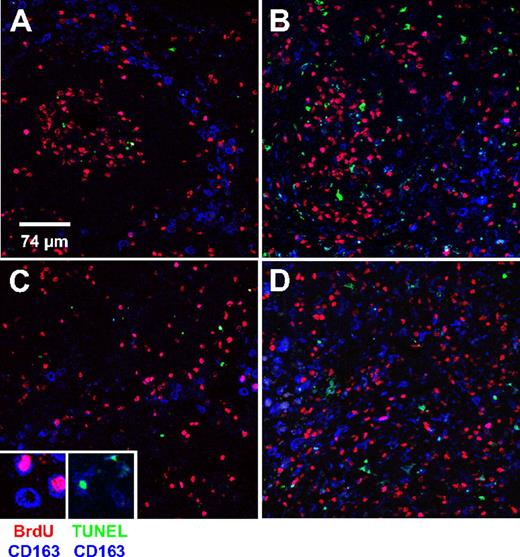
Homeostatic regulation of the number of peripheral blood monocytes is a result of equilibrium between monocyte release from bone marrow into circulation and the exit from circulation into tissues. To directly examine whether the high monocyte turnover we observed in SIV-infected macaques is due to increased recruitment of those cells into tissues, we examined recruitment and apoptosis of tissue macrophages in mesenteric lymph node. This was done by multilabel confocal microscopy with the use of CD163 to identify macrophages, BrdU labeling to identify newly recruited cells, and TUNEL to identify apoptotic cells. CD163 is restricted to cells of the monocyte/macrophage lineage and is up-regulated as monocytes differentiate into macrophages.25,26 The analysis of newly recruited (BrdU+CD163+) and apoptotic (TUNEL+CD163+) macrophages in mesenteric lymph node was performed on 2 SIV-infected and 2 uninfected macaques. Representative data are shown in Figure 4 and Table 3. Representative images of follicular (Figure 4A-B) and cortical sinus (Figure 4C-D) areas from the lymph nodes of the control (Figure 4A,C) and infected (Figure 4B,D) animals are shown. The number of BrdU+CD163+ cells per 375 × 375 μm2 field in the lymph node of the SIV-infected animal was approximately 15 times higher than that of the uninfected control. As expected, a higher percentage of TUNEL+CD163+ cells was also observed in the infected animal, providing direct evidence of high macrophage death rate as the direct cause of high monocyte turnover (Table 3).
Increased macrophages turnover in mesenteric lymph node from SIV-infected (AIDS) macaque. Lymph nodes were collected 24 hours after BrdU injection from uninfected and SIV-infected macaques. The percentages of BrdU+ monocytes in the uninfected and infected monkeys shown in this figure were 3.0% and 45.3%, respectively. Group data are shown in Table 3. Triple-label confocal microscopy was performed for CD163 (macrophage marker, blue), BrdU (red) to identify recently arrived macrophages, and TUNEL (green) to identify apoptotic macrophages. (A,B) From follicular areas and (C,D) from the cortical sinus areas in mesenteric lymph nodes from an uninfected animal (A,C) and an SIV-infected animal (B,D). Each panel is representative of 5 different fields of the follicular and the cortical sinus areas. Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems). An oil objective 40× fluotar/NA 1.0 was used to image the slides. Scale bar, 74 μm. Magnification, × 40.
Increased macrophages turnover in mesenteric lymph node from SIV-infected (AIDS) macaque. Lymph nodes were collected 24 hours after BrdU injection from uninfected and SIV-infected macaques. The percentages of BrdU+ monocytes in the uninfected and infected monkeys shown in this figure were 3.0% and 45.3%, respectively. Group data are shown in Table 3. Triple-label confocal microscopy was performed for CD163 (macrophage marker, blue), BrdU (red) to identify recently arrived macrophages, and TUNEL (green) to identify apoptotic macrophages. (A,B) From follicular areas and (C,D) from the cortical sinus areas in mesenteric lymph nodes from an uninfected animal (A,C) and an SIV-infected animal (B,D). Each panel is representative of 5 different fields of the follicular and the cortical sinus areas. Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems). An oil objective 40× fluotar/NA 1.0 was used to image the slides. Scale bar, 74 μm. Magnification, × 40.
High macrophage turnover in mesenteric lymph node of SIV-infected (AIDS) monkey
| Animal/area . | Total . | CD163+ cells (macrophage) . | ||||
|---|---|---|---|---|---|---|
| BrdU+ . | TUNEL+ . | Total . | BrdU+ . | TUNEL+ . | TUNEL+BrdU+ . | |
| Uninfected (3.0%)* | ||||||
| Sinus | 73.8 ± 26.6‡ | 6.6 ± 2.3 | 40.8 ± 2.9 | 2.0 ± 1.6 | 0.4 ± 0.5 | 0 |
| Follicle | 90.8 ± 25.1 | 7.4 ± 3.9 | 37.0 ± 18.4 | 2.0 ± 1.4 | 0.4 ± 0.5 | 0.2 ± 0.4 |
| Infected (45.3%) | ||||||
| Sinus | 189.0 ± 25.1 | 27.6 ± 7.8 | 194.6 ± 11.0 | 33.6 ± 3.5 | 11.0 ± 1.4 | 2.0 ± 1.0 |
| Follicle | 169.2 ± 87.5 | 33.8 ± 11.9 | 162.4 ± 43.2 | 27.0 ± 7.0 | 10.2 ± 1.8 | 1.0 ± 0.7 |
| P† | ||||||
| Sinus | .0001‖ | .0004 | < .0001 | < .0001 | < .0001 | na§ |
| Follicle | .0904‖ | .0015 | .0003 | < .0001 | < .0001 | .0650‖ |
| Animal/area . | Total . | CD163+ cells (macrophage) . | ||||
|---|---|---|---|---|---|---|
| BrdU+ . | TUNEL+ . | Total . | BrdU+ . | TUNEL+ . | TUNEL+BrdU+ . | |
| Uninfected (3.0%)* | ||||||
| Sinus | 73.8 ± 26.6‡ | 6.6 ± 2.3 | 40.8 ± 2.9 | 2.0 ± 1.6 | 0.4 ± 0.5 | 0 |
| Follicle | 90.8 ± 25.1 | 7.4 ± 3.9 | 37.0 ± 18.4 | 2.0 ± 1.4 | 0.4 ± 0.5 | 0.2 ± 0.4 |
| Infected (45.3%) | ||||||
| Sinus | 189.0 ± 25.1 | 27.6 ± 7.8 | 194.6 ± 11.0 | 33.6 ± 3.5 | 11.0 ± 1.4 | 2.0 ± 1.0 |
| Follicle | 169.2 ± 87.5 | 33.8 ± 11.9 | 162.4 ± 43.2 | 27.0 ± 7.0 | 10.2 ± 1.8 | 1.0 ± 0.7 |
| P† | ||||||
| Sinus | .0001‖ | .0004 | < .0001 | < .0001 | < .0001 | na§ |
| Follicle | .0904‖ | .0015 | .0003 | < .0001 | < .0001 | .0650‖ |
Percentages of BrdU+ monocytes in blood are indicated.
Data were analyzed for statistical significance by the unpaired t test.
Each value represents mean ± SD of the cell number per area obtained from 5 randomly selected areas.
na indicates not applicable.
Not significant (P > .05).
Early high monocyte turnover correlates with AIDS progression in SIV-infected rhesus macaques
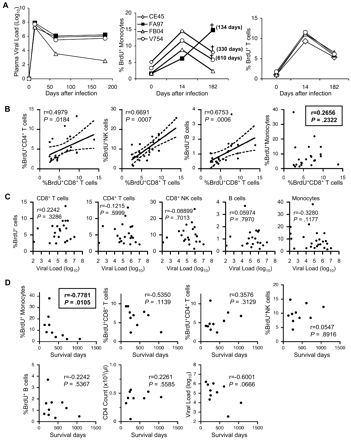
We next performed a longitudinal study in a cohort of 4 SIV-infected rhesus macaques to determine whether the kinetics of monocyte release from bone marrow or turnover as shown above correlated with disease progression. As previously found, plasma viral load in all 4 infected monkeys peaked within 14 days after infection.27,–29 One monkey (FB04) gradually controlled virus replication and stably maintained low viral load (100-500 copies/mL) at 120 days after infection, showing a classic pattern of virus replication seen in long-term nonprogressor animals. The remaining 3 animals reached their virus set point of nearly 106 virus copies/mL at 60 to 90 days after infection (Figure 5A left). As expected from previous studies,30,31 in all 4 animals, the CD4+CCR5+ (memory CD4+ T cells) cell count in blood and the percentage of CD4+ cells in intestine were greatly decreased by 21 days after infection (data not shown). Three of these animals had a high virus set point and low CD4+ T lymphocyte counts, consistent with animals progressing to AIDS. These animals received a single BrdU injection at different time points before and after SIV infection to monitor monocyte turnover as a function of time. All 4 animals had an increased percentage of BrdU+ blood monocytes at 14 days after infection (at the peak of viremia) compared with before infection (Figure 5A center). The next BrdU analysis was performed at 182 days after infection at a time when viral load had reached set point. Notably, 3 of the infected animals showed a drop in the level of monocyte turnover shown by the percentage of BrdU+ monocytes. Only 1 animal (FA97) showed a dramatic increase in the percentage of BrdU+ monocytes. Interestingly, this animal died of AIDS 134 days later. No correlation between viral load and the level of monocyte turnover was observed in these animals: 3 of the animals (FA97, CE45, and V754) had very similar viral loads throughout, but only FA97 showed a dramatic increase in monocyte turnover and rapid disease progression. Two of these animals (CE45 and V754) with high viral load showed a drop in monocyte turnover similar to the animal (FB04) that controlled virus replication and is still alive 904 days after infection. Both animals CE45 and V754 have died of AIDS at 330 and 610 days after BrdU injection. The high percentage of the BrdU+ monocytes in animal FA97 was not due to a difference in BrdU labeling efficiency because T lymphocytes from all animals showed homogeneous labeling kinetics in all 4 animals as previously reported (Figure 5A right).
High monocyte turnover predicts AIDS progression in SIV-infected rhesus macaques. (A) Kinetics of plasma viral load (left) in 4 SIV-infected rhesus macaques at multiple time points after SIV infection. The percentages of BrdU-labeled monocytes (middle) and CD3+ T lymphocytes (right) at indicated time points from the same 4 SIV-infected animals are shown. BrdU staining of peripheral blood was performed at 24 hours after BrdU injection at indicated time points. †Death of the animal. In parentheses is indicated the date the animal died after the last BrdU injection experiment (182 days after infection). (B) Correlation of BrdU+CD8+ T cells with other BrdU-labeled cells in infected monkeys. Note that the turnover of monocytes is not correlated with that of other cell types examined. (C) Relation between the percentage of BrdU-labeled cells and plasma viral loads in chronically SIV- or SHIV-infected rhesus macaques. Peripheral bloods were obtained from 21 infected macaques at 24 hours after BrdU inoculation and stained for BrdU. Dots represent individual animals. The Spearman rank test was used to determine the correlation between the percentage of BrdU+ cells and viral loads. (D) Correlation between the survival time after the in vivo BrdU experiment and the percentage of BrdU+ cells, CD4 counts, and plasma viral load at the time of the BrdU injection experiment. The only parameter that predicted progression to AIDS with statistical significance was high monocyte turnover. The Spearman rank correlation test was used to determine correlations and P values. The regression line and the 95% confidence intervals are shown with continuous and broken lines, respectively (applied only for panel B).
High monocyte turnover predicts AIDS progression in SIV-infected rhesus macaques. (A) Kinetics of plasma viral load (left) in 4 SIV-infected rhesus macaques at multiple time points after SIV infection. The percentages of BrdU-labeled monocytes (middle) and CD3+ T lymphocytes (right) at indicated time points from the same 4 SIV-infected animals are shown. BrdU staining of peripheral blood was performed at 24 hours after BrdU injection at indicated time points. †Death of the animal. In parentheses is indicated the date the animal died after the last BrdU injection experiment (182 days after infection). (B) Correlation of BrdU+CD8+ T cells with other BrdU-labeled cells in infected monkeys. Note that the turnover of monocytes is not correlated with that of other cell types examined. (C) Relation between the percentage of BrdU-labeled cells and plasma viral loads in chronically SIV- or SHIV-infected rhesus macaques. Peripheral bloods were obtained from 21 infected macaques at 24 hours after BrdU inoculation and stained for BrdU. Dots represent individual animals. The Spearman rank test was used to determine the correlation between the percentage of BrdU+ cells and viral loads. (D) Correlation between the survival time after the in vivo BrdU experiment and the percentage of BrdU+ cells, CD4 counts, and plasma viral load at the time of the BrdU injection experiment. The only parameter that predicted progression to AIDS with statistical significance was high monocyte turnover. The Spearman rank correlation test was used to determine correlations and P values. The regression line and the 95% confidence intervals are shown with continuous and broken lines, respectively (applied only for panel B).
To further examine whether high monocyte/macrophage turnover could be a unique indicator for AIDS progression, we systematically compared it with the turnover of other cell types, which have been linked to immune activation. We also examined other parameters that have been linked to disease progression, including CD4+ T-cell counts and viral load. First, we examined the in vivo incorporation of BrdU in different subsets of cells involved in the immune response at 24 hours after BrdU injection. Because a linear correlation between BrdU and Ki-67 staining was observed in different cell subsets, these markers could be used interchangeably to measure actively proliferating lymphocytes (r = 0.9022, P < .001; supplemental Figure 1A). As previously described,21,22,32 SIV- or SHIV-infected monkeys clearly showed statistically significant higher turnover of CD4 (P < .001) and CD8+ T cells (P < .001), natural killer (NK) cells (P < .001), and B cells (P = .007) shown by BrdU staining compared with the uninfected control group (supplemental Figure 2). Peripheral CD4+ T-cell numbers from the same infected animals were also significantly lower than that observed in control uninfected animals (P < .001; supplemental Figure 3). Importantly, because the percentage of BrdU+ T cells directly correlated with the percentage of MHC class II-DR molecule–expressing cells from the same animal, the degree of BrdU incorporation could similarly indicate activation status in peripheral lymphocytes (r = 0.7159, P < .001; supplemental Figure 1B). This is in agreement with previous studies that have shown generalized immune activation and increased cell turnover in SIV- or HIV-infected persons.2,21,22,32,,–35 Thus, when the BrdU status was compared among cell subsets for each individual animal, the degree of BrdU incorporation was directly correlated (CD8 vs CD4, r = 0.4979, P = .018; CD8 vs NK, r = 0.6691, P < .001; CD8 vs B cells, r = 0.6753, P < .001), suggesting that the turnover of these cell subsets may have a common driver (Figure 5B). Interestingly, however, monocyte turnover shown by the BrdU incorporation did not correlate with the turnover of the CD8+ T cells from the same animal (P = .232; Figure 5B). These data strongly suggest that the level of monocyte turnover is driven by an independent mechanism other than the generalized immune activation described for lymphocyte populations.2,33,–35 Furthermore, there was no correlation between the turnover of lymphocytes or monocytes and viral load (CD4+ T cells, P = .6; CD8+ T cells, P = .329; NK cells, P = .701; B cells, P = .797; and monocytes, P = .118; Figure 5C). However, when we compared the level of BrdU incorporation in these cell subsets with the length of time to succumb to AIDS after the assessment, the only parameter that predicted progression to AIDS with statistical significance was high monocyte turnover (r = −0.7781, P = .011; Figure 5D). These data strongly suggest that progressive damage in the monocyte/macrophage lineage reflected by high monocyte turnover during HIV/SIV infection may be an important factor dictating the tempo of AIDS progression. The high turnover of monocyte/macrophages may also play a role in development of some opportunistic infections, particularly those controlled largely by innate immunity.
Discussion
This study shows a high rate of monocyte turnover linked to macrophage destruction in tissues during SIV infection irrespective of CD4 count, T-cell turnover, or viral load in the macaque model of AIDS. More importantly, in our longitudinal study, the monkey with greatest monocyte turnover among a group of animals with similar peak viral load and viral set points rapidly progressed to AIDS. No differences in other parameters, including CD4+ lymphocyte counts and T-cell turnover in blood and gastrointestinal tissues including the CCR5+CD4+ cell subset, were found among these animals (data not shown). These data were strongly supported by the fact that chronically infected animals with significant increases in monocyte turnover die of AIDS faster compared with those infected monkeys with low monocyte turnover (Figure 5D). Importantly, the cell turnover found in other cell subsets (CD4+ T cells, CD8+ T cells, NK cells, and B cells) indicative of general immune activation was not correlated with viral load or survival time (Figure 5C-D). These data indicate that monocyte turnover may be an important parameter that predicts the tempo of AIDS disease. The high turnover of monocyte/macrophages may also play a role in the development of some opportunistic infections, particularly those controlled largely by innate immunity.
It is important to interpret our results in light of previous studies on leukocyte turnover in human HIV infection.22,24 In one study, which used deuterated glucose labeling, there seemed to be no differences between monocyte turnover in uninfected and infected subjects.22 However, that study only analyzed 3 uninfected and 2 infected persons, and in our study, we observed a substantial overlap between the turnover of monocytes in individual animals (supplemental Figure 2); thus, any significant differences may have been masked by the small number of people studied. In a second study,24 there were no uninfected subjects included; therefore, direct comparison cannot be made with our findings in uninfected versus infected macaques. However, that latter study, which also used BrdU, did find a negative correlation between monocyte production (s) and viral load. In our study, we found a similar trend (Figure 5C), but this did not reach statistical significance.
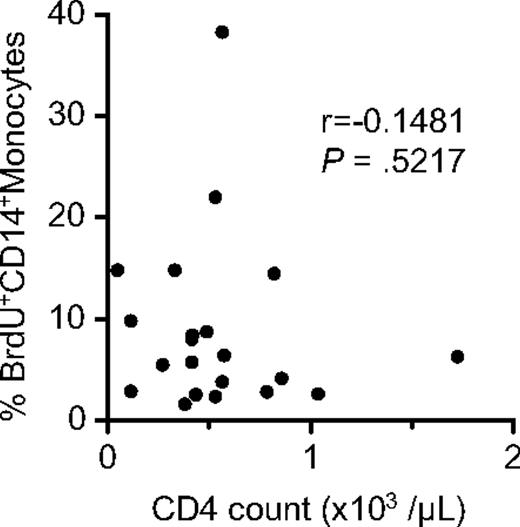
Because the degree of monocyte turnover was independent of CD4+ T-cell count and turnover, indicative of the CD4 damage (Figure 6), it will be important to elucidate the causes of tissue macrophage destruction that presumably drives increased production of monocytes during HIV and SIV infection. Destruction of tissue macrophages or any other end-stage cells derived from monocytes could be caused either directly through virus infection or by bystander effects of virus replication. It will also be important to show whether direct virus infection to monocytes contributes to increase the monocyte turnover.
No correlation between monocyte turnover and blood CD4 T-cell count in SIV- or SHIV-infected rhesus macaques. Peripheral blood samples were obtained from 21 chronically infected rhesus macaques at 24 hours after BrdU inoculation, and the percentage of BrdU-labeled monocytes and blood CD4 count were measured. The Spearman rank test was used to determine the correlation between the percentage of BrdU+CD14+ monocytes and CD4+ T-cell counts.
No correlation between monocyte turnover and blood CD4 T-cell count in SIV- or SHIV-infected rhesus macaques. Peripheral blood samples were obtained from 21 chronically infected rhesus macaques at 24 hours after BrdU inoculation, and the percentage of BrdU-labeled monocytes and blood CD4 count were measured. The Spearman rank test was used to determine the correlation between the percentage of BrdU+CD14+ monocytes and CD4+ T-cell counts.
The nonpathogenic model of SIV infection has been recently widely accepted as an important experimental model to understand the pathogenesis of SIV/HIV infection. Studies that used these models have started providing insight into the mechanism of AIDS disease progression. Therefore, determining whether the degree of macrophage destruction manifested by monocyte turnover can explain the lack of disease progression in the nonpathogenic SIV/monkey model may provide strong evidence to support the present study.
It is important to note that monocyte numbers in peripheral blood were efficiently maintained by newly formed monocytes from bone marrow despite ongoing destruction of their end-stage product (eg, macrophages). The ability of the body to maintain this important cell subset despite ongoing loss of tissue macrophages for long periods of time may be yet another part of the explanation of why AIDS progression is slow despite persistent high-level viral replication.
Currently, the mechanism of immunodeficiency caused by HIV-1 infection is based on the elimination of CD4+ T cells that orchestrate acquired immunity and the degree of immune activation caused by the virus.33,–35 However, not every patient with low CD4 counts or high immune activation progresses to AIDS similarly. Our study offers new insights into the pathogenesis of AIDS and suggests that other components of the immune system may also be critically and independently affected by HIV-1 infection and ultimately contributes to progression to AIDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Peter Mottram, Erin M. Haupt, Ashley N. Leach, and Toni P. Penney of the Division of Immunology and Jason P. Dufour of the Division of Veterinary Medicine at the TNPRC for technical assistance.
This work was supported by grants from NIH to K.C.W. (NS040237, NS037654), M.J.K. (AI58882), and the Tulane National Primate Center (RR000164) and by AIDS research grants from the Health Sciences Research Grants, the Ministry of Health, Labor, and Welfare in Japan (M.J.K.).
National Institutes of Health
Authorship
Contribution: A.H. designed and performed the research and analyzed and interpreted data; H.L. performed research; B.L. provided animal samples and viral load data; J.T.B., X.A., C.S., W.-K.K., and K.C.W participated in the discussion of the results; H.V.-O. and R.M.R. performed the mathematical model; A.A.L. and R.S.V. provided animal samples and participated in the discussion of the results; and M.J.K. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcelo J. Kuroda, Division of Immunology, Tulane National Primate Research Center, Tulane University Health Sciences Center, 18703 Three Rivers Rd, Covington, LA 70433; e-mail: mkuroda@tulane.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal