Abstract
The CXCR4/SDF-1 axis has been studied extensively because of its role in development and hematopoiesis. In acute myeloid leukemia (AML), elevated expression of CXCR4 has been shown to correlate with shortened survival. Hy-poxia increases CXCR4 in several tumor models, but the impact of reduced O2 partial pressure (pO2) on expression and biologic function of CXCR4 in AML is unknown. We determined pO2 in bone marrows of AML patients as 6.1% (±1.7%). At this pO2, CXCR4 surface and total expression were up-regulated within 10 hours in leukemic cell lines and patient samples as shown by Western blotting, fluorescence-activated cell sorting, and microscopy. Interestingly, hypoxic cells failed to internalize CXCR4 in response to SDF-1, and upon reoxygenation at 21% O2, surface and total expression of CXCR4 rapidly decreased independent of adenosine triphosphate or proteasome activity. Instead, increased pO2 led to alteration of lipid rafts by cholesterol depletion and structural changes and was associated with increased shedding of CXCR4-positive microparticles, suggesting a novel mechanism of CXCR4 regulation. Given the importance of CXCR4 in cell signaling, survival, and adhesion in leukemia, the results suggest that pO2 be considered a critical variable in conducting and interpreting studies of CXCR4 expression and regulation in leukemias.
Introduction
In recent years, hypoxia has emerged as an important component of the microenvironment of cancer and its influence on the pathophysiology of malignant cells has attracted major attention. In solid tumors, it has been well established that areas with low oxygen tension represent an independent risk factor for poor prognosis and increased risk for metastases.1-4 Although the term “hypoxia” is used frequently, a clear definition is still lacking; in vitro studies comprise oxygen conditions ranging from 0.1% to 3% O2.5-7 Most importantly, what may be true for solid neoplasias remains questionable for hematologic malignancies, especially acute leukemias. Here, the impact of hypoxia on leukemia development and maintenance is less understood, and the oxygen tension of the acute myeloid leukemia (AML) bone marrow is unknown. While animal studies suggest O2 levels less than 10 mm Hg by indirect measurement,8 the 2 studies investigating the actual pO2 of the bone marrow in humans found mean values between 48 and 54.9 mm Hg9,10 and were carried out in patients with chronic pulmonary disease (COPD) and healthy volunteers, respectively. Although these levels are “hypoxic” compared with normal air with approximately 160 mm Hg O2 (equals standard laboratory conditions), the study by Harrison et al9 points out that a pO2 of 54.9 mm Hg or 7.2% in healthy humans is considered “physiologic.” Taken together, therefore, it is reasonable to assume that gradients of O2 from 1% (in hypoxic niches) to 6% (in the sinusoidal cavity) exist within the bone marrow
The chemokine receptor CXCR4 and its ligand SDF-1 have attracted attention for their physiologic role in AIDS and in the development and maintenance of the hematopoietic and immune system,11,12 as well as the development of metastases in solid tumors and the trafficking of hematologic malignant cells like chronic lymphocytic leukemia (CLL) and AML.11 For the latter, patients whose blasts express high levels of CXCR4 display a worse prognosis than do those with little or no expression.13,14 As a potential explanation for this correlation, the crosstalk between the bone marrow stroma and the leukemic blasts mediated via the CXCR4/ SDF-1 axis has been proposed; and indeed, inhibition of CXCR4 signal transduction can overcome stroma-mediated chemoresistance in AML and CLL.15
The connection between CXCR4 and hypoxia has been suggested after the recognition that the CXCR4 and SDF-1 genes are targets of hypoxia-inducible factor 1 (HIF-1), and the observation that at 1% O2, CXCR4 is up-regulated on monocytes, monocyte-derived macrophages, tumor-associated macrophages, endothelial cells, and cancer cell lines (eg, ovarian, breast, and renal carcinoma),16 as well as on normal and malignant B lymphocytes.5 However, the effects of the physiologic pO2 of the bone marrow on the expression of CXCR4 in AML have not been investigated.
In order to function, CXCR4 must be localized within the lipid rafts of cellular membranes.17,18 In these cholesterol-rich plasma membrane fractions, proteins like receptors and their second messengers have to reside for proper function, for example, the T-cell receptor with the adjacent Lck kinase.19,20 The observations that reactive oxygen species (ROS) and oxygen levels themselves can alter cholesterol in cellular membranes were made nearly 20 years ago,21-23 before the discovery of lipid rafts. However, as is known from experiments using methyl-β-cyclodextrin (MβCD), depletion of cholesterol from lipid rafts leads to destruction of raft structure with various consequences for surface expression and signaling of raft-dependent proteins. Thus, recent observations are linking lipid raft and subsequent signaling with oxygen/ROS.24
The purposes of this study were (1) to determine the physiologic pO2 in the bone marrow of AML patients and (2) to evaluate the influence of this pO2 on CXCR4 expression and biologic function of the CXCR4/ SDF-1 axis in AML cell lines and primary samples. Our findings demonstrate that AML bone marrows display a reduced oxygen tension, and, at this oxygen tension, CXCR4 surface expression is increased and associated with lipid rafts. Most interestingly, however, is the observation that during reoxygenation, CXCR4 expression is lost within minutes, via in part, by shedding of CXCR4-positive microparticles. These effects were independent of proteasome or adenosine triphosphate (ATP) levels and appeared to be mediated directly by oxygen tension. Taken together, these results suggest that reduced pO2 of physiologic relevance promotes profound changes in membrane structure and CXCR4 signaling.
Methods
Measurement of pO2 in the bone marrow
Bone marrow aspirates were collected from AML patients who underwent routine bone marrow aspiration into heparinized syringes. The samples, comprising approximately 2 mL of aspirate, were collected immediately after aspiration, and all air was evacuated from the syringe. The sample was closed airtight without any air bubbles and analyzed within 5 to 10 minutes after aspiration for pO2 by the Portable Clinical Analyzer (i-STAT, Princeton, NJ) with G3+ cartridges (Abbott Laboratories, East Windsor, NJ). Sequential measurements performed on 3 samples demonstrated no significant change in pO2 from 5 to 15 minutes while the syringe remained capped (data not shown).
Cell culture and patients
U937 cells were purchased from ATCC (Rockville, MD) and OCI-AML3 cells were obtained from Dr M.D. Minden (Ontario Cancer Institute, Toronto, ON). Cell lines were maintained in RPMI 1640 containing 10% FCS (Gemini Bio-Products, Woodland, CA) and 1% penicillin-streptomycin(Life Technologies Laboratories, Grand Island, NY). Peripheral blood samples from patients with AML were collected during routine diagnostic procedures after informed consent was obtained in accordance with Institutional Review Board regulations of The University of Texas M. D. Anderson Cancer Center and the Declaration of Helsinki. Mononuclear cells were separated by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density-gradient centrifugation.
Standard laboratory (normoxic) conditions comprised 21% O2, 5% CO2, and 37°C. For experiments in a reduced oxygen environment, the hypoxic Workstation INVIVO2 400 from Ruskinn Technology (Bridgend, United Kingdom) was used. Cells were incubated in 6% O2, 5% CO2, and 37°C. Cell lines were incubated for at least one week before experiments for adjustment to hypoxic conditions. Patient samples were acclimated for 24 hours.
Antibodies and reagents
CXCR4 Antibody (extracellular loop) was obtained from Chemicon (Millipore, Billerica, MA). Recombinant human SDF-1α was purchased from R&D Systems (Minneapolis, MN). Fluorescein isothiocyanate (FITC)–labeled anti-human CXCR4 (12G5) was purchased from BD PharMingen (San Diego, CA); antibodies to human Akt, Ser473-phosphorylated Akt, Erk, and phosphorylated Erk were purchased from Cell Signaling Technology (Beverly, MA). AMD3100, lactacystin, and MβCD were from Sigma-Aldrich. Cholera toxin subunit B conjugated with Alexa 488 was from Invitrogen (Carlsbad, CA).
Flow cytometry and live imaging
Flow cytometric data were acquired using a FACSCalibur cytometer (BD Biosciences, San Jose, CA). For assessment of CXCR4 expression, the mean fluorescent intensity was measured using a FITC-labeled antibody compared with isotype or unstained control. Analysis of expression was performed after scatter-gating for live cells using BD CellQuest Pro software. Cells in 6% O2 were kept under hypoxia until immediately before data acquisition.
For detection of microparticles, forward and sidescatter were set to logarithmic amplification and 2 gates were defined: 1 including the cellular fraction and 1 including the fraction comprising subcellular particles. For detection of microparticles, cells were washed twice with prefiltered (0.5 μm) phosphate-buffered saline (PBS), then either reoxygenated with 21% O2 or kept in hypoxia until measurement.
For live imaging, an Olympus IX81 Microscope with DSU confocal attachment (Center Valley, PA) and a Hamamatsu Orca II-ER camera (Bridgewater, NJ) was used with Slidebook Software from Intelligent Imaging Innovations (Denver, CO). Cells were incubated with RPMI (Gemini Bio Products, Woodland, CA) and CXCR4 antibody (BD Pharmingen) or quantum dots (Qtracker 565; Invitrogen) and kept at hypoxic conditions. For imaging, cells were removed from the hypoxic incubator in a parafilm-sealed plate.
Sucrose density centrifugation
OCI-AML3 cells (3 × 107) were washed with PBS and cell pellets were resuspended in 300 μL of lysis buffer (25 mM Tris [tris(hydroxymethyl)aminomethane], 2 mM ethylenediaminetetraacetic acid [EDTA], 150 mM NaCl, 1% Triton-X). Lysates were mixed with equal volume of 80% sucrose, placed in a centrifuge tube on top of 300 μL 80% sucrose and layered with 900 μL of 30% sucrose and 600 μL of 10% sucrose. Tubes were then centrifuged at 100 000g for 16 hours. Fractions (240 μL) were collected from the top of the tube and frozen at −20°C.
Assays for measurement of protein concentration and cholesterol
Protein concentration was measured with the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) and the readout was carried out in a photometer set to absorbance mode (BMG Labtech, Offenburg, Germany). Cholesterol measurement was performed similarly with the Amplex Red Cholesterol Assay Kit from Molecular Probes (Eugene, OR).
Western blotting and RT-PCR
For Western blot analysis, cells were lysed in phosphoprotein lysis buffer (150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM NaF, 5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1% Triton ×-100, 10 mM iodoacetamide, 1 mM Na3VO4, 0.1% NaN3, and 3 mM phenylmethylsulfonyl fluoride) in either normoxia or hypoxia. Lysis buffer was supplemented with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). For Western blotting of supernatants from cell culture, 2 mL of supernatant of 20 × 106 cells was concentrated with Centricon Centrifugal Filter Devices YM-10 (Millipore). Lysates were then separated on a 10% to 12% polyacrylamide gel, transferred to Hybond-P membranes (GE Healthcare, Little Chalfont, United Kingdom), probed with the appropriate antibodies, visualized using an enhanced chemiluminescence plus kit (GE Healthcare) and analyzed on a STORM-860 system using Imagequant software (Molecular Dynamics, Sunnyvale, CA).
Total RNA was obtained using Trizol reagent (Invitrogen, Eugene, OR) from OCI-AML3 and U937 cells. From 2 μg of RNA, cDNA was made using the commercially available First Strand cDNA Synthesis Kit for RT-PCR (AMV) from Roche Diagnostics. One microgram of cDNA was used for real time RT-PCR using the TaqMan Fast Universal PCR Master-Mix and TaqMan Gene Expression Assay for CXCR4 from Applied Biosystems (Foster City, CA). Real-time polymerase chain reaction (RT-PCR) for ABL was run as control. PCR was performed in the 7900HT Fast-Real-Time PCR System with SDS2.3 Software from Applied Biosystems.
Migration assays
A total of 106 cells in a volume of 100 μL were added to the top chamber of 6.5-mm diameter Transwell Culture Inserts (Costar, Corning, NY) with a pore size of 5.0 μm. Inserts were placed in wells containing 0.6 mL serum-free RPMI with 0 to 100 ng/mL of SDF-1α. Chemotaxis assays were done at 37°C for 24 hours. Cells migrating to the lower chamber were counted by flow cytometry using CountBright Absolute Counting beads from Invitrogen.
Statistical analysis
Results are shown as the mean plus or minus SD or SE of at least 3 experiments (in duplicates) each. Paired data were analyzed using the paired Student t test. Microsoft Excel was used for data acquisition and storage. A P value less than .05 was considered statistically significant.
Results
Oxygen partial pressure in the leukemic bone marrow
Recent reports have suggested the O2 content of the bone marrow is 7.2% to 6.3% in healthy volunteers and patients with COPD.9,10 To approximate the oxygen content in AML bone marrow, 19 leukemia patients (12 in CR and 7 with active AML) were analyzed. The average age was 57 years (range, 33-77 years). The average level of pO2 in the bone marrow of these patients was 46.05 mm Hg (range, 35-63 mm Hg; 6.06% ± 1.7%). Thus, a level of 6% was chosen for subsequent experiments.
Oxygen partial pressure regulates CXCR4 expression in AML
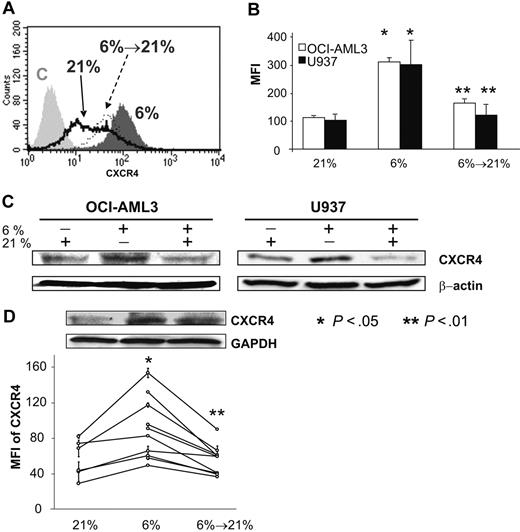
To investigate the expression of CXCR4 on leukemic cell lines in a reduced-oxygen environment, we used U937 and OCI-AML3 cells. From these cell lines, we established hypoxia-conditioned clones by adjustment to 6% O2 for at least one week before further analysis. As shown in Figure 1A,B, these hypoxia-adapted cell lines displayed significantly increased CXCR4 expression compared with their normoxic counterparts by flow cytometry. OCI-AML3 cells showed an average increase of 2.8-fold (average mean fluorescence intensity [MFI], 112.26-310.83; P < .05) and U937 of 2.9-fold (average MFI, 102.94-300.42; P < .05) at 6% O2. Notably, Figure 1A,B also shows that reoxygenation of these cell lines with 21% O2 leads to a decrease of CXCR4 expression to a level similar to the normoxic cells. In U937 cells, expression dropped to an average MFI of 121.70 or 37.8% of the hypoxic value (P < .01) and OCI-AML3 dropped to an average MFI of 149.96 or 52.8% (P < .01).
Reduced oxygen tension of 6% O2 induces increases in both surface and total CXCR4 expression in AML cell lines and patient samples. Reoxygenation with 21% O2 induces loss of CXCR4 on hypoxic samples. (A) Histogram of representative experiment showing CXCR4 expression of OCI-AML3 cells under 21% (black line), 6% (dark gray), and reoxygenation with 21% O2 of at least 1 hour (of cells adjusted to hypoxia; black dotted line). Surface expression is shown relative to an irrelevant IgG control (C, light gray). (B) Up-regulation and loss of CXCR4 surface expression depends on O2 in AML cell lines OCI-AML3 and U937. (C) In OCI-AML3 and U937 cell lines, physiologic hypoxia increases total amount of CXCR4 as shown by Western blotting, which is lost when cells are reexposed to 21% O2. (D) Samples from patients with AML (n = 10) show a concordant pattern: using flow cytometry, hypoxia leads to a statistically significant increase in the MFI of CXCR4 expression (n = 6). Increase of O2 from 6% to 21% leads to a significant loss in MFI (n = 10). Western blotting also shows the changes (1.6-fold increase in OD at 6% O2 and 0.7-fold decrease after reoxygenation) in total protein in 1 primary AML sample. Graphs show mean values and SD.
Reduced oxygen tension of 6% O2 induces increases in both surface and total CXCR4 expression in AML cell lines and patient samples. Reoxygenation with 21% O2 induces loss of CXCR4 on hypoxic samples. (A) Histogram of representative experiment showing CXCR4 expression of OCI-AML3 cells under 21% (black line), 6% (dark gray), and reoxygenation with 21% O2 of at least 1 hour (of cells adjusted to hypoxia; black dotted line). Surface expression is shown relative to an irrelevant IgG control (C, light gray). (B) Up-regulation and loss of CXCR4 surface expression depends on O2 in AML cell lines OCI-AML3 and U937. (C) In OCI-AML3 and U937 cell lines, physiologic hypoxia increases total amount of CXCR4 as shown by Western blotting, which is lost when cells are reexposed to 21% O2. (D) Samples from patients with AML (n = 10) show a concordant pattern: using flow cytometry, hypoxia leads to a statistically significant increase in the MFI of CXCR4 expression (n = 6). Increase of O2 from 6% to 21% leads to a significant loss in MFI (n = 10). Western blotting also shows the changes (1.6-fold increase in OD at 6% O2 and 0.7-fold decrease after reoxygenation) in total protein in 1 primary AML sample. Graphs show mean values and SD.
To investigate whether these changes represent changes of surface expression of CXCR4, Western blotting for CXCR4 was performed to estimate total levels of protein in the 3 different stages. As shown in Figure 1C, hypoxia of 6% increased total levels of the CXCR4 band at 42 kD compared with normoxic (ie, 21%) parental cells both in OCI-AML3 and U937 (average increase in optical density [OD] on multiple blots, 42.7% ± 2%). Reoxygenation of cells adjusted to 6% with 21% O2 lead to a loss of total protein in both cell lines (average decrease in OD, 50.3% ± 15%).
To investigate whether this phenomenon was a cell line–specific event, we performed similar experiments with 10 samples from patients with AML. Reduced O2 content led to a 1.55-fold increase of CXCR4 (average MFI, 56.78-88.29; P < .05) on 6 samples within 24 hours. In these and an additional 4 samples (kept only at hypoxia), the effect of increase of O2 on CXCR4 expression was analyzed. Here, a decrease to 61.3% of the hypoxic value was observed (average MFI in hypoxia, 90.60 vs average MFI after reoxygenation, 55.57; P < .01). Figure 1D shows the results individually for all of the 10 samples. Hypoxia, however, did not change the percentage of CXCR4-positive cells (normoxia 85.5% ± 12.0%, hypoxia 86.8% ± 11.1%, reoxygenated 90.1% ± 15.7%; P = not significant). The above data were confirmed by Western blot of primary AML blasts, as shown in Figure 1D (1.6-fold increase in OD at 6% O2 and 0.7-fold decrease after reoxygenation), suggesting that primary samples from patients with AML show behavior similar to AML cell lines.
Reduced pO2 promotes increase in CXCR4 expression
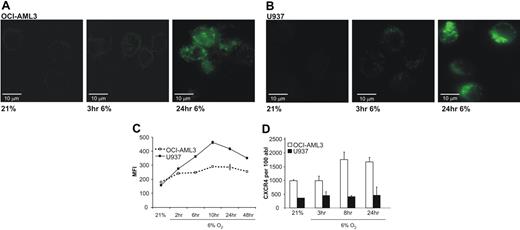
To determine the kinetics of up-regulation of CXCR4 under reduced oxygen tension, U937 and OCI-AML3 cells were investigated by live imaging and flow cytometry. For live imaging, cells were incubated with the antibody under 21% and then exposed to hypoxia of 6% and imaged at the given time points. Figure 2A,B shows the results for OCI-AML3 and U937 cells. As early as 3 hours after hypoxia, cells show an increase in CXCR4 expression reaching a maximum after 24 hours.
AML cell lines up-regulate CXCR4 within the first 2 – 10 hours of physiologic hypoxia. Up-regulation of transcription seems not to be involved as a major factor of increased expression. (A) Live microscopy (40×/1.5 NA) of OCI-AML3 cells after incubation with FITC-labeled CXCR4 antibody. Images show not only an increase of total CXCR4 as early as 3 hours after start of hypoxia, but also a distinct punctate pattern. (B) U937 cells show an identical behavior with rapid increase of CXCR4 and characteristic pattern. (C) Flow cytometry reveals increased CXCR4 as soon as 2 hours after initiation of hypoxia. (D) Real time RT-PCR for CXCR4 cDNA of OCI-AML3 and U937 cells. Transcriptional activity increases only 8 hours after hypoxia and only to a moderate extent (∼2-fold) in OCI-AML3, and not at all in U937. Graphs show mean and SD.
AML cell lines up-regulate CXCR4 within the first 2 – 10 hours of physiologic hypoxia. Up-regulation of transcription seems not to be involved as a major factor of increased expression. (A) Live microscopy (40×/1.5 NA) of OCI-AML3 cells after incubation with FITC-labeled CXCR4 antibody. Images show not only an increase of total CXCR4 as early as 3 hours after start of hypoxia, but also a distinct punctate pattern. (B) U937 cells show an identical behavior with rapid increase of CXCR4 and characteristic pattern. (C) Flow cytometry reveals increased CXCR4 as soon as 2 hours after initiation of hypoxia. (D) Real time RT-PCR for CXCR4 cDNA of OCI-AML3 and U937 cells. Transcriptional activity increases only 8 hours after hypoxia and only to a moderate extent (∼2-fold) in OCI-AML3, and not at all in U937. Graphs show mean and SD.
As shown in Figure 2C, CXCR4 expression is already increased 1.4-fold in OCI-AML3 and 1.8-fold in U937 cells after 2 hours, reaching maximal expression after 10 hours. No further increase was observed during an additional 38 hours of incubation.
Real-time RT-PCR was performed to answer the question whether the increase was due to increased transcriptional activity. In summary, as shown in Figure 2D, no significant increase in CXCR4 mRNA standardized to ABL mRNA was observed during prolonged hypoxia of 6%. While surface expression increased as early as 3 hours by microscopy, no relevant increase in the amount of mRNA could be observed by RT-PCR. While U937 showed a 1.3-fold increase after 3 hours, the maximal 1.8-fold increase in OCI-AML3 was achieved only after 8 hours. These data suggest that an increased transcriptional activity does not play a critical role in the hypoxia-induced rapidly increased expression of CXCR4.
Reduced pO2 changes the response of SDF-1/CXCR4 signaling in AML
Next, we addressed whether these changes in CXCR4 surface expression also have functional consequences. As CXCR4 is known to be internalized upon ligand-binding under normoxia, this was investigated under 6% O2 (Figure 3A). Whereas SDF-1 led to a significant decrease in CXCR4 surface expression in normoxia, the chemokine induced a significant increase in CXCR4 surface expression in OCI-AML3 and U937 cells under hypoxia. Migration of OCI-AML3 and U937 cells toward increasing doses of SDF-1 was significantly augmented under hypoxia after 24 hours (Figure 3B), most likely due to increased expression of CXCR4. However, as the receptor is not internalized in hypoxia, internalization is likely not important for chemotaxis.
The SDF-1/CXCR4 axis displays different features under 6% and 21% O2 levels. (A) SDF-1 (50 ng/mL) leads to a statistically significant decrease of CXCR4 under normoxia in OCI-AML3 and U937 cell lines. However, under 6% O2, exposure to the same amount of SDF-1 leads to a significant increase of CXCR4 expression. (B) Dose-response curves of leukemic cell lines reveal a significant increase (OCI-AML3 maximum 1.6-fold, U937 maximum 2.2-fold) in migration toward SDF-1 at 6% O2. Graphs show mean and SD. (C) Western blotting of OCI-AML3 after stimulation of CXCR4 with SDF-1 under 6% and 21% O2. Hypoxic cells show a dramatic increase in Akt and Erk stimulation, but no response to SFD-1.
The SDF-1/CXCR4 axis displays different features under 6% and 21% O2 levels. (A) SDF-1 (50 ng/mL) leads to a statistically significant decrease of CXCR4 under normoxia in OCI-AML3 and U937 cell lines. However, under 6% O2, exposure to the same amount of SDF-1 leads to a significant increase of CXCR4 expression. (B) Dose-response curves of leukemic cell lines reveal a significant increase (OCI-AML3 maximum 1.6-fold, U937 maximum 2.2-fold) in migration toward SDF-1 at 6% O2. Graphs show mean and SD. (C) Western blotting of OCI-AML3 after stimulation of CXCR4 with SDF-1 under 6% and 21% O2. Hypoxic cells show a dramatic increase in Akt and Erk stimulation, but no response to SFD-1.
Figure 3C shows a Western blot of serum-starved OCI-AML3 cells under 6% and 21% O2 before and after treatment with 100 ng/mL SDF-1. Although SDF-1 leads to activation of Akt as shown by increased phosphorylation in normoxia (average increase in OD on 3 blots, 2.9 ± 1.3-fold), which has been reported previously,15 no such effect was observed under 6% O2, perhaps due to the lack of internalization. Interestingly, OCI-AML3 blasts acclimated to 6% O2 showed a much higher level of endogenous phosphorylation both for Akt and Erk.
Increase in O2 partial pressure leads to loss of CXCR4 and structural changes in cellular membranes
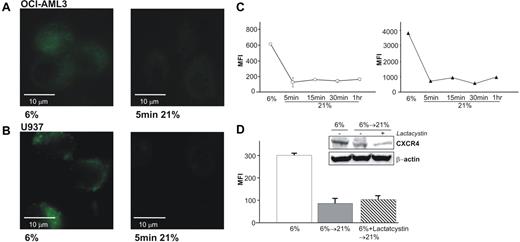
As shown in Figure 1, reoxygenation of hypoxic AML blasts with 21% O2 leads to a rapid loss of CXCR4. Figures 4A and 4B show the loss of CXCR4 on living, unfixed OCI-AML3 and U937 cells. Noteworthy is the rapid kinetics of this phenomenon. After 5 minutes of 21% O2, the cells show an expression level similar to that of normoxic cells (as shown in Figure 2A,B). As can be seen by microscopy, the punctuated pattern observed under reduced oxygen tensions is lost. Figure 4C shows the timeline of this process in fixed OCI-AML3 cells. Surface CXCR4 (left subpanel, white circles) is lost within 5 minutes, and total amount of the protein (right subpanel, black triangles) is decreased in the same time.
Reexposure of AML cells adjusted to 6% O2 result in a very rapid loss of surface and total CXCR4. (A) Live imaging (40×/1.5 NA) of OCI-AML3 cells at 6% (left) and 5 minutes reoxygenation with 21% O2 (right) show the loss of bound FITC-labeled CXCR4 antibody. (B) Similar results are found for U937 cells. (C) Flow cytometric analysis of OCI-AML3, either surface-stained or fixed with 2% PFA and methanol permeabilized for total CXCR4, shows a simultaneous loss of surface CXCR4 (left, ○) and total protein (right, ▴) within 5 minutes. Graphs show means and SD. (D) Treatment of OCI-AML3 with proteasome inhibitor lactacystin (10 mM) before reoxygenation prevents neither loss of surface expression of CXCR4 by flow cytometry nor total CXCR4 by Western blotting.
Reexposure of AML cells adjusted to 6% O2 result in a very rapid loss of surface and total CXCR4. (A) Live imaging (40×/1.5 NA) of OCI-AML3 cells at 6% (left) and 5 minutes reoxygenation with 21% O2 (right) show the loss of bound FITC-labeled CXCR4 antibody. (B) Similar results are found for U937 cells. (C) Flow cytometric analysis of OCI-AML3, either surface-stained or fixed with 2% PFA and methanol permeabilized for total CXCR4, shows a simultaneous loss of surface CXCR4 (left, ○) and total protein (right, ▴) within 5 minutes. Graphs show means and SD. (D) Treatment of OCI-AML3 with proteasome inhibitor lactacystin (10 mM) before reoxygenation prevents neither loss of surface expression of CXCR4 by flow cytometry nor total CXCR4 by Western blotting.
To investigate whether the rapid intracellular degradation is due to cytoplasmatic proteasome activity, we used 10 mM lactacystin to inhibit proteasome activity. As shown in Figure 4D, lactacystin could neither prevent loss of surface expression upon reoxygenation with 21% O2 nor loss of total protein as shown by Western blotting (instead, lactacystin seems to promote CXCR4 degradation upon increase in O2). Similar experiments using the proteasome inhibitor MG-132 and potassium cyanide for disruption of ATP production revealed no effect of these compounds on degradation of CXCR4 (data not shown).
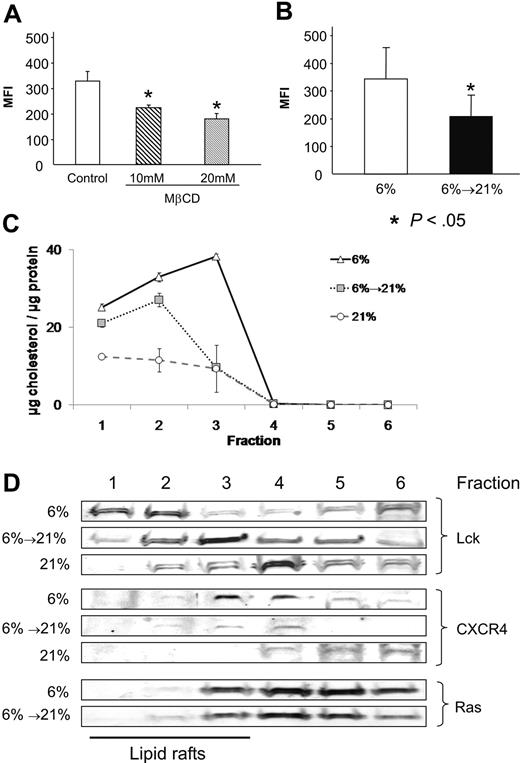
To clarify whether CXCR4 is located in lipid rafts under a reduced O2 environment as suggested by the punctuated, localized expression pattern, OCI-AML3 cells were exposed to MβCD, which disrupts lipid rafts by sequestration of cholesterol from the cholesterol-rich rafts. Similar to what has been reported for cells at 21%,17 MβCD showed a dose-dependent statistically significant (P = .01) decrease in CXCR4 expression at 6% (10 mM MβCD: 68%, 20 mM MβCD: 55% MFI compared with untreated control; Figure 5A), suggesting that CXCR4 is at least partially located in lipid rafts at 6% O2. To establish whether increase of O2 leads to changes in lipid rafts in hypoxic cells, we used the cholera toxin subunit B (conjugated with Alexa488), which is known to bind to GM1-gangliosides, an important component of lipid rafts. The result shown in Figure 5B demonstrates that reoxygenated cells showed a significantly lower binding of cholera toxin subunit B compared with hypoxic cells (average MFI, 210.25 vs 343.87) in OCI-AML3 cells.
CXCR4 is located in lipid rafts in physiologic hypoxia and reoxygenation changes lipid rafts. (A) Treatment of hypoxic OCI-AML3 cells with MβCD results in a dose-dependent decrease of CXCR4 expression. (B) OCI-AML3 stained with FITC-labeled β-subunit cholera toxin show a statistically significant loss of GM1-Ganglioside upon reoxygenation. (C) Reoxygenation leads to loss of cholesterol in lipid raft fractions 1 to 3 in OCI-AML3 cells after sucrose density centrifugation, mimicking MβCD treatment. Cells kept at 21% O2 show the lowest cholesterol per protein content. (D) Western blots of fractions after sucrose density centrifugation: Lck shows redistribution in reoxygenated cells; CXCR4 is lost from lipid rafts during reoxygenation, while Ras is unaltered. Cells kept at 21% are shown as control. Graphs show mean and SD.
CXCR4 is located in lipid rafts in physiologic hypoxia and reoxygenation changes lipid rafts. (A) Treatment of hypoxic OCI-AML3 cells with MβCD results in a dose-dependent decrease of CXCR4 expression. (B) OCI-AML3 stained with FITC-labeled β-subunit cholera toxin show a statistically significant loss of GM1-Ganglioside upon reoxygenation. (C) Reoxygenation leads to loss of cholesterol in lipid raft fractions 1 to 3 in OCI-AML3 cells after sucrose density centrifugation, mimicking MβCD treatment. Cells kept at 21% O2 show the lowest cholesterol per protein content. (D) Western blots of fractions after sucrose density centrifugation: Lck shows redistribution in reoxygenated cells; CXCR4 is lost from lipid rafts during reoxygenation, while Ras is unaltered. Cells kept at 21% are shown as control. Graphs show mean and SD.
To establish whether reoxygenation itself affects lipid rafts, we isolated lipid raft fractions by sucrose density centrifugation.25 By measurement of protein and cholesterol content of the different fractions, the first 3 fractions were attributed to containing the raft fraction. Interestingly, Figure 5C shows that during reoxygenation lipid rafts lose cholesterol. Detailed analysis of the individual fractions was performed by Western blotting and is shown in Figure 5D. The formation of Lck, a member of the Src family of tyrosine kinases closely associated with lipid rafts, was altered in hypoxic and reoxygenated cells. While the total amount was not decreased, Lck was reorganized from the first 2 fractions in hypoxia to the heavier fractions in normoxia. CXCR4 localization was unchanged, but the total amount was decreased in the first 3 fractions already after short-time reoxygenation, which was even more significant compared with cells kept at 21% O2. The majority of CXCR4 in normoxia is not localized in lipid rafts but in the cytoplasma, as recently reported.26 Notably, Ras, which is localized to the inner leaflet of the cell membrane in its biologically relevant form, was not changed during the process.
Taken together, these data strongly suggest that increased O2 in the surrounding environment changes cholesterol content in the cellular membrane, thus affecting lipid rafts with fundamental changes in their architecture and potential function.
AML blasts release CXCR4 and CXCR4-containing microparticles upon increase of pO2
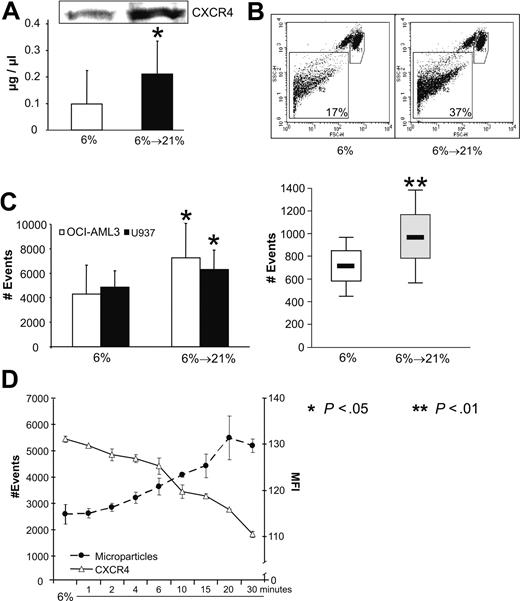
The observed changes in the membrane and lipid raft fractions cannot by themselves elucidate the reason for the decrease of CXCR4. As internalization and subsequent proteasome-dependent degradation can be largely ruled out, another possible mechanism is the shedding of CXCR4. To address this question, we first measured the protein content of the supernatant of 2 × 107 hypoxic and reoxygenated cells. As shown in Figure 6A protein concentration of PBS conditioned by reoxygenated cells was significantly increased compared with that conditioned by hypoxic cells (mean, 0.99 μg/μL vs 0.21 μg/μL; P < .05). As shown by Western blotting, CXCR4 was among the proteins increased in culture supernatants derived from reoxygenated cells.
Reoxygenation of cells acclimated to 6% O2 leads to shedding of CXCR4 and release of microparticles. (A) Protein concentration in the supernatant of 2 × 107 OCI-AML3 cells under 6% and after reexposure with 21% shows a significant increase. The protein fraction also contains CXCR4 as shown by Western blotting. (B) Dot plot of flow cytometry analysis of OCI-AML3 cells. R1 indicates gate for cells. A total of 10 000 cells were counted. R2 indicates gate for microparticles. (C) Number of events in R2 are significantly (P < .05) increased upon reoxygenation in OCI-AML3 and U937 cell lines (left) and patient samples (n = 4, right). (D) OCI-AML3 cells show a highly significant correlation between increase in microparticles and decrease of CXCR4 expression over a period of 30 minutes. Graphs show mean values and SD.
Reoxygenation of cells acclimated to 6% O2 leads to shedding of CXCR4 and release of microparticles. (A) Protein concentration in the supernatant of 2 × 107 OCI-AML3 cells under 6% and after reexposure with 21% shows a significant increase. The protein fraction also contains CXCR4 as shown by Western blotting. (B) Dot plot of flow cytometry analysis of OCI-AML3 cells. R1 indicates gate for cells. A total of 10 000 cells were counted. R2 indicates gate for microparticles. (C) Number of events in R2 are significantly (P < .05) increased upon reoxygenation in OCI-AML3 and U937 cell lines (left) and patient samples (n = 4, right). (D) OCI-AML3 cells show a highly significant correlation between increase in microparticles and decrease of CXCR4 expression over a period of 30 minutes. Graphs show mean values and SD.
The presence of CXCR4 positive microparticles has recently been reported.27 This finding, together with the alterations of the cellular membrane and the lipid rafts, could therefore support a possible mechanism of shedding a transmembrane protein. Therefore, we measured the amount of microparticles released by AML cell lines and primary blast under hypoxia and after reoxygenation with 21% O2. Figure 6B shows a dot plot of OCI-AML3 cells and the gating strategy (R1 indicates cells; R2, microparticles). After reoxygenation with 21% O2, the number of microparticles is increased, and Figure 6C shows the respective statistics for cell lines (left) and patient samples (right). In OCI-AML3 and U937 cells, significant 1.7- and 1.3-fold increases were observed, and similarly in 5 patient samples, a 1.3-fold increase (P < .01). Similarly, disruption of lipid rafts of normoxic OCI-AML3 cells with MβCD leads to an increase in microparticles as well (data not shown). Figure 6D depicts the correlation between the loss of CXCR4 and the concomitant increase in microparticles over a time period of 30 minutes in OCI-AML3. With a correlation coefficient r = 0.92, these data suggest that the loss of CXCR4 may be mediated, at least in part, by the shedding of microparticles when pO2 is increased.
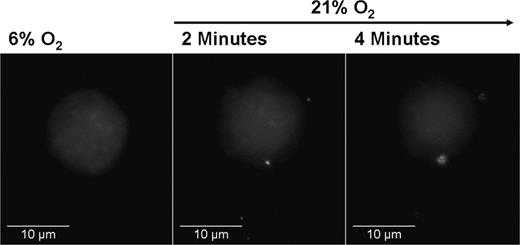
In accordance with previous reports, we found that these microparticles stained positive for CXCR4, however, there was no significant difference in the surface expression of CXCR4 between those released by hypoxic and those released by reoxygenated cells (data not shown). At least some of these microparticles seem to have a vesicular structure, as is shown in Figure 7, where OCI-AML3 cells were loaded with quantum dots (nanoparticles accumulating in the cytoplasm) in reduced oxygen conditions and then reoxygenated. As soon as 2 minutes after the increase in O2, the cells released particles containing fluorescent quantum dots. This indicates that the microparticles, at least in part, do not only comprise membrane particles, but also contain cytoplasmic components. The detailed structure of these particles, however, needs further investigation.
OCI-AML3 cell loaded with cytoplasmic quantum dots. (Left) Representative cell at 6% O2. (Middle) The same cell after 2 minutes of reoxygenation with 21% O2. Four Qdot-containing particles leaving the cell can be seen in the picture. (Right) The cell loses fluorescence, indicating loss of Qdots. (100×/1.4 NA oil.)
OCI-AML3 cell loaded with cytoplasmic quantum dots. (Left) Representative cell at 6% O2. (Middle) The same cell after 2 minutes of reoxygenation with 21% O2. Four Qdot-containing particles leaving the cell can be seen in the picture. (Right) The cell loses fluorescence, indicating loss of Qdots. (100×/1.4 NA oil.)
Discussion
The data presented here are the first to show a rapid, direct influence of O2 partial pressure on the expression of CXCR4 on AML blasts by alterations of environmental O2. The influence of hypoxia on the expression of CXCR4 has been shown on several normal and malignant cells and the mechanism responsible for the observed up-regulation has been proposed to be mediated by HIF-1.16 In contrast, here we show that transcriptional activity does not seem to be paramount for the increase of expression, but rather that these changes already take place under conditions in which the O2 content exceeds the conventional “hypoxic” conditions of less than 3%.
These findings lead to a new paradigm. Conventional studies investigate hypoxia and its effect by comparing cells under normoxic (ie, 21% O2) with hypoxic conditions (ie, < 3% O2). In some studies, even lower O2 conditions are applied, sometimes being close to the margin of anoxia.5 (Notably, this last condition of anoxia was used less than 20 years ago as a model for ischemia and ischemia-induced cell death.28-30 ) However, what may be true for epithelial tumors may not apply equally to hematopoeitic cells, which are usually mobile and, therefore, can evade an inhospitable environment. The bulk of leukemic cells will prefer an environment that supports their biologic needs and reside in the bone marrow and the peripheral blood in the majority of patients. Whether the bone marrow itself is a hypoxic environment has long been speculated and investigations have been focused rather on identification of elusive hypoxic regions8 without ever considering the overall pO2 of the bone marrow. Here we show that this parameter in 19 leukemic patients is 6.06% and, therefore, identical to the already published data.9,10
The observed O2 levels of 6% should therefore be considered “physiologic” O2 partial pressure, as this is the normal pO2 of the bone marrow of AML patients. The amount of 6% is considerably lower than the 21% O2, which is presently considered laboratory standard. Therefore, we recommend the term “physiologic hypoxia” should be used in further discussions to refer to oxygen environments in the 6% range.
The data described here emphasize the difference between normal laboratory standard and physiologic conditions. Under physiologic hypoxia, CXCR4 expression is higher and the expression pattern differs. The biology is also different: cell migration toward SDF-1 is increased, the cells fail to decrease surface expression of the chemokine receptor upon binding, and exposure to SDF-1 does not result in increased phosphorylation of Akt. However, regulation of intracellular signal transduction pathways is altered under physiologic conditions, as shown by increased phosphorylation of Akt and Erk at 6% O2 compared with cells cultured at 21%. The mechanisms for this variable behavior toward SDF-1 and the intracellular signaling are unclear and warrant further investigation. Thus, it would be interesting to see which of the changes attributed to classic hypoxia (ie, < 3% O2) are not associated with hypoxia at all, but can also be found at the physiologic 6% O2.
The other important finding of this study is the realization that changes in the O2 partial pressure affects cells not only when oxygen is lowered, but also when it is increased. While long-term changes have not been investigated in this project, we clearly demonstrate that an increase from 6% to 21% O2 affects the cellular content of CXCR4. Surprisingly, this took place within 5 to 10 minutes and could be found both in AML cell lines and in patient samples. This finding is relevant in several ways, not only as a novel biologic mechanism, but also from a clinical point of view. The change from 6% resembles the oxygen content of the peripheral blood, which means that by drawing venous blood with subsequent processing under standard laboratory conditions (ie, 21% O2) our experimental setting of 6% and reoxygenation with 21% O2 is mimicked. This raises the question whether the measured CXCR4 levels of AML blasts really represent actual CXCR4 expression levels or may strongly underestimate expression in the published reports.13,14 However, these findings may be sustained; although absolute expression levels were higher at 6%, relative expression of CXCR4 was mostly similar at 21% O2. Nevertheless, further studies are warranted before any final conclusions can be drawn.
The event that we observed and that is at least in part responsible for the loss of CXCR4 is unexpected and has not been described for AML cells before: our data imply an oxygen-dependent loss of cholesterol in the cellular membranes resulting in a disruption of lipid rafts followed by shedding of CXCR4 positive microparticles into the environment. Whether this is an active process requiring additional enzymes (but not ATP as indicated by the inability of potassium cyanide to prevent it) or a direct reaction of O2 with cholesterol, is a question that still has to be answered. It should be mentioned, though, that the number of detectable microparticles may imply that not every cell releases them. Whether this means that other cells release microparticles too small to be detected by flow cytometry, or that not every cell is capable of a controlled release, is unknown.
Receptors can be shed of membranes via multiple ways: by proteolytic cleavage via a “sheddase” where parts of the receptor (eg, epidermal growth factor receptor) are cleaved and released31 ; by exosome-associated shedding were intracellular vesicles (∼100 nm in diameter and containing, eg, tumor necrosis factor receptor-1) are first formed in multivesicular bodies (MVP) and then released upon fusion of the MVP with the cellular membrane32 ; or via microvesicles (∼100-1000 nm in diameter) that are shed directly from the plasma membrane containing, for example, CCR6.33 The finding that part of the microparticles observed by us in fact contain cytoplasma implies that at least some of them represent microvesicles (ie, larger structures rather than membrane parts).
CXCR4 positive microparticles have been described recently in AML,27 where the number of microparticles correlated with the blast count. However, no mechanism was provided on the origin of these microparticles, and it must be assumed that the observed fraction of microparticles itself comprises a very heterogeneous group with cell debris, apoptotic bodies, exosomes, and microvesicles. Therefore, our observations do not necessarily imply that the patient sample microparticles described are all shed membrane particles (although they were capable of transferring functional active CXCR4 to receptor-negative cell lines), although it seems likely that part of the microparticles were created either during the collection of the sample (by drawing venous blood and exposing it to 21% O2) or during the continuous changes in pO2 that take place in the human body. A thorough characterization of these microparticles is certainly necessary.
Taken together, all these data imply a need for recognition of a new degree of reduced O2 condition, in addition to standard hypoxia, for studying cells in their natural environment: that of “physiologic” hypoxia, as this itself can profoundly change the biology. Moreover, we describe a novel biologic mechanism of rapid regulation of CXCR4 of AML blasts by shedding of microparticles/vesicles as a response to increases in environmental O2.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Seshagiri Duvvuri, Liran Zhou, Twee Tsao, Teresa McQueen, Leslie Calvert, Vinod Shah, and Herbert Fritsche, PhD, for invaluable support.
This research was supported by National Cancer Institute grants PO1 CA55164 and P30 CA016672 (M.A.). Support was also received from the Paul and Mary Haas Chair in Genetics of the University of Texas (to M.A.). M.F. is supported by a grant from the Deutsche Forschungsgemeinschaft (grant FI 1487).
National Institutes of Health
Authorship
Contribution: M.F., I.S., K.C.D., J.B., and Z.M. performed experiments; M.F., I.S., and M.A. designed the research; and M.F. analyzed the data, made the figures, and, together with I.S., wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Andreeff, MD, PhD, Departments of Stem Cell Transplantation & Cellular Therapy and Leukemia, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: mandreef@mdanderson.org.
References
Author notes
*M.F. and I.S. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal