Abstract
Therapeutic regulation of globin genes is a primary goal of translational research aimed toward hemoglobinopathies. Signal transduction was used to identify chromatin modifications and transcription factor expression patterns that are associated with globin gene regulation. Histone modification and transcriptome profiling were performed using adult primary CD34+ cells cultured with cytokine combinations that produced low versus high levels of gamma-globin mRNA and fetal hemoglobin (HbF). Embryonic, fetal, and adult globin transcript and protein expression patterns were determined for comparison. Chromatin immunoprecipitation assays revealed RNA polymerase II occupancy and histone tail modifications consistent with transcriptional activation only in the high-HbF culture condition. Transcriptome profiling studies demonstrated reproducible changes in expression of nuclear transcription factors associated with high HbF. Among the 13 genes that demonstrated differential transcript levels, 8 demonstrated nuclear protein expression levels that were significantly changed by cytokine signal transduction. Five of the 8 genes are recognized regulators of erythropoiesis or globin genes (MAFF, ID2, HHEX, SOX6, and EGR1). Thus, cytokine-mediated signal transduction in adult erythroid cells causes significant changes in the pattern of globin gene and protein expression that are associated with distinct histone modifications as well as nuclear reprogramming of erythroid transcription factors.
Introduction
In patients with sickle cell disease and β-thalassemia syndromes, the perinatal phenomenon of hemoglobin switching ultimately causes the manifestations of pathology or death. Sickle cell disease patients who have high levels of fetal hemoglobin usually manifest milder clinical courses because fetal hemoglobin (HbF) interferes with hemoglobin S polymerization and prevents red blood cell sickling.1 Bulk HbF levels of more than 20% may be sufficient for a significant reduction of the clinical sequelae.2 In β-thalassemia, increased expression of γ-globin chain synthesis at all levels decreases the degree of α- to non–α-globin chain imbalance and consequently improves the anemia.3 Hence, intense focus is placed on understanding and increasing fetal globin gene expression as a preventative therapy.
HbF reaches its maximal expression in human erythrocytes during fetal development, but those peak levels begin to fall before birth. Thereafter, HbF expression is nearly completely silenced during the first year of postnatal life. In a subset of clinical conditions, expression of HbF may be temporarily increased at later stages of life. “Stressed” erythropoiesis associated with hypoxia or a hypoxic response causes increased expression of HbF in apes, but the HbF-augmenting effects of erythropoietin or mild erythroid stress are less robust in humans. Significant elevations in HbF among adult humans are associated with more extreme stressed erythropoiesis, such as recovery from bone marrow ablation in the setting of transplantation.4 HbF is also increased by drugs, such as hydroxyurea, azacytidines, and butyrates.4 In addition to the temporary effects of erythroid stress and drugs, HbF can be permanently elevated by heritable traits. In cases of hereditary persistence of fetal hemoglobin, genetic determinants that affect transcription include point mutations or large deletions within the β-globin locus5 as well as quantitative trait loci on chromosomes 2p15,6 6q23,7 and 8q11.8 It is assumed that the capacity to induce HbF synthesis in patients may be modulated by polymorphisms or other features in these genomic regions. Although the increased HbF is attributable to changes in globin gene transcription, the molecular bases for the modified transcription remain largely unsolved.
A growing body of evidence has shown that histone tail modifications play an important role in the control of transcriptional activity.9 In mammalian globin loci, marks for active chromatin, such as dimethylation and trimethylation of lysine 4 and acetylation of lysine 9 on histone H3, are strongly associated with the locus control region (LCR) and the actively transcribed β-like globin genes.10 In contrast, transcriptionally silenced globin genes lack these positive marks; and instead, repressive histone modifications, such as dimethylation of lysine 9 on histone H3, localize to these regions. Histone deacetylase inhibitors including butyrate are thought to reactivate embryonic or fetal globin expression among adult erythroblasts through epigenetic modification within the β-globin locus.
Signal transduction also has the potential to up-regulate γ-globin gene transcription in adult erythroblasts. Interestingly, signal transduction cascades involved in growth and cellular stress response may modify globin gene regulation. In experimental models, activation of mitogen-activated protein (MAP) kinase11 and cyclic nucleotide pathways (cyclic guanosine monophosphate12,13 and cyclic adenosine monophosphate)14 modulate globin gene expression to some degree. Cytokines provide a means to coordinate several cascades and cause robust augmentation of γ-globin mRNA and HbF expression ex vivo. In particular, the combination of erythropoietin (EPO), stem cell factor, and transforming growth factor-β was shown to increase HbF expression in adult human erythroblasts to levels more than 20% without dramatic changes in the viability, growth, or differentiation of the cells.15 These cytokines act via phosphorylation cascades that originate at the cell surface. However, the nuclear mechanisms responsible for the cytokine-induced increase in γ-globin transcript are not understood. To begin to address this topic, chromatin immunoprecipitation (ChIP) assays and transcriptome profiles were used to identify globin locus histone modifications and patterns of transcription factor expression that are associated with cytokine signaled changes in globin gene expression.
Methods
Primary erythroblast cultures
Primary CD34+ cells from 15 healthy donors (collected after National Institutes of Health Institutional Review Board approval and informed consent obtained in accordance with the Declaration of Helsinki) were cultured for 14 days as described previously,15 with low-HbF culture conditions; EPO alone (4 U/mL; Amgen), versus high-HbF culture conditions; EPO, stem cell factor (50 ng/mL; R&D Systems), and transforming growth factor-β (1.25 ng/mL; R&D Systems). Eagle minimum essential medium was utilized containing 30% fetal bovine serum, 1% deionized bovine serum albumin, 40 mM glutamine, 1 U/mL penicillin-streptomycin, 100 μM β-mercaptoethanol, 1 μM dexamethasone, and 0.3 mg/mL holotransferrin. All reagents, except fetal bovine serum (HyClone Laboratories), glutamine, and penicillin-streptomycin (both from Biosource), were obtained from Sigma-Aldrich. Cellular growth and differentiation were confirmed by flow cytometry and cytospin observation as previously described.16
Quantitative real-time RT-PCR
RNA was extracted from cell cultures using the RNeasy Plus Micro kit (QIAGEN) and reverse transcribed using a SuperScript III reverse transcriptase (Invitrogen). Real-time polymerase chain reaction (RT-PCR) was performed on the ABI PRISM 7700 sequence detection system instrument and software (Applied Biosystems), using the manufacturer's recommended conditions. Quantitative PCR assays were performed with primers, probes, and PCR conditions described previously for γ-, β-, μ-, and α-globin.17 In addition, amplifications for ϵ-, δ-, ζ-, and θ-globin transcripts were performed using Assays-on-Demand Gene Expression Products (Applied Biosystems) according to the manufacturer's instructions. Individual globin copy numbers were calculated by comparison with standard curves generated from a plasmid DNA encoding each globin template. Statistical significance was determined by paired Student t test analyses. The transcript levels of other genes were measured by quantitative PCR using the Assays-on-Demand products (Applied Biosystems). The relative fold changes were calculated using the ΔΔC(T) method by normalizing against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Information on PCR primers and probes is available in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
ChIP and antibodies
ChIP was performed as previously described18 with minor modification to accommodate lower cell numbers available for each experiment (typically 1-2 × 106 cells per ChIP). Protein G Dynabeads (Invitrogen) were used for preclearing sonicated chromatin and for the capture of protein-DNA complexes. Beads bound to immunoprecipitated chromatin were washed 3 times in modified radioimmunoprecipitation assay buffer (10 mM Tris, pH 7.5, 1 mM ethylenediaminetetraacetic acid [EDTA], 0.5 mM ethyleneglycoltetraacetic acid [EGTA], 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 0.1% sodium deoxycholate, 140 mM NaCl) and twice with Tris-EDTA buffer. Formaldehyde cross-links were reversed and DNA-protein complexes were eluted in a single step using modified elution buffer (20 mM Tris, pH 7.5, 5 mM EDTA, pH 8.0, 50 mM NaCl, 1% SDS, and 50 μg/mL proteinase K) for 2 hours at 65°C followed by a second elution in an equal volume of elution buffer for 30 minutes at 65°C. Three to 6 donor-matched cultures were examined under both low-HbF and high-HbF conditions on day 9 of culture. Anti-dimethyl H3K9 (Abcam), anti-dimethyl H3K4, anti-trimethyl H3K4, anti-acetyl H3K9 (all from Millipore), and RNA Polymerase II (pol II) antibody (Santa Cruz Biotechnology) were used. Quantitative PCR primers, probes, and PCR conditions have been previously described.18 Because of the high level of sequence homology, primers do not distinguish the Gγ- and Aγ-globin genes, and the results reported reflect an average across both.
Transcriptional profiling
For microarray evaluation, equivalent amounts of total RNA were pooled from RNA obtained on cultured day 7 from CD34+ cells of 3 donors grown in low-HbF versus high-HbF culture conditions. Five such pools were prepared (15 donors total). RNA was isolated with TRIzol LS (Invitrogen) and purified in the RNeasy Mini Kit (QIAGEN) according to the manufacturer's protocols. RNA qualities were checked by Agilent Bioanalyzer (Agilent Technologies). Microarray analyses were performed using 3 μg total RNA from each pool after one cycle of complementary RNA amplification, using the GeneChips Human Genome U133 Plus 2.0 Array (Affymetrix), according to the manufacturer's protocol. After hybridization and washing, microarray chips were scanned using Affymetrix MAS 5.0 software. Raw gene expression data are available at http://www.ncbi.nlm.nih.gov/geo (accession no. series GSE7874). Gene expression data were analyzed using GeneSpring, Version 7.3 (Agilent Technologies) with Affymetrix grade A probesets (identified from NetAffx database; http://www.affymetrix.com/analysis/index.affx) and normalized using the software default criteria. Transcription factor lists were generated from public databases and Genomatix MatBase matrix library 7.1 (http://www.genomatrix.de; Genomatix Software). The array data were filtered by discarding probe sets that failed to meet confidence criteria based on signal intensity.
Western blot analysis
Nuclear extracts were prepared from CD34+ cells differentiated in low-HbF and high-HbF culture conditions, using nuclear and cytoplasmic extraction reagents (Pierce Biotechnology) according to the manufacturer's protocol. Equal amounts of protein (10-20 μg) were separated by 10% SDS–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Invitrogen). Blots were probed with antibodies against ζ-globin (Abnova), ϵ-globin (Cortex Biochem), EGR1 and ELK1 (both from Cell Signaling Technology), ID2, LRRFIP1, MAFF, MNDA, and THRB (all from Santa Cruz Biotechnology), BCL11A, CBFB, HHEX, and SOX6 (all from Abcam), SMAD1 (R&D Systems), or GATA-1 (Active Motif), and the appropriate horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology). Immunoreactive proteins were detected and visualized using ECL Plus Western blotting detection reagents (GE Healthcare). All blots were probed for β-actin (Abcam) or GAPDH (Research Diagnostics) antibody to ensure equivalent loading.
Results
Changes in globin gene and protein expression patterns in response to cytokine signaling
The culture model chosen for these studies was previously characterized.15 The combination of stem cell factor and transforming growth factor-beta signal transduction produces a marked increase in γ-globin transcript and protein expression. Because cytokine signaling may affect the expression of the additional globin genes, the study began by examining the expression patterns of each gene. Although gene-specific primers were used for the ϵ-, δ-, β-, μ-, and θ-globin transcripts, degenerate quantitative PCR primers were used for the paired γ-, ζ-, and α-globin transcripts. Quantitation of the globin mRNA was performed on culture days 7 (proliferating erythroid progenitor cells) and 14 (hemoglobinized erythroid precursor cells) for both donor-matched low-HbF and high-HbF conditions as shown in Table 1. The high-HbF culture condition was associated with a 13- to 14-fold increased expression of γ-globin gene mRNA (day 7; low-HbF = 9.1 ± 4.1 × 10E5 vs high-HbF = 1.2 ± 0.2 × 10E7; and day 14; low-HbF = 4.3 ± 2.7 × 10E5 vs high-HbF = 6.0 ± 3.8 × 10E6 copies/ng total RNA, n = 15). Expression of β- and δ-globin mRNAs was reduced significantly on both culture days in the high-HbF compared with the low-HbF conditions. Unexpectedly, the high-HbF culture condition was associated with increased expression of embryonic globin genes. Induction of ϵ-globin mRNA in the high-HbF condition was as high as 27-fold and 18-fold in culture days 7 and 14, respectively. Increased amounts of ζ-globin mRNA expression were found in culture day 7 (low-HbF; 6.9 ± 5.5 × 10E1 vs high-HbF; 1.3 ± 0.8 × 10E4 copies/ng total RNA). Transcript levels of the remaining globin genes (μ-, α-, and θ-globins) were not significantly altered in the high-HbF cultures. Although cytokines changed the transcript levels of several individual globin genes, the total levels from the α-like or β-like loci remained stable.
Transcript levels of the globin genes in the beta- and alpha-like globin clusters
| . | Cultured erythroblasts day 7 . | Cultured erythroblasts day 14 . | ||||
|---|---|---|---|---|---|---|
| Low-HbF (L) . | High-HbF (H) . | H/L ratio . | Low-HbF (L) . | High-HbF (H) . | H/L ratio . | |
| Beta-like globin cluster | ||||||
| ϵ-globin | 1.0 ± 0.6 × 10E3 | 2.7 ± 1.1 × 10E4* | 27 | 4.4 ± 1.5 × 10E2 | 7.9 ± 4.0 × 10E3* | 18 |
| γ-globin | 9.1 ± 4.1 × 10E5 | 1.2 ± 0.2 × 10E7* | 13 | 4.3 ± 2.7 × 10E5 | 6.0 ± 3.8 × 10E6* | 14 |
| δ-globin | 5.0 ± 4.4 × 10E5 | 1.9 ± 1.8 × 10E5* | 0.4 | 2.0 ± 2.0 × 10E5 | 7.4 ± 10.0 × 10E4* | 0.4 |
| β-globin | 1.3 ± 0.4 × 10E7 | 5.5 ± 1.8 × 10E6* | 0.4 | 1.5 ± 0.8 × 10E7 | 8.5 ± 4.9 × 10E6* | 0.6 |
| Total | 1.5 ± 0.5 × 10E7 | 1.8 ± 0.4 × 10E7 | 1.2 | 1.6 ± 0.8 × 10E7 | 1.5 ± 0.9 × 10E7 | 0.9 |
| Alpha-like globin cluster | ||||||
| ζ-globin | 6.9 ± 5.5 × 10E1 | 1.3 ± 0.8 × 10E4* | 190 | 2.7 ± 1.9 × 10E1 | 2.2 ± 2.4 × 10E2* | 8.1 |
| μ-globin | 4.0 ± 1.4 × 10E4 | 3.5 ± 1.4 × 10E4 | 0.9 | 1.4 ± 0.5 × 10E4 | 1.3 ± 1.0 × 10E4 | 0.9 |
| α-globin | 7.9 ± 2.0 × 10E6 | 8.7 ± 1.6 × 10E6 | 1.2 | 1.3 ± 0.6 × 10E7 | 1.1 ± 0.6 × 10E7 | 0.8 |
| θ-globin | 1.1 ± 0.5 × 10E3 | 1.4 ± 0.7 × 10E3 | 1.2 | 8.7 ± 2.9 × 10E2 | 5.5 ± 3.6 × 10E2 | 0.6 |
| Total | 7.9 ± 2.0 × 10E6 | 8.7 ± 1.6 × 10E6 | 1.1 | 1.3 ± 0.7 × 10E7 | 1.1 ± 0.7 × 10E7 | 0.8 |
| . | Cultured erythroblasts day 7 . | Cultured erythroblasts day 14 . | ||||
|---|---|---|---|---|---|---|
| Low-HbF (L) . | High-HbF (H) . | H/L ratio . | Low-HbF (L) . | High-HbF (H) . | H/L ratio . | |
| Beta-like globin cluster | ||||||
| ϵ-globin | 1.0 ± 0.6 × 10E3 | 2.7 ± 1.1 × 10E4* | 27 | 4.4 ± 1.5 × 10E2 | 7.9 ± 4.0 × 10E3* | 18 |
| γ-globin | 9.1 ± 4.1 × 10E5 | 1.2 ± 0.2 × 10E7* | 13 | 4.3 ± 2.7 × 10E5 | 6.0 ± 3.8 × 10E6* | 14 |
| δ-globin | 5.0 ± 4.4 × 10E5 | 1.9 ± 1.8 × 10E5* | 0.4 | 2.0 ± 2.0 × 10E5 | 7.4 ± 10.0 × 10E4* | 0.4 |
| β-globin | 1.3 ± 0.4 × 10E7 | 5.5 ± 1.8 × 10E6* | 0.4 | 1.5 ± 0.8 × 10E7 | 8.5 ± 4.9 × 10E6* | 0.6 |
| Total | 1.5 ± 0.5 × 10E7 | 1.8 ± 0.4 × 10E7 | 1.2 | 1.6 ± 0.8 × 10E7 | 1.5 ± 0.9 × 10E7 | 0.9 |
| Alpha-like globin cluster | ||||||
| ζ-globin | 6.9 ± 5.5 × 10E1 | 1.3 ± 0.8 × 10E4* | 190 | 2.7 ± 1.9 × 10E1 | 2.2 ± 2.4 × 10E2* | 8.1 |
| μ-globin | 4.0 ± 1.4 × 10E4 | 3.5 ± 1.4 × 10E4 | 0.9 | 1.4 ± 0.5 × 10E4 | 1.3 ± 1.0 × 10E4 | 0.9 |
| α-globin | 7.9 ± 2.0 × 10E6 | 8.7 ± 1.6 × 10E6 | 1.2 | 1.3 ± 0.6 × 10E7 | 1.1 ± 0.6 × 10E7 | 0.8 |
| θ-globin | 1.1 ± 0.5 × 10E3 | 1.4 ± 0.7 × 10E3 | 1.2 | 8.7 ± 2.9 × 10E2 | 5.5 ± 3.6 × 10E2 | 0.6 |
| Total | 7.9 ± 2.0 × 10E6 | 8.7 ± 1.6 × 10E6 | 1.1 | 1.3 ± 0.7 × 10E7 | 1.1 ± 0.7 × 10E7 | 0.8 |
Values are copies/ng total RNA. The globin copy number per nanogram of total RNA was determined by quantitative real-time PCR in adult human cultured erythroblasts on days 7 and 14. Information on PCR primers and probes is available in supplemental Table 1.
P < .05, comparing low-HbF (L) vs high-HbF (H) culture conditions (n = 15).
The globin expression patterns were validated by protein analyses using cation exchange (Figure 1A-C) and reverse-phase high-performance liquid chromatography (Figure 1D-F) for quantitation of hemoglobin and globin chains, respectively. Erythroblasts cultured in the high-HbF condition revealed a significant enhancement of HbF/(HbF + HbA) levels (low-HbF = 3.7% ± 2.4% vs high-HbF = 42.2% ± 5.8%, P = 8.8 × 10−9; Figure 1C) without significant effects on erythroblast maturation (supplemental Figure 1). Consistent with increased transcript level in quantitative PCR, both γ-globins were increased (compare Figure 1D and E). In vivo, the Gγ- to Aγ-globin ratio in erythroid cells is normally less than one in adults and greater than one in the fetus.19 Despite lower levels of HbF produced in low-HbF cultures, quantitation was possible and the Gγ- to Aγ-globin ratio reached levels of greater than one in association with the increased HbF (Figure 1F). Globin chain analyses additionally confirmed the reduction of β-globin expression and stability of α-globin expression in the high-HbF cultures as predicted from quantitative PCR analyses. Neither HBZ- nor HBE1-derived globins were detected by high-performance liquid chromatography analyses. However, more sensitive Western analyses clearly revealed both ζ- and ϵ-globins exclusively in high-HbF cultures on days 8 to 14 (Figure 1G).
Determination of hemoglobin and globin chain expression. (A-C) Cation exchange and (D-F) reverse-phase chromatography analyses of cells cultured in (A,D) low-HbF versus (B,E) high-HbF culture conditions. The major peaks are labeled on each graph (y-axis, absorbance; x-axis, elution time). (C) The HbF/HbF + HbA ratios expressed as a percentage (y-axis) from 9 separate donor-matched erythroblasts cultured for 14 days in low-HbF (□) and high-HbF (■) conditions. Average values (Ave) are also shown. (F) Gγ/Aγ globin ratios (y-axis) correspond to the results in panel C. *P < .05. (G) Western blot analyses were performed using total protein extract (25 μg/lane) from erythroblasts cultured in low-HbF (L) and high-HbF (H) conditions and harvested on days 2, 4, 6, 8, 10, 12, and 14. Antibodies to ζ-globin chain (ζ), ϵ-globin chain (ϵ), and GAPDH were used for detection. A vertical line between d6 and d8 was inserted to indicate the transition between 2 blots.
Determination of hemoglobin and globin chain expression. (A-C) Cation exchange and (D-F) reverse-phase chromatography analyses of cells cultured in (A,D) low-HbF versus (B,E) high-HbF culture conditions. The major peaks are labeled on each graph (y-axis, absorbance; x-axis, elution time). (C) The HbF/HbF + HbA ratios expressed as a percentage (y-axis) from 9 separate donor-matched erythroblasts cultured for 14 days in low-HbF (□) and high-HbF (■) conditions. Average values (Ave) are also shown. (F) Gγ/Aγ globin ratios (y-axis) correspond to the results in panel C. *P < .05. (G) Western blot analyses were performed using total protein extract (25 μg/lane) from erythroblasts cultured in low-HbF (L) and high-HbF (H) conditions and harvested on days 2, 4, 6, 8, 10, 12, and 14. Antibodies to ζ-globin chain (ζ), ϵ-globin chain (ϵ), and GAPDH were used for detection. A vertical line between d6 and d8 was inserted to indicate the transition between 2 blots.
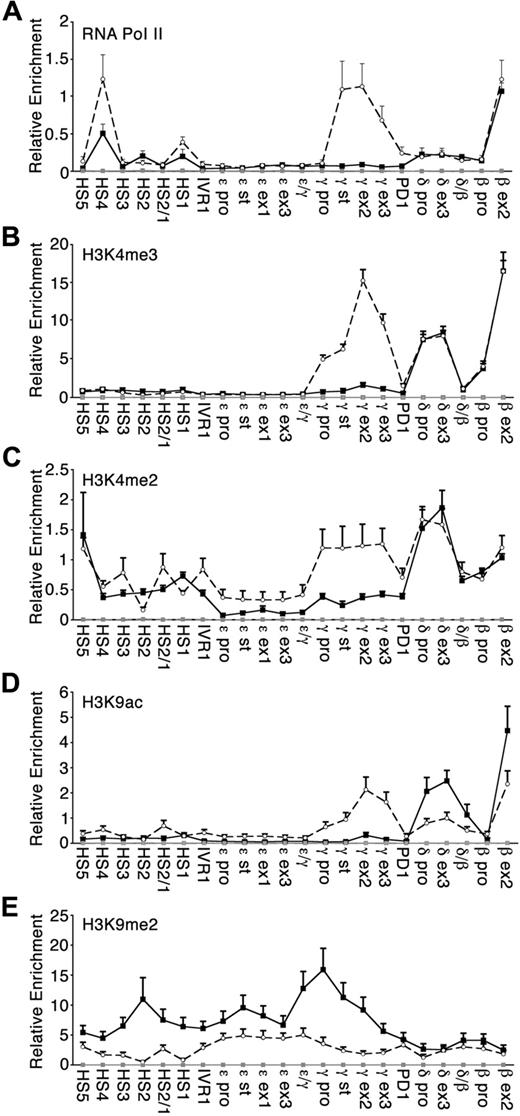
Cytokine-dependent differences in histone modification within the β-globin locus
Experiments were performed to determine whether the RNA pol II occupancy and histone tail modifications in the β-globin locus were reflective of the different globin transcriptional profiles observed under low-HbF and high-HbF culture conditions. To ensure a sufficient number of differentiating erythroid cells for ChIP studies, day 9 cultured cells were used. Globin gene expression ratios on day 9 of erythroblast cell culture were equivalent to day 7 (data not shown). pol II levels at the β-globin gene were similar in both low-HbF and high-HbF culture conditions (Figure 2A). High levels of pol II were recruited to γ-globin genes when their transcription was up-regulated under the high-HbF condition, whereas pol II was essentially undetected at these genes under the low-HbF condition. pol II was detectable over the LCR in both culture conditions, with an increase at HS4 in high-HbF erythroblasts.
RNA polymerase II occupancy and histone modifications across the human β-globin locus. ChIPs were performed on culture day 9 using antibodies specific to (A) RNA polymerase II, (B) trimethylated histone H3 lysine 4 (H3K4me3), (C) dimethylated histone H3 lysine 4 (H3K4me2), (D) acetylated histone 3 lysine 9 (H3K9ac), and (E) dimethylated histone H3 lysine 9 (H3K9me2). Plotted values are relative enrichments (y-axis) measured for sites in the β-globin locus (x-axis) in low-HbF (■, black solid line) and high-HbF (○, black broken line). Antibody against IgG was used as a nonspecific control (gray line). Three to 6 separate donor-matched experiments were analyzed for each antibody under each condition; SE is indicated. Note that the scales on the y-axis differ between the panels.
RNA polymerase II occupancy and histone modifications across the human β-globin locus. ChIPs were performed on culture day 9 using antibodies specific to (A) RNA polymerase II, (B) trimethylated histone H3 lysine 4 (H3K4me3), (C) dimethylated histone H3 lysine 4 (H3K4me2), (D) acetylated histone 3 lysine 9 (H3K9ac), and (E) dimethylated histone H3 lysine 9 (H3K9me2). Plotted values are relative enrichments (y-axis) measured for sites in the β-globin locus (x-axis) in low-HbF (■, black solid line) and high-HbF (○, black broken line). Antibody against IgG was used as a nonspecific control (gray line). Three to 6 separate donor-matched experiments were analyzed for each antibody under each condition; SE is indicated. Note that the scales on the y-axis differ between the panels.
Histone modifications that mark actively transcribed regions were also examined. Trimethylation of lysine 4 on histone H3 (H3K4me3) was tightly correlated with the actively transcribed globin genes, as expected (Figure 2B). Under low-HbF culture condition, H3K4me3 was enriched over the adult globin genes (δ- and β-globin). By comparison, H3K4me3 levels were increased over the actively transcribed γ-globin genes only in high-HbF cultures. Similarly, the level of histone H3 lysine 4 dimethylation (H3K4me2) was comparable between low-HbF and high-HbF culture conditions, with the exception of the marked increase over the γ-globin genes (Figure 2C). H3K4me2 was prominent in the LCR, consistent with earlier work.18 Acetylation of lysine 9 on histone H3 (H3K9ac) was likewise higher over the γ-globin genes under high-HbF compared with low-HbF culture conditions (Figure 2D). This modification was reduced over the δ- and β-globin genes in high-HbF erythroblasts where transcription of these genes was reduced compared with low-HbF cells.
Histone H3 lysine 9 dimethylation (H3K9me2) is a mark of silent euchromatin. Under high-HbF culture conditions, H3K9me2 was relatively low across the entire β-globin locus region (Figure 2E). In contrast, H3K9me2 under low-HbF conditions was more elevated across the locus but dropped significantly over the active δ- and β-globin genes. The changes in H3K9me2 marks are consistent with their expression levels under low-HbF culture conditions. A direct comparison of methyl-lysine histone marks for active (H3K4me2) and silenced (H3K9me2) chromatin revealed that dimethylation at these lysines is reciprocal and reflective of the transcriptional state of the β-like globin genes (supplemental Figure 2). One interesting exception is within the LCR of high-HbF erythroblasts where the levels of dimethylation of lysine 4 and lysine 9 appear to follow the same pattern of enrichment.
Correlation of globin gene expression pattern and histone modification with expression of erythroblast transcription factors
As shown in Figures 1 and 2, the cytokine signaling in these cultured adult human primary erythroid cells resulted in significant changes in globin locus chromatin, transcripts, and proteins in adult erythroblasts. The changes in globin transcripts and proteins were consistently opposite in direction to those defined by the fetal-to-adult transition of human ontogeny. Based on these consistent changes, it was hypothesized that cytokine signaling may change the expression levels of transcription regulating factors. Based on the clear differences in globin mRNA levels and the transition in embryonic globin protein expression from adult human erythroblast culture days 6 to 8, culture day 7 was chosen for transcriptome studies (supplemental Figure 3A). Total RNA was extracted from donor-matched cultures of erythroblasts that express low (< 5%, low-HbF) versus high (> 25%, high-HbF) fetal hemoglobin levels (supplemental Figure 3B and C, respectively). A consistent increase in HbF was detected from each donor, and the HbF levels were significantly increased to levels above 30% in each of the high-HbF pools. For each sample, nondegraded erythroblast RNAs from 15 donors were combined in equimolar concentration to obtain 5 donor-matched pools (each pool containing RNA from 3 separate donors) for transcriptome analysis using Affymetrix Genechips (HG-U133 plus 2.0).
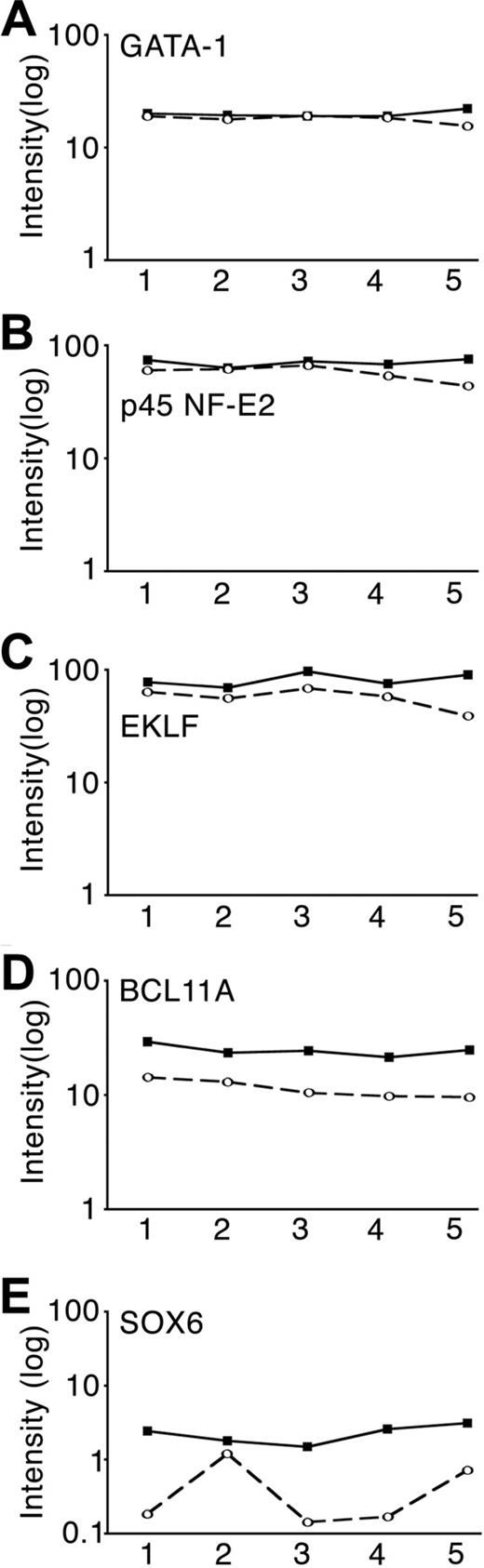
Of interest, the expression patterns of 40 candidate transcription factors that earlier work had shown associated with globin gene expression, switching or silencing, including GATA, KLF, and NF-E families, were initially examined (supplemental Table 2). Among those candidate globin gene expression regulators, BCL11A and SOX-6 showed major expression changes according to the array data: 2.2-fold and 7.0-fold down-regulated in the high-HbF condition, respectively. Decreased expression of BCL11A in the high-HbF condition was consistently identified in 5 of 5 pools, but the decreases in SOX6 were less consistent among the pools (Figure 3). EKLF and p45 NF-E2 showed slightly decreased expression in the high-HbF condition, whereas GATA-1 showed a similar expression level in low-HbF and high-HbF conditions.
Comparison of candidate globin transcription factor expression levels using oligonucleotide arrays. Array signal intensities graphed according to cultured erythroblasts day 7 RNA pool for (A) GATA-1, (B) p45 NF-E2, (C) EKLF (KLF1), (D) BCL11A, and (E) SOX6 are shown. The lines connect the normalized intensity values from each of the 5 matched pools (3 separate donors per pool, x-axis) of RNA from day 7 cultured erythroblasts expressing low-HbF (■) versus high-HbF (○).
Comparison of candidate globin transcription factor expression levels using oligonucleotide arrays. Array signal intensities graphed according to cultured erythroblasts day 7 RNA pool for (A) GATA-1, (B) p45 NF-E2, (C) EKLF (KLF1), (D) BCL11A, and (E) SOX6 are shown. The lines connect the normalized intensity values from each of the 5 matched pools (3 separate donors per pool, x-axis) of RNA from day 7 cultured erythroblasts expressing low-HbF (■) versus high-HbF (○).
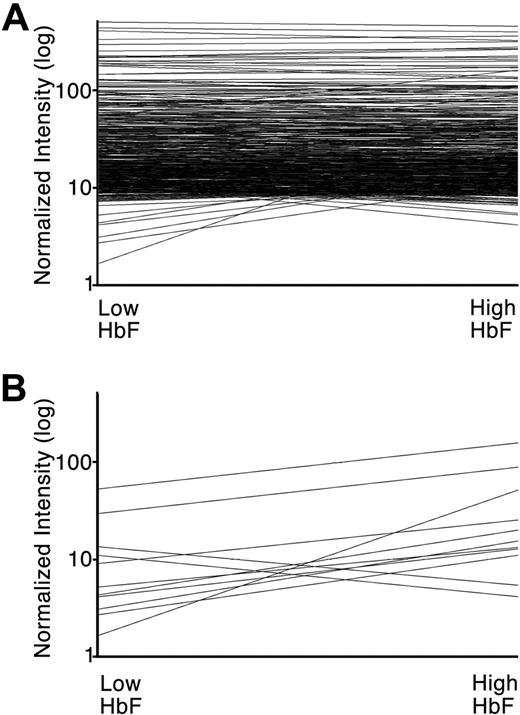
To identify additional transcription factors affected by cytokine-induced increases in HbF, the entire list of 2977 probe sets matching 1523 transcription factor genes were examined. A total of 534 probe sets representing 369 genes were identified that demonstrated relatively high level expression (defined here by raw intensity of > 1000 in at least 5 of the 10 arrays; Figure 4A; supplemental Table 3). In addition to GATA-1, EKLF, and p45 NF-E2, several dozen transcription factors previously reported as erythroid regulators, including ATF2, ATF4, CREB, GFI1B, HIF1A, LMO2, MAX, MYB, MYC, TAL1, and YY1, demonstrated similar transcript levels in low-HbF and high-HbF erythroblasts. Interestingly, among a larger group of transcription factors expressed at high levels, only 11 genes demonstrated a greater than 2.5-fold change in the average normalized intensities of the low-HbF arrays versus the high-HbF arrays (Figure 4B; supplemental Table 3 bold font). Quantitative RT-PCR with gene-specific primer and probe was carried out to validate cytokine-mediated changes in mRNA levels of these candidate genes selected from array analysis as well as BCL11A and SOX6 (Table 2). ATF7IP2, CBFB, EGR1, ELK1, HHEX, ID2, LRRFIP1, and MAFF transcripts were confirmed by quantitative PCR as up-regulated in high-HbF culture conditions. BCL11A, SMAD1, SOX6, and THRB were confirmed by quantitative PCR to be down-regulated in the high-HbF condition.
Differential transcription factor genes expression associated with increased HbF expression. (A) Array normalized intensity level (y-axis) for 534 probe sets representing 369 transcription factors. The lines attach average normalized intensity levels for all RNA pools of adult human erythroblasts cultured under low-HbF (left) versus high-HbF (right) conditions are shown. (B) Comparable image of 11 probe sets demonstrating greater than 2.5-fold change in normalized intensity by comparison of low-HbF (left) versus high-HbF (right) culture conditions. Probe identities and intensity levels are shown in Table 2 and supplemental Table 3 (bold font).
Differential transcription factor genes expression associated with increased HbF expression. (A) Array normalized intensity level (y-axis) for 534 probe sets representing 369 transcription factors. The lines attach average normalized intensity levels for all RNA pools of adult human erythroblasts cultured under low-HbF (left) versus high-HbF (right) conditions are shown. (B) Comparable image of 11 probe sets demonstrating greater than 2.5-fold change in normalized intensity by comparison of low-HbF (left) versus high-HbF (right) culture conditions. Probe identities and intensity levels are shown in Table 2 and supplemental Table 3 (bold font).
Cytokine-induced changes in transcription factor mRNA levels
| Probe set ID . | Gene name . | GenBank no. . | Microarray . | Quantitative PCR . |
|---|---|---|---|---|
| 201565_s_at | ID2: inhibitor of DNA binding 2, dominant-negative helix-loop-helix protein | BC030639 | (+) 3.1 ± 0.7 | (+) 2.3 ± 1.2 |
| 201694_s_at | EGR1: early growth response 1 | X52541 | (+) 3.5 ± 1.7 | (+) 3.8 ± 2.6 |
| 201862_s_at | LRRFIP1: leucine-rich repeat (in FLII) interacting protein 1 | AJ223075 | (+) 2.9 ± 0.6 | (+) 2.1 ± 0.3 |
| 202370_s_at | CBFB: core-binding factor, beta subunit | BC018509 | (+) 4.7 ± 0.5 | (+) 2.5 ± 0.2 |
| 203617_x_at | ELK1: ELK1, member of ETS oncogene family | BC056150 | (+) 2.7 ± 1.2 | (+) 3.3 ± 0.4 |
| 204959_at | MNDA: myeloid cell nuclear differentiation antigen | BC032319 | (+) 36.0 ± 18.7 | ND |
| 215933_s_at | HHEX: hematopoietically expressed homeobox | BC015110 | (+) 4.4 ± 1.2 | (+) 3.0 ± 1.2 |
| 219498_s_at | BCL11A: B-cell CTT/lymphoma 11A (zinc finger protein) | AJ404611 | (−) 2.2 ± 0.3 | (−) 1.7 ± 0.2 |
| 223865_at | SOX-6: SRY (sex determining region Y)-box 6 | BC047064 | (−) 7.0 ± 4.2 | (−) 5.3 ± 1.5 |
| 227798_at | SMAD1: SMAD family member 1 | BC001878 | (−) 2.6 ± 0.8 | (−) 2.3 ± 0.5 |
| 228381_at | ATF7IP2: activating transcription factor 7 interacting protein 2 | BC033891 | (+) 3.1 ± 0.5 | (+) 2.7 ± 0.4 |
| 229657_at | THRB: thyroid hormone receptor, beta (erythroblastic leukemia viral [v-erb-a] oncogene homolog 2, avian) | M26747 | (−) 2.7 ± 0.7 | (−) 4.4 ± 1.5 |
| 36711_at | MAFF: v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | AJ010857 | (+) 5.3 ± 1.6 | (+) 4.5 ± 3.7 |
| Probe set ID . | Gene name . | GenBank no. . | Microarray . | Quantitative PCR . |
|---|---|---|---|---|
| 201565_s_at | ID2: inhibitor of DNA binding 2, dominant-negative helix-loop-helix protein | BC030639 | (+) 3.1 ± 0.7 | (+) 2.3 ± 1.2 |
| 201694_s_at | EGR1: early growth response 1 | X52541 | (+) 3.5 ± 1.7 | (+) 3.8 ± 2.6 |
| 201862_s_at | LRRFIP1: leucine-rich repeat (in FLII) interacting protein 1 | AJ223075 | (+) 2.9 ± 0.6 | (+) 2.1 ± 0.3 |
| 202370_s_at | CBFB: core-binding factor, beta subunit | BC018509 | (+) 4.7 ± 0.5 | (+) 2.5 ± 0.2 |
| 203617_x_at | ELK1: ELK1, member of ETS oncogene family | BC056150 | (+) 2.7 ± 1.2 | (+) 3.3 ± 0.4 |
| 204959_at | MNDA: myeloid cell nuclear differentiation antigen | BC032319 | (+) 36.0 ± 18.7 | ND |
| 215933_s_at | HHEX: hematopoietically expressed homeobox | BC015110 | (+) 4.4 ± 1.2 | (+) 3.0 ± 1.2 |
| 219498_s_at | BCL11A: B-cell CTT/lymphoma 11A (zinc finger protein) | AJ404611 | (−) 2.2 ± 0.3 | (−) 1.7 ± 0.2 |
| 223865_at | SOX-6: SRY (sex determining region Y)-box 6 | BC047064 | (−) 7.0 ± 4.2 | (−) 5.3 ± 1.5 |
| 227798_at | SMAD1: SMAD family member 1 | BC001878 | (−) 2.6 ± 0.8 | (−) 2.3 ± 0.5 |
| 228381_at | ATF7IP2: activating transcription factor 7 interacting protein 2 | BC033891 | (+) 3.1 ± 0.5 | (+) 2.7 ± 0.4 |
| 229657_at | THRB: thyroid hormone receptor, beta (erythroblastic leukemia viral [v-erb-a] oncogene homolog 2, avian) | M26747 | (−) 2.7 ± 0.7 | (−) 4.4 ± 1.5 |
| 36711_at | MAFF: v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (avian) | AJ010857 | (+) 5.3 ± 1.6 | (+) 4.5 ± 3.7 |
Thirteen genes that were identified to be differentially expressed by the microarray analysis (15 separate donor-matched) were studied using quantitative real-time RT-PCR to verify. Quantitative PCR was performed in RNA from day 7 cultured erythroblasts (3 separate donor-matched). Average expression fold change compared in high-HbF versus low-HbF culture conditions is shown with SD.
(−) indicates down-regulated in high-HbF condition; (+), up-regulated in high-HbF condition; and ND, not determined.
Cytokine-signaled changes in nuclear transcription factor proteins
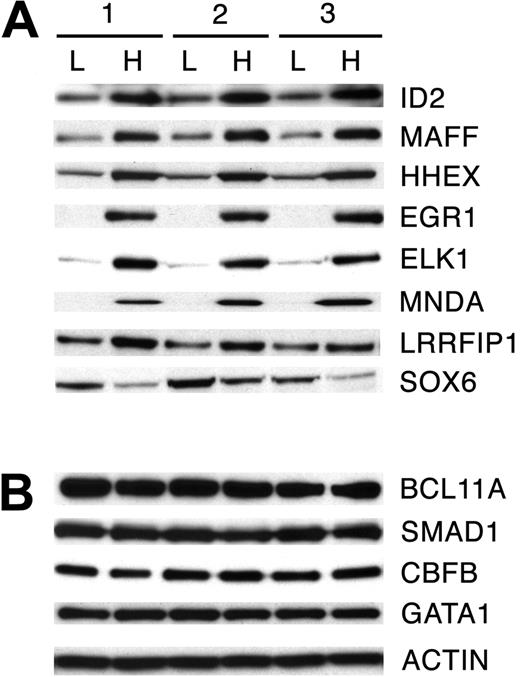
Based on the transcriptome comparisons, Western analyses were performed in nuclear extracts (3 independent donors) from culture day 7 erythroblasts. The nuclear protein expression levels of 12 genes of interest were analyzed: BCL11A, CBFB, EGR1, ELK1, HHEX, ID2, LRRFIP1, MAFF, MNDA, SMAD1, SOX6, and THRB, and compared in low-HbF and high-HbF culture conditions (ATF7IP2 antibody not available). β-Actin was used as a protein loading control for Western analyses. Because GATA-1 showed no transcript level changes in low-HbF versus high-HbF conditions in microarray study, GATA-1 protein expression was examined as an additional control. Western blotting showed that the nuclear protein expression patterns for 8 of the 12 selected transcription factors were consistent with the transcript expression patterns determined by array and quantitative PCR studies. As shown in Figure 5A, ID2, MAFF, HHEX, EGR1, ELK1, MNDA, and LRRFIP1 showed up-regulation in the high-HbF culture condition. A remarkably reproducible protein expression pattern of all proteins was observed in 3 separate nuclear extract samples. SOX6 protein expression was down-regulated by the cytokines, but the donor variation in its expression level was noted. Unlike the transcriptome analyses, the nuclear level of BCL11A, CBFB, and SMAD1 proteins showed no robust change in erythroid progenitor cells with the additional cytokines (Figure 5B). No expression above background level of THRB was detected with the available antibodies (not shown). Therefore, cytokine signaling of fetal hemoglobin caused consistent and demonstrable changes in the transcription and nuclear protein levels of only 8 transcription factors identified by transcriptome comparisons.
Nuclear protein expression of reprogrammed transcription factors. Validation of gene expression profiles obtained by microarray and quantitative PCR analysis in low-HbF (L) and high-HbF (H) culture conditions by Western analysis. Nuclear protein extracts were used from erythroblasts grown in low-HbF versus high-HbF conditions for 7 days in 3 separate donor-matched cultures. (A) Eight transcription factors with confirmed expression changes in the nuclear protein extracts. (B) Three transcription factors with reprogramming demonstrated by RNA transcript levels that were not confirmed in the nuclear protein extracts. GATA-1 and β-actin controls are provided for comparison (bottom).
Nuclear protein expression of reprogrammed transcription factors. Validation of gene expression profiles obtained by microarray and quantitative PCR analysis in low-HbF (L) and high-HbF (H) culture conditions by Western analysis. Nuclear protein extracts were used from erythroblasts grown in low-HbF versus high-HbF conditions for 7 days in 3 separate donor-matched cultures. (A) Eight transcription factors with confirmed expression changes in the nuclear protein extracts. (B) Three transcription factors with reprogramming demonstrated by RNA transcript levels that were not confirmed in the nuclear protein extracts. GATA-1 and β-actin controls are provided for comparison (bottom).
Discussion
The goal of this study was aimed toward understanding how specific cytokines, when added to culture medium ex vivo, modify the globin gene expression program of adult human erythroblasts. Previously, it was shown that the globin-regulating effects of signal transduction were pancellular among erythroid progenitor and precursor cells rather than restricted to a cellular subset.15 In humans, the α-like globin genes (5′-ζ, μ, α2, α1, and θ–3′) located on chromosome 16 and β-like genes (5′-ϵ, Gγ, Aγ, δ, and β-3′) on chromosome 11 are expressed in a developmental stage-specific manner. During the fetal-to-adult transition, the embryonic ζ- and ϵ-globins are silenced, the fetal γ-globin genes are down-regulated or silenced, and the δ- and β-globin genes are up-regulated. As a result, the major hemoglobin type in circulating cells switches from fetal (HbF; α2γ2) to adult (HbA; α2β2) types. Consistent with previous reports,15 the cytokine combination of erythropoietin, stem cell factor, and transforming growth factor-β resulted in a reversal of the down-regulated pattern of HbF expression. Increased expression from both γ-globin genes with a preferential enhancement of Gγ- over Aγ-globin chains was observed. In addition, silencing of the ζ- and ϵ-globin gene expression was reversed, but the absolute expression level of the embryonic globins expression remained low compared with the fetal and adult globins. The expression of δ- and β-globins was reduced. Because gene regulation at the α- and β-globin loci is thought to be autonomous,20,21 signal transduction must act in a coordinated fashion according to trends that are opposite to the characteristic changes observed during the fetal-to-adult transition of human life.
Recent reports hint at a role for cytokine signaling in gene transcription through changes in histone tail modifications at specific target loci.22-25 In this study, cytokine signal transduction resulted in multiple histone modifications at the globin genes concomitant with their significantly altered expression. Acetylation of H3K9 was the most reflective of the overall differences in globin gene transcription induced by cytokines in adult erythroblast culture. At the adult genes, H3K9 acetylation under low-HbF culture conditions was approximately twice the level observed in high-HbF conditions. In contrast, acetylation was significantly higher over the γ-globin genes and slightly higher over ϵ-globin gene in high-HbF erythroblasts. The ϵ- and γ-globin genes were additionally marked by the repressive H3K9 dimethylation mark in low-HbF erythroblasts. In contrast, the H3K9 dimethylation mark was replaced by H3K4 dimethylation and trimethylation over the actively expressed γ-globin genes in high-HbF erythroblasts. Further, H3K4 dimethylation increased over the ϵ-globin gene in high-HbF erythroblasts correlating with the low but detectable increase in ϵ-globin expression. Several of the histone marks modifications remained unchanged near the adult globin genes, despite the significant reductions in δ- and β-globin gene expression that were associated with increased ϵ- and γ-globin gene expression. These data indicate that cytokine signal transduction is able to orchestrate complex changes in the methylation and acetylation of histone proteins within the β-globin gene cluster that are coordinated with changes in ϵ-, γ-, δ-, and β-globin gene expression.
In addition to histone modifications, it was shown that cytokines change the nuclear protein expression levels of a subset of transcription factors. Previously, it was determined that MAP kinase signal transduction is involved, but the nuclear events that are downstream of the cytoplasmic signaling cascades were not explored.15 To assist with the experimental design (choice of proerythroblast stage culture for profiling) and the eventual interpretation of the transcription factor profiles, a complete profile of globin transcript and protein expression was generated. Among the 369 transcription factors expressed at relatively high levels, 8 transcription factors consistently demonstrated a nuclear protein expression change in association with the changing globin gene expression patterns. HHEX26 and SOX627 were previously reported as globin gene regulators during ontogeny. Three others were identified in the context of erythroid biology by drug screening or ex vivo model systems (ID2,28 MAFF,29 and EGR130 ). The differential expression patterns of EGR1 and BCL11A are especially intriguing. EGR1 (also named ZIF268) and EKLF share major structural similarities and the oligonucleotides encoding the EGR1 binding motif compete with EKLF in gel shift assays.31 The induction of Egr-1 correlated with suppression of β-globin mRNA expression in Epo-responsive murine erythroblast cell line.30 Moreover, EGR1 is up-regulated in sickle cell disease in response to hydroxyurea.32 EGR1 may play a role in hemoglobin switching by competing with EKLF and suppressing β-globin expression. EGR1 is additionally a downstream target of ELK1,33 another transcription factor identified in this study that is a substrate for all 3 distinct classes of MAP kinase signal transduction pathways.34 Similar to EKLF and EGR1, BCL11A is a C2H2 domain-containing transcription factor.35 Recently, the BCL11A gene was clinically and experimentally linked to fetal hemoglobin regulation.36,37 However, neither splice variation nor major changes in the nuclear expression of this protein were detected in this study (supplemental Figure 4).
Cytokines are essential for the growth and differentiation of hematopoietic cells.38 Erythropoietin is particularly important for the survival and expansion of erythoblasts, and other cytokines further affect the growth and differentiation of the cells through a complex network of signal transduction pathways.39 In this study, cytokines were shown to signal distinct changes in chromatin and the expression patterns in the globin genes. The signaling effects were pancellular, and the differentiation kinetics of the erythroblasts grown under the low-HbF and high-HbF culture conditions were nearly identical (supplemental Figure 1).15,40 With combinatorial signal transduction, it is thus suggested that coordinate regulation in globin gene expression and histone modifications may be achieved in the absence of overt changes in cell growth and differentiation. Although the molecular mechanisms responsible for the modified histone marks and globin gene expression patterns remain vague, the associated change in transcription factor expression supports the notion that at least some of the factors may be involved at the globin locus. Based on their DNA-binding domain diversity (basic leucine zipper, helix-loop-helix, high-mobility-group, homeobox, zinc finger), it is proposed that cytokine signaling may change the transcription of globin genes through modifications in the large regulatory complexes that are localized at the globin loci.41 Experiments involving genetic-based manipulation are underway to determine whether the transcription factors identified here directly associate with the globin locus chromatin or contribute to the cytokine-mediated changes in globin gene expression. Recently, it was demonstrated that manipulation of a small subset of transcription factors was sufficient to induce pluripotency of mammalian cells.42 In that study, transcription factor reprogramming resulted in the generation of stem cells from fibroblasts. We hypothesize that more subtle changes in cellular fate that regulate globin gene expression may be accomplished by cytokine signal transduction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Department of Transfusion Medicine at the National Institutes of Health for assistance in the collection of blood samples, members of the National Institute of Diabetes and Digestive and Kidney Diseases Microarray Core Facility for their assistance, and Andrew Perkins for helpful conversations regarding globin gene regulation.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: O.S., C.M.K., and N.V.B. performed research and wrote the paper; T.T., S.-J.N., and S.-H.G. analyzed and interpreted the informatic data; J.E.R. contributed reagents; C.-N.O. contributed reagents and analyzed data; C.L.R., P.A.O., and E.R.M. assisted with globin and hemoglobin analyses; N.M.G. and C.B. assisted with flow cytometry; Y.T.L. performed research; A.D. supervised and interpreted the ChIP experiments; and J.L.M. assisted and supervised the research team, performed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffery L. Miller, Molecular Medicine Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bldg 10, Rm 9N311, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: jm7f@nih.gov.
References
Author notes
*O.S., C.M.K., and N.V.B. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal