Abstract
Platelet integrin αIIbβ3 activation is tightly controlled by intracellular signaling pathways, and several molecules, including talin, have been identified as critical for αIIbβ3 activation. However, the whole pathway associated with αIIbβ3 activation remains to be determined. To address this issue, we established a Chinese hamster ovary cell line (parental cells) that expresses constitutively activated chimeric integrin αIIbα6Bβ3, and then obtained mutant cells expressing inactivated αIIbα6Bβ3 by genome-wide mutagenesis. We have performed expression cloning to isolate signaling molecules responsible for integrin activation in the mutant cells. We show that integrin-linked kinase (ILK) complements defective integrin activation in the mutant cells. ILK mRNAs in the mutant cells contained 2 nonsense mutations, R317X and W383X, in a compound heterozygous state, resulting in a complete loss of ILK expression. Moreover, the mutant cells showed partially impaired activation of endogenous β1 integrins. Knockdown of ILK in parental cells significantly suppressed the activated state of αIIbα6Bβ3. However, ILK overexpression did not rescue the impaired integrin activation in talin knocked-down parental cells, whereas overexpression of talin-F3, a subdomain of the talin head domain, restored the function. Our present data suggest that ILK contributes to inside-out integrin activation.
Introduction
Integrins are cell -surface transmembrane receptors that serve as heterodimeric complexes composed of an α- and a β-subunit.1 Integrins are expressed in various cells and mediate the binding of cells to adhesive proteins. Integrin αIIbβ3 is mainly expressed in megakaryocyte/platelet lineage cells and functions as a major receptor for fibrinogen and von Willebrand factor (VWF), enabling crosslinking between platelets in the process of thrombus growth.2 In patients with Glanzmann thrombasthenia, platelet aggregation is impaired and a bleeding tendency is observed.3 In addition, a blockade of platelet aggregation using αIIbβ3 antagonists beneficially reduced thrombotic events in pathologic states, such as percutaneous coronary intervention or acute coronary syndromes.4,5 Thus, αIIbβ3 plays a critical role in both physiologic and pathologic thrombus formation.
Activation of αIIbβ3 is regulated by an intracellular process called inside-out signaling, which is triggered by agonist stimulation of G protein-coupled receptors or binding of VWF and collagen to their receptors glycoprotein Ib-V-IX and glycoprotein VI, respectively.6-8 Activated αIIbβ3 shows a shift from a low- to high-affinity state for ligands, fibrinogen, and VWF. The bound ligands accelerate αIIbβ3 clustering and promote further conformational changes that initiate intracellular signal pathways through the cytoplasmic domains, termed outside-in signaling. Studies of platelet integrin activation have recently been advanced by RNA interference technology and knockout mice. For example, talin, a major cytoskeletal actin-binding protein, is composed of an N-terminal head domain, and a large C-terminal rod domain is associated with β integrin cytoplasmic domains. The talin head domain contains a FERM (protein 4.1, ezrin, radixin, moesin) domain and plays a critical role in the final common step in integrin activation.9-11 The FERM domain consists of 3 subdomains: F1, F2, and F3. The F3 subdomain binds to the β3 cytoplasmic domain with high affinity and results in αIIbβ3 activation.12 Recently, kindlin-3, another FERM domain-containing protein, one of the kindlin family (kindlin-1, -2, and -3), was shown to be an essential factor for platelet integrin activation,13 and kindlin-2 also proved to function as a coactivator of β3 integrins.14 However, although those intensive efforts have been done, the whole signaling pathway for platelet integrin activation has not been fully elucidated.
Because anucleated platelets are not suitable for the manipulation of gene expression, Chinese hamster ovary (CHO) cells have been used on the study of integrin activation. For example, αIIbα6Aβ3β1 expressed in CHO cells containing the extracellular and transmembrane domains of αIIbβ3 fused to the cytoplasmic domains of α6Aβ1 is constitutively activated and useful for expression cloning to isolate the molecules that regulate integrin function. Indeed, using this cell system, H-Ras,15 CD98,16 and PEA-1517 were ingeniously identified as a molecule regulating integrin activation, and also integrin activation-deficient CHO cell lines have been isolated using a genome-wide mutagenesis approach and characterized.18 Furthermore, the critical role of talin or the talin head domain for integrin activation has been demonstrated in CHO cell systems.9,19
In the present study, to identify the signaling molecules that contribute to activation of integrin αIIbβ3, we performed expression cloning in which a cDNA library was transfected into mutant cells expressing inactivated integrin as follows: (1) the establishment of CHO cell lines (parental cells) expressing constitutively activated chimeric integrin αIIbα6Bβ3, in which the cytoplasmic domain of αIIb was replaced by that of α6B; (2) the establishment of mutant cell lines with inactivated integrin by introduction of genome-wide random mutagenesis into the parental cells with an alkylating agent, ethyl methane sulfonate (EMS); and (3) the identification of signaling molecules responsible for integrin activation using expression cloning by cDNA library transfection into the mutant cells. We successfully isolated an integrin-linked kinase (ILK) cDNA that restored the function of inactivated integrin αIIbα6Bβ3 expressed in mutant cells. The mutant cells were deficient in the ILK protein. ILK knockdown in parental cells significantly suppressed integrin activation. These data suggest that ILK contributes to inside-out integrin activation in CHO cells.
Methods
Plasmids
Human αIIb and β3 cDNAs cloned into mammalian expression vector pcDNA3 (Invitrogen, Carlsbad, CA) were gifts from Dr Peter J. Newman (Blood Research Instititute, BloodCenter of Wisconsin, Milwaukee, WI) and Dr Gilbert C. White II (Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI), respectively. pcDNA3.1, including talin-F3 or talin-F23, was a gift from Dr Mark H. Ginsberg (Department of Medicine, University of California, San Diego). Another vector, pcDNA3-αIIbα6B, was generated using polymerase chain reaction (PCR)-based mutagenesis.20 A chimeric Sse8387 I/XbaI fragment (385 bp) containing part of the αIIb and the whole α6B cytoplasmic domain was amplified by PCR using sense (19 bp; the nucleotide sequence at position 2650-2669 of αIIb) and antisense (196 bp; the nucleotide sequence at position 3041-3058 [21 bp] of αIIb followed by the downstream sequence, including the whole α6B cytoplasmic domain [176 bp]) primers, and was introduced into pcDNA3-αIIb. Sequence numbers of nucleotides and amino acids begin with the start codon (ATG) and the initial Met residue, respectively. The full-length or truncated constructs (ankyrin repeat domain, residues 1-179; kinase domain, residues 213-452; ankyrin repeat and pleckstrin homology [PH] domains, residues 1-212; PH and kinase domains, residues 180-452) of rat ILK cDNA were constructed by PCR, and the PCR fragments were subcloned into green fluorescent protein (GFP) containing vector pEGFP-N1 (Clontech, Mountain View, CA). Point mutations in rat ILK mutants (K220M and S343A) were generated by an overlap extension PCR technique, and the fragments were subcloned into GFP containing vector pAcGFP1-Hyg-C1 (Clontech). All PCR-generated DNA inserts were verified by sequencing using a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA).
Cells and cultures
CHO-K1 cells were cultured in Dulbecco modified Eagle medium (Nacalai Tesque, Kyoto, Japan) containing 10% fetal bovine serum and 1% nonessential amino acids (Sigma-Aldrich, St Louis, MO).
A CHO cell line stably expressing constitutively activated αIIbα6Bβ3 was established by the following procedure. CHO-K1 cells were cotransfected with pcDNA3-αIIbα6B and pcDNA3-β3 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Transfectants were selected with 1 mg/mL G418 (Nacalai Tesque). To obtain CHO cells with high expression of αIIbα6Bβ3, G418-resistant cells were incubated with 10 μg/mL activation-specific mouse anti-αIIbβ3 monoclonal antibody (mAb), PAC-1 (BD Biosciences, San Jose, CA), for 30 minutes at room temperature, washed once, and then incubated with 10 μg/mL fluorescein isothiocyanate–conjugated mouse anti–β3 mAb, Y2/51 (Santa Cruz Biotechnology, Santa Cruz, CA), and 10 μg/mL phycoerythrin (PE)-conjugated anti–mouse IgM (μ-chain specific; eBioscience, San Diego, CA) for PAC-1 for 30 minutes at 4°C. After washing, cells were stained with 1 μg/mL 7-aminoactinomycin D (Sigma-Aldrich) to discriminate dead cells. The live cells that expressed high levels of αIIbα6Bβ3 and that significantly bound PAC-1 were sorted by the fluorescence-activated cell sorter (FACS) FACSAria (BD Biosciences). Sorted cells were cloned to isolate parental cells by a limiting dilution method.
The mutant cell lines expressing αIIbα6Bβ3 and showing a low affinity for PAC-1 were established by the following method. Parental cells (107) expressing activated αIIbα6Bβ3 were mutagenized with 0.4 mg/mL EMS for 20 hours and cultured further for 7 days in fresh media without EMS.18,21 The mutagen-exposed cells were stained with PAC-1 and Y2/51. The mutant cells that expressed high levels of αIIbα6Bβ3 but failed to bind PAC-1 were sorted out. After these cells were cultured further for several days, cells were sorted again, and then the selected cells were cloned by the limiting dilution method. Finally, approximately 20 mutant cell lines expressing inactivated αIIbα6Bβ3 were isolated.
Expression cloning
To identify the molecules responsible for integrin activation, cloned mutant cells (107) were transiently cotransfected with 7 μg of a rat C6 glioma cDNA library22 introduced into an episomal expression vector pMEPyori, including a polyoma origin of replication and 7 μg pcDNA-PyT lacking the polyoma replication origin, pcDNA-PyT(ori-)22 using the Nucleofector II electroporation device (Amaxa Biosystems, Gaithersburg, MD). After 72 hours, approximately 700 cells that bound high levels of PAC-1 were collected by FACSAria, and plasmids were rescued from the collected cells by Hirt's method.23 The isolated plasmids were transformed into Escherichia coli by electroporation and amplified in liquid culture. After plasmid preparation, plasmid DNAs (7 μg) were transiently cotransfected with 7 μg pcDNA-PyT(ori-) into 107 mutant cells, and a second sorting and plasmid rescue was performed by the same procedure. After the third sorting and plasmid rescue, the plasmids were introduced into E coli, and 960 independent colonies were individually separated into 10 96-well plates. Five positive clones induced significant PAC-1 binding in the mutant cells. The cDNA inserts of the 5 positive clones were sequenced.
Flow cytometry
Cell staining and fluorescence analysis were performed as described with minor modifications.24 Adherent cells were detached with 0.02% ethylenediaminetetraacetic acid solution, washed with phosphate-buffered saline (PBS), and then resuspended in Tyrode buffer containing 1.5 mM CaCl2, 1 mM MgCl2, and 1% bovine serum albumin. A total of 50 μL of 106 cells were incubated with one of the following primary antibodies for 30 minutes at 4°C: 10 μg/mL of a mouse mAb specific for αIIb, SZ22 (Beckman Coulter, Fullerton, CA), 5 μg/mL of a mouse mAb specific for β3, Y2/51, 5 μg/mL of a mouse mAb specific for αIIbβ3, HIP8 (BD Biosciences), 5 μg/mL of a mouse mAb specific for hamster integrin β1, 7E2 (Developmental Studies Hybridoma Bank, Iowa City, IA), and 2 μg/mL of a rat mAb specific for activated β1, 9EG7 (BD Biosciences). After washing with Tyrode buffer, cells were incubated with the secondary Ab of 10 μg/mL Alexa Fluor 488–conjugated anti–mouse IgG (Invitrogen, Carlsbad, CA), 10 μg/mL PE-conjugated anti–mouse IgG1 (BD Biosciences), or 10 μg/mL PE-conjugated anti–rat IgG2a (BD Biosciences) for 30 minutes at 4°C and then analyzed on a FACSCalibur flow cytometer (BD Biosciences). As a negative control, cells were incubated with the secondary Ab alone. As a positive control for PAC-1 binding, cells were incubated with 15 mM dithiothreitol (DTT) for 10 minutes at 37°C to activate integrin αIIbα6Bβ325,26 and incubated with PAC-1 as mentioned in “Cells and cultures.” Short interfering RNA (siRNA) and short hairpin RNA (shRNA) transfectants were incubated with PAC-1 in the absence or presence of 10 μM of a peptidomimetic antagonist of αIIbβ3, Ro44-9883 (a gift from Astellas Pharma, Tokyo, Japan).24 The amount of PAC-1 binding was determined by flow cytometry. Integrin activation was quantified as an activation index calculated using the following formula: 100 × (F minus Fo)/(Fmax minus Fo), where F is the mean fluorescence intensity (MFI) of PAC-1 binding, Fo is the PAC-1 binding in the presence of Ro44-9883, and Fmax is the maximal PAC-1 binding in the cells treated with DTT. For binding of recombinant PAC-1 Fab (a gift from Dr Sanford J. Shattil, Department of Medicine, University of California, San Diego), an experiment was performed according to the procedure of PAC-1 binding, except for use of 10 μg/mL R-PE–conjugated anti–mouse IgG (Invitrogen) as a secondary antibody.
Immunoblotting
Preparation of cell lysate, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting was performed by procedures previously described.27 In brief, adherent cells were rinsed with PBS and directly lysed with SDS sample buffer. Samples were electrophoresed on a SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% skim milk in 0.05% Tween 20/PBS overnight at 4°C and incubated with one of the following primary antibodies: 0.5 μg/mL rabbit polyclonal Ab specific for ILK (Upstate Biotechnology, Charlottesville, VA), mouse mAb specific for talin, 8D4 (ascites fluid; 1:2000; Sigma-Aldrich), 0.5 μg/mL rabbit polyclonal Ab specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), FL-335 (Santa Cruz Biotechnology), or horseradish peroxidase–conjugated rabbit polyclonal Ab specific for GFP (1:3000; Clontech) for 90 minutes at room temperature. After washing, bound Abs (except for the horseradish peroxidase–conjugated anti-GFP Ab) were incubated with peroxidase-conjugated secondary Abs (Kirkegaard and Perry Laboratories, Gaithersburg, MD), and detection was performed using a chemiluminescence kit (Immobilon Western; Millipore, Billerica, MA). Chemiluminescence was visualized by an image analyzer, LAS-3000PLUS (Fuji Photo Film, Kanagawa, Japan).
Reverse transcription–polymerase chain reaction and genomic polymerase chain reaction
Total RNA from cultured cells was extracted using Trizol reagent (Invitrogen) and amplified by one-step reverse transcription–polymerase chain reaction (RT-PCR) kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. PCR fragments covering the coding regions of αIIbα6Bβ3 mRNA and ILK mRNA were amplified and directly sequenced using specific primers. For genomic PCR, chromosomal DNA from cultured cells was isolated by DNeasy tissue kit (QIAGEN) and amplified by PCR using Hot Start Taq DNA polymerase (QIAGEN). The region covering exon 9 through exon 12 was amplified by the following primer pair: sense (21 bp; 798-818 of hamster ILK) and antisense (21 bp; 1223-1243 of hamster ILK). The amplified DNA fragments were subcloned into pcDNA4-TOPO (Invitrogen) and the inserted DNA fragments from 5 independent colonies were sequenced using vector- and sequence-specific primers.
siRNA and shRNA
siRNAs were synthesized as Stealth RNAi by Invitrogen. The siRNA sequences to target hamster ILK are as follows: ILK (Ilk) 467 sense 5′-UAUGGAAUACGGUUGAGAUUCUGGC-3′ and antisense 5′-GCCAGAAUCUCAACCGUAUUCCAUA-3′, ILK (Ilk) 467 scrambled control sense 5′-UAUCUGGAAUACGGUUGAGAUUGGC-3′ and antisense 5′-GCCAAUCUCAACCGUAUUCCAGAUA-3′; ILK (Ilk) 1255 sense 5′-UCUUCAUGAGCUUACACACGUGUGG-3′ and antisense 5′-CCACACGUGUGUAAGCUCAUGAAGA-3′, ILK (Ilk) 1255 scrambled control sense 5′-UCUGUUGUACUCGAACAUGCACUGG-3′ and antisense 5′-CCAGUGCAUGUUCGAGUACAACAGA-3′. To target hamster talin, we used the specific sequence for both mouse and rat talin as follows: Talin (Tln) 5465 sense 5′-UAUAGCAACUGCAAGGCAGACUCUG-3′ and antisense 5′-CAGAGUCUGCCUUGCAGUUGCUAUA-3′, Talin (Tln) 5465 scrambled control sense 5′-UAUUCAAACGCGUCGGAAAGACCUG-3′ and antisense 5′-CAGGUCUUUCCGACGCGUUUGAAUA-3′. Parental cells cultured in 6-well plates were transfected with 100 nM siRNA using Lipofectamine 2000. After 72 hours, transfectants were used for flow cytometry or immunoblotting. For shRNAs, we used the plasmids expressing talin and its mismatched control shRNAs whose abilities were examined and confirmed elsewhere.9 Transfections were performed using Lipofectamine 2000. In 6-well plates, parental cells were transfected with 4 μg shRNA plasmid, 2 μg plasmid encoding talin-F3, ILK, or empty plasmid (pcDNA3), and 0.5 μg pEGFP-C1 (Clontech). Cells were used for flow cytometry at 72 hours after transfection.
Adhesion studies
Cell adhesion experiments were performed as described previously.28 Briefly, wells of 96-well microtiter plates were coated with fibrinogen. After blocking with 1% bovine serum albumin, cells (105) were added into wells in triplicate and incubated for 60 minutes at 37°C. Wells were washed with PBS, and the adherent cells were quantified by measuring endogenous cellular acid phosphatase activity in an enzyme-linked immunosorbent assay using p-nitrophenyl phosphate (Calbiochem, San Diego, CA) as a chromogenic substrate.
Results
Establishment of mutant cell lines that express inactivated αIIbα6Bβ3
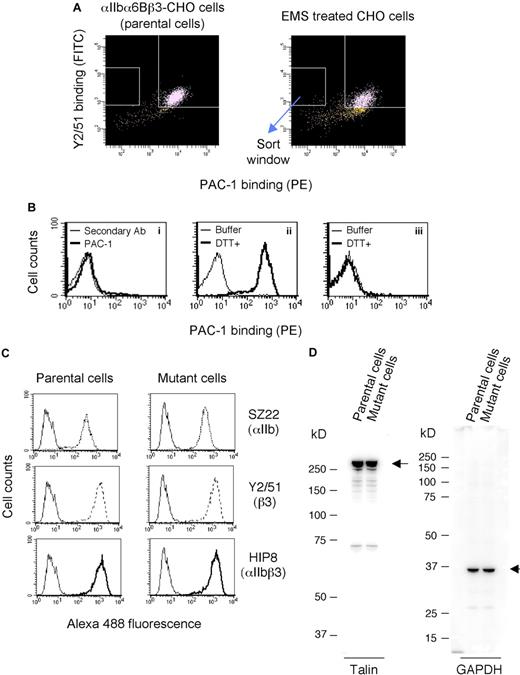
We initially established a parental CHO cell line expressing constitutively activated αIIbα6Bβ3 as described in “Cells and cultures.” It has been reported that the activation state of this chimeric integrin is dependent on cellular signaling.29 Hence, the established cell line can be appropriate to identify signaling molecules ascribable to integrin activation. To obtain mutant cells with defective activation of αIIbα6Bβ3, the parental cell line was treated with the chemical mutagen EMS that randomly introduces point mutations into DNA, and mutant cells that failed to bind PAC-1 specific for activated αIIbβ3 but retained αIIbα6Bβ3 expression were collected by cell sorting (Figure 1A). Approximately 6000 cells were sorted, expanded for several days, sorted again by the same procedure, and then cloned. Among the cloned mutant cells, most showed impaired PAC-1 binding (Figure 1Bi) and failed to increase their PAC-1 binding after DTT treatment to activate αIIbα6Bβ3 (Figure 1Biii), suggesting that these mutant cells expressed αIIbα6Bβ3 in which mutations had been introduced in the ligand-binding domain.30 However, PAC-1 binding in a small number of mutant cells was effectively increased by DTT treatment (Figure 1Bii). Accordingly, we selected 20 mutant cell lines showing effective PAC-1 binding after DTT treatment. The DNA sequence of integrin αIIbα6Bβ3 expressed in these cells was confirmed by the direct sequencing of RT-PCR products. The mutant cell lines showed similar levels of αIIbα6Bβ3 expression to those of parental cells. One representative mutant cell line was examined for talin expression to confirm that inactivated αIIbα6Bβ3 was not caused by impaired talin expression. A mutant cell line in which αIIbα6Bβ3 (Figure 1C) and talin (Figure 1D) were expressed at similar levels to parental cells was used for expression cloning.
Establishment of mutant cells expressing inactivated αIIbα6Bβ3. (A) FACS sorting of mutant cells. CHO cells expressing constitutively activated αIIbα6Bβ3 (parental cells) were treated with a chemical mutagen EMS for 20 hours. After cell culture for 7 days, the cells were incubated with an activation-specific mAb against αIIbβ3, PAC-1 (conjugated with PE), and a β3-specific mAb Y2/51 (conjugated with fluorescein isothiocyanate). The cells that showed high levels of Y2/51 binding but did not bind PAC-1 were sorted out by a FACS. These cells were expanded and resorted, and then the mutant cells were cloned. (B) Characterization of mutant clones. Clones defective in PAC-1 binding were confirmed by flow cytometry (i), and the clones were examined for a phenotypic restoration of PAC-1 binding by DTT treatment (ii,iii). Nonspecific binding was shown by cells incubated with the secondary Ab alone (i). Mutant cells were treated with DTT or buffer and washed once, and then the cells were incubated with PAC-1 (ii,iii). (C) Flow cytometry showing expression of αIIbα6Bβ3 on parental or mutant cells. Cells were incubated with mAbs against αIIb (SZ22), β3 (Y2/51), and αIIbβ3 (HIP8). After washing, bound mAbs were detected with an AlexaFluor 488–conjugated secondary Ab. Nonspecific binding is shown by the cells stained with the secondary Ab alone (thin solid line). Results are a representative of 3 independent experiments. (D) Immunoblotting analysis of talin in the parental and mutant cells. Whole-cell lysates were electrophoresed, transferred to a polyvinylidene difluoride membrane, incubated with antitalin mAb or anti-GAPDH polyclonal Ab, and then detected by chemiluminescence.
Establishment of mutant cells expressing inactivated αIIbα6Bβ3. (A) FACS sorting of mutant cells. CHO cells expressing constitutively activated αIIbα6Bβ3 (parental cells) were treated with a chemical mutagen EMS for 20 hours. After cell culture for 7 days, the cells were incubated with an activation-specific mAb against αIIbβ3, PAC-1 (conjugated with PE), and a β3-specific mAb Y2/51 (conjugated with fluorescein isothiocyanate). The cells that showed high levels of Y2/51 binding but did not bind PAC-1 were sorted out by a FACS. These cells were expanded and resorted, and then the mutant cells were cloned. (B) Characterization of mutant clones. Clones defective in PAC-1 binding were confirmed by flow cytometry (i), and the clones were examined for a phenotypic restoration of PAC-1 binding by DTT treatment (ii,iii). Nonspecific binding was shown by cells incubated with the secondary Ab alone (i). Mutant cells were treated with DTT or buffer and washed once, and then the cells were incubated with PAC-1 (ii,iii). (C) Flow cytometry showing expression of αIIbα6Bβ3 on parental or mutant cells. Cells were incubated with mAbs against αIIb (SZ22), β3 (Y2/51), and αIIbβ3 (HIP8). After washing, bound mAbs were detected with an AlexaFluor 488–conjugated secondary Ab. Nonspecific binding is shown by the cells stained with the secondary Ab alone (thin solid line). Results are a representative of 3 independent experiments. (D) Immunoblotting analysis of talin in the parental and mutant cells. Whole-cell lysates were electrophoresed, transferred to a polyvinylidene difluoride membrane, incubated with antitalin mAb or anti-GAPDH polyclonal Ab, and then detected by chemiluminescence.
Expression cloning of ILK cDNA that induces integrin activation
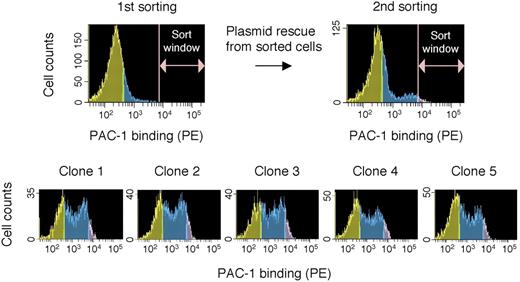
We transiently cotransfected a rat C6 glioma cDNA library and pcDNA-PyT(ori-) into mutant cells defective in PAC-1 binding and screened for cDNA clones that restored the function of integrin activation (PAC-1 binding) to the transfected cells (Figure 2). In a single round of screening, cells with restored PAC-1 binding were sorted out, plasmids in these cells were rescued by a cell lysis procedure, and then amplified in E coli. Approximately 0.006% of the total stained cells were sorted out as PAC-1 binding–positive in the first sorting and 0.41% of the total cells were collected in the second sorting, indicating that positive cDNAs were enriched 68-fold in the single round of screening. After 3 rounds of screening for the plasmid clones that restored PAC-1 binding, we successfully isolated 5 clones exhibiting strong PAC-1 binding. DNA sequencing revealed that the rat full-length ILK cDNA was inserted. The length and sequence of the inserted ILK cDNA were the same in all 5 plasmid clones, suggesting that these clones were derived from the same clone.
cDNA cloning of a molecule that restores PAC-1 binding. The mutant cells transiently transfected with a cDNA library were analyzed in PAC-1 binding, and the cells that showed high levels of PAC-1 binding within a window were sorted. Plasmids were rescued from these sorted cells and amplified by E coli into which cDNA plasmids were transformed. After 3 rounds of cDNA transient transfection, cell sorting, and plasmid rescue, finally, 5 cDNA clones that exhibited significant restoration of PAC-1 binding in the mutant cells were isolated.
cDNA cloning of a molecule that restores PAC-1 binding. The mutant cells transiently transfected with a cDNA library were analyzed in PAC-1 binding, and the cells that showed high levels of PAC-1 binding within a window were sorted. Plasmids were rescued from these sorted cells and amplified by E coli into which cDNA plasmids were transformed. After 3 rounds of cDNA transient transfection, cell sorting, and plasmid rescue, finally, 5 cDNA clones that exhibited significant restoration of PAC-1 binding in the mutant cells were isolated.
Evaluation of hamster ILK in mutant cells
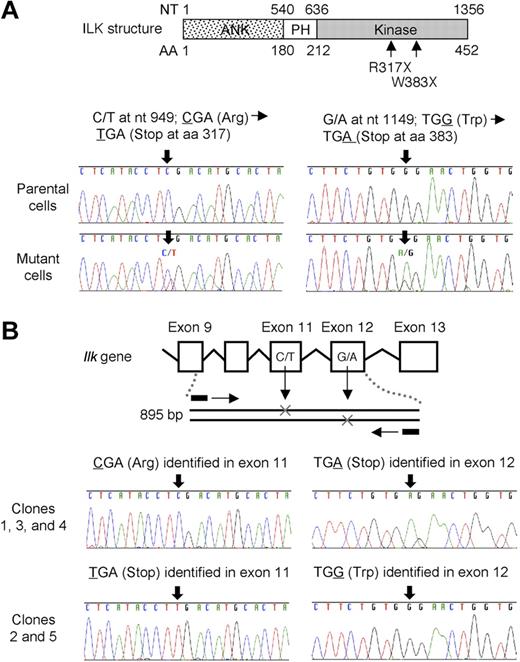
Using RT-PCR, we determined the hamster ILK cDNA sequences of both mutant cells used in expression cloning and parental cells not exposed to EMS. Hamster ILK cDNA was shown to consist of 452 amino acid residues and to exhibit high amino acid identity to human (99.3%), mouse (99.3%), and rat (99.8%) ILK (data not shown). The hamster ILK cDNA sequence was deposited in the DDBJ/EMBL/GenBank31 nucleotide sequence database under accession number AB443452. ILK mRNA in mutant cells exhibited 2 nonsense mutations, R317X and W383X, in the kinase domain in a heterozygous state (Figure 3A). To confirm whether these mutations are compound heterozygous or not, we examined the ILK gene, in which R317X and W383X are predicted to be located, within exon 11 and exon 12, respectively (Figure 3B). We amplified the 895-bp region of the hamster gene, including both mutations. PCR fragments were then subcloned, and 5 independent colonies were sequenced. Each clone insert contained one of the 2 mutations, indicating that R317X and W383X were present in a compound heterozygous state.
Sequence analysis of ILK mRNA and gene in mutant cells. (A) Analysis of ILK mRNA sequence. ILK mRNA of parental cells and mutant cells was amplified by RT-PCR and the PCR products were directly sequenced. Two different nonsense mutations R317X and W383X were identified in the ILK kinase domain in the mutant cells in a heterozygous state. (B) Analysis of the Ilk gene sequence. The 895-bp region encompassing exon 9 through exon 12 in the Ilk gene was amplified by PCR. The PCR products were subcloned into pCR4-TOPO vector and sequenced. The vector inserts included one or the other mutation.
Sequence analysis of ILK mRNA and gene in mutant cells. (A) Analysis of ILK mRNA sequence. ILK mRNA of parental cells and mutant cells was amplified by RT-PCR and the PCR products were directly sequenced. Two different nonsense mutations R317X and W383X were identified in the ILK kinase domain in the mutant cells in a heterozygous state. (B) Analysis of the Ilk gene sequence. The 895-bp region encompassing exon 9 through exon 12 in the Ilk gene was amplified by PCR. The PCR products were subcloned into pCR4-TOPO vector and sequenced. The vector inserts included one or the other mutation.
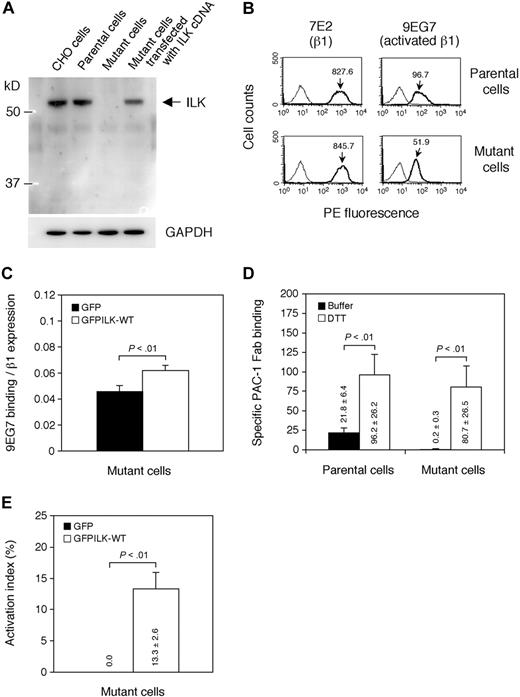
We examined the ILK protein expression of mutant cells by immunoblotting (Figure 4A). CHO cells and parental cells showed similar levels of the ILK protein band detected by a rabbit anti-ILK polyclonal Ab that recognizes ankyrin repeat domain of ILK (see next paragraph). In contrast, mutant cells did not exhibit any bands of the 2 truncated mutant proteins of ILK (R317X and W383X). Mutant cells transfected with wild-type rat ILK cDNA clearly showed a band having the same size as endogenous hamster ILK. These data suggest that the compound heterozygous nonsense mutations cause complete ILK deficiency.
Analysis of ILK in mutant cells. (A) Immunoblotting using anti-ILK Ab. Whole-cell lysates obtained from CHO cells, parental cells, mutant cells, and mutant cells transiently transfected with rat ILK cDNA were electrophoresed on SDS-PAGE and immunoblotted with rabbit anti-ILK polyclonal or rabbit anti-GAPDH polyclonal Ab. (B) Flow cytometry showing expression of activated β1 integrins. Cells were incubated with mAb specific for hamster β1 (7E2) or activated β1 (9EG7). Bound mAbs were detected with PE-conjugated secondary Ab. A thin solid line represents nonspecific binding to cells stained with the secondary Ab alone. Results are representative of 3 independent experiments. The mean fluorescence intensities of 7E2 and 9EG7 binding are indicated on the histograms. (C) Effects of ILK expression on β1 integrin activation. Mutant cells were transiently transfected with GFP-fused wild-type ILK (GFPILK-WT) cDNA or GFP cDNA. After 72 hours, 9EG7 binding to transfectants was analyzed by flow cytometry. 9EG7 binding was expressed as binding normalized to β1 expression (7E2 binding). Data are mean plus or minus SD of 3 independent experiments. (D) Specific binding of PAC-1 Fab fragment to parental cells and mutant cells. Nonspecific binding of PAC-1 Fab fragment to cells was measured in the presence of Ro44-9883, and specific PAC-1 Fab binding was estimated by subtracting the MFI of nonspecific binding from the MFI of PAC-1 Fab binding to the indicated cells. Data are mean plus or minus SD of 3 experiments, and the calculated values are displayed on the graph. (E) The activation index of ILK-transfected mutant cells. The activation index using PAC-1 Fab was calculated by the formula shown in “Flow cytometry.” A value of 100% represents maximal PAC-1 Fab binding to the DTT-treated cells. Data are mean plus or minus SD of 3 independent experiments, and the values are displayed on the graph.
Analysis of ILK in mutant cells. (A) Immunoblotting using anti-ILK Ab. Whole-cell lysates obtained from CHO cells, parental cells, mutant cells, and mutant cells transiently transfected with rat ILK cDNA were electrophoresed on SDS-PAGE and immunoblotted with rabbit anti-ILK polyclonal or rabbit anti-GAPDH polyclonal Ab. (B) Flow cytometry showing expression of activated β1 integrins. Cells were incubated with mAb specific for hamster β1 (7E2) or activated β1 (9EG7). Bound mAbs were detected with PE-conjugated secondary Ab. A thin solid line represents nonspecific binding to cells stained with the secondary Ab alone. Results are representative of 3 independent experiments. The mean fluorescence intensities of 7E2 and 9EG7 binding are indicated on the histograms. (C) Effects of ILK expression on β1 integrin activation. Mutant cells were transiently transfected with GFP-fused wild-type ILK (GFPILK-WT) cDNA or GFP cDNA. After 72 hours, 9EG7 binding to transfectants was analyzed by flow cytometry. 9EG7 binding was expressed as binding normalized to β1 expression (7E2 binding). Data are mean plus or minus SD of 3 independent experiments. (D) Specific binding of PAC-1 Fab fragment to parental cells and mutant cells. Nonspecific binding of PAC-1 Fab fragment to cells was measured in the presence of Ro44-9883, and specific PAC-1 Fab binding was estimated by subtracting the MFI of nonspecific binding from the MFI of PAC-1 Fab binding to the indicated cells. Data are mean plus or minus SD of 3 experiments, and the calculated values are displayed on the graph. (E) The activation index of ILK-transfected mutant cells. The activation index using PAC-1 Fab was calculated by the formula shown in “Flow cytometry.” A value of 100% represents maximal PAC-1 Fab binding to the DTT-treated cells. Data are mean plus or minus SD of 3 independent experiments, and the values are displayed on the graph.
It has been reported that CHO cells express activated integrin α5β1.29 We evaluated the activation state of endogenous hamster β1 integrins using 9EG7, a mAb specific for activated β132,33 and 7E2, a mAb specific for β1. Mutant cells expressed similar levels of β1 integrins compared with those of parental cells when detected β1 by mAb 7E2 (Figure 4B). However, the binding of 9EG7 to mutant cells was reduced. In addition, transfection of GFP-fused wild-type ILK (GFPILK-WT) cDNA into mutant cells increased 9EG7 binding to mutant cells (Figure 4C). These findings suggest that ILK is in part involved in activation of endogenous β1 integrins.
Because PAC-1 Fab recognizes an activated conformation of αIIbβ3 rather than its clustering,34 we also assessed PAC-1 Fab binding to parental cells and mutant cells. PAC-1 Fab bound to parental cells but not mutant cells (Figure 4D). When mutant cells were transfected with GFPILK-WT cDNA, the transfectants were able to bind PAC-1 Fab (Figure 4E), suggesting that ILK is associated with the activated state conformation of αIIbα6Bβ3.
Role of ILK domains for integrin activation
To examine the role of each domain of ILK for integrin activation, we expressed constructs, including an ankyrin repeat domain, a kinase domain, an ankyrin repeat/PH domain, and a PH/kinase domain fused to GFP. We confirmed by immunoblotting using a rabbit anti-GFP polyclonal Ab that these ILK domains were properly expressed. We also found that the rabbit anti-ILK polyclonal Ab used for immunoblotting recognized the ankyrin repeat domain of ILK (data not shown). However, transfection of these constructs into mutant cells or parental cells resulted in no significant effect on PAC-1 binding (data not shown). Thus, none of the ILK domains was sufficient for the induction of integrin activation in the mutant cells or the inhibition of integrin activation in the parental cells.
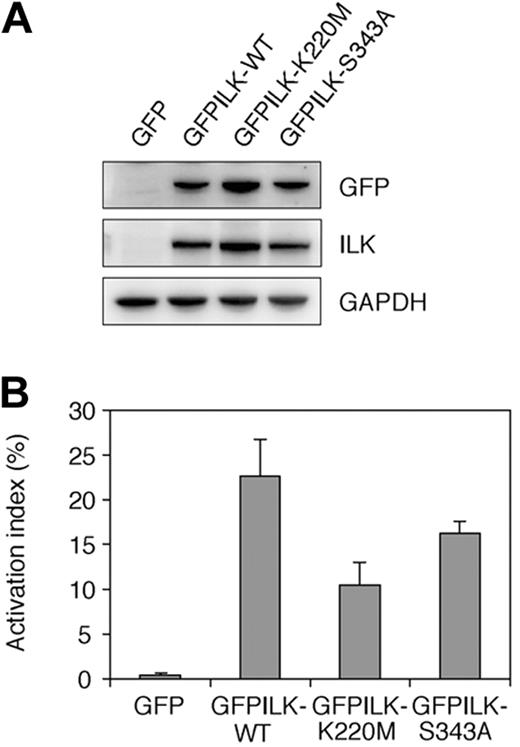
To examine the contribution of ILK kinase activity to integrin activation in mutant cells, we expressed GFPILK-WT and 2 GFP-fused mutant ILKs (GFPILK-K220M and GFPILK-S343A) lacking the kinase activity.35,36 Transfection of these GFP-fused constructs into the mutant cells resulted in proper protein expression (Figure 5A). GFPILK-S343A transfected mutant cells increased their PAC-1 binding to a just below that of GFPILK-WT transfectants (Figure 5B). GFPILK-K220M also allowed mutant cells to increase PAC-1 binding by approximately 50% compared with that of GFPILK-WT. These data suggest that the kinase activity of ILK is not essential for integrin activation in the mutant cells.
Effects of mutant ILKs lacking kinase activity in mutant cells. (A) Immunoblotting showing protein expression of GFPILK-WT and kinase-dead ILK mutants (GFPILK-K220M and GFPILK-S343A). Whole-cell lysates were electrophoresed on SDS-PAGE and immunoblotted with rabbit anti-GFP polyclonal, rabbit anti-ILK polyclonal, or rabbit anti-GAPDH polyclonal Ab. (B) The activation indexes of transfectants. The activation index was determined by the formula shown in “Flow cytometry.” A value of 100% represents maximal PAC-1 binding in the cells treated with DTT. Data are mean plus or minus SD of 3 independent experiments.
Effects of mutant ILKs lacking kinase activity in mutant cells. (A) Immunoblotting showing protein expression of GFPILK-WT and kinase-dead ILK mutants (GFPILK-K220M and GFPILK-S343A). Whole-cell lysates were electrophoresed on SDS-PAGE and immunoblotted with rabbit anti-GFP polyclonal, rabbit anti-ILK polyclonal, or rabbit anti-GAPDH polyclonal Ab. (B) The activation indexes of transfectants. The activation index was determined by the formula shown in “Flow cytometry.” A value of 100% represents maximal PAC-1 binding in the cells treated with DTT. Data are mean plus or minus SD of 3 independent experiments.
ILK knockdown in parental cells expressing activated αIIbα6Bβ3
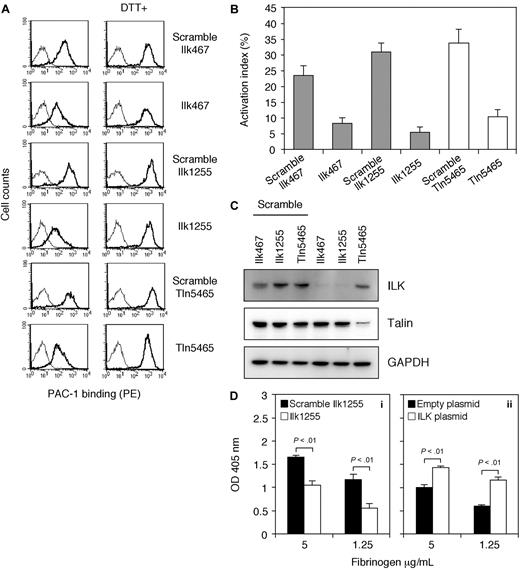
To confirm that ILK is essential for integrin activation in αIIbα6Bβ3-expressing cells, parental cells were studied for the effects of ILK knockdown on PAC-1 binding (Figure 6A-C). ILK siRNA transfectants using 2 different siRNAs (Ilk467 and Ilk1255) showed a significant reduction of PAC-1 binding compared with that of the scrambled siRNA transfectants (Figure 6A,B). ILK protein expression was also suppressed by these 2 siRNAs specific for ILK despite constant protein expression of talin (Figure 6C). In contrast, scrambled ILK siRNAs had no significant effects on the protein expression. It is well known that talin is required for the final common step in integrin activation. Thus, we also examined the effects of talin knockdown on PAC-1 binding. As expected, the talin siRNA transfectants exhibited a decrease in PAC-1 binding but not the scrambled talin siRNA transfectants (Figure 6A,B). In addition, talin protein expression was inhibited by talin siRNA, whereas scrambled talin siRNA displayed no remarkable effects on the protein expression (Figure 6C).
Effects of ILK siRNA on activated αIIbα6Bβ3 in parental cells. (A) Flow cytometric analysis of PAC-1 binding to siRNA transfectants. Parental cells were transiently transfected with scrambled siRNAs, ILK-specific siRNAs (Ilk467 and Ilk1255), or talin-specific siRNA (Tln5465) at a final concentration of 100 nM. After 72 hours, PAC-1 binding to transfectants was analyzed by flow cytometry in either DTT-treated or nontreated condition. The thin line histogram represents cells incubated with a PE-conjugated secondary Ab alone. Results are representative of 3 independent experiments. (B) The activation index of transfectants. The activation index was determined by the formula shown in “Flow cytometry.” Data are mean plus or minus SD of 3 experiments. (C) Immunoblotting of ILK and talin showing knockdown effects of siRNA transfection. Whole-cell lysates from the indicated transfectants were electrophoresed on SDS-PAGE and immunoblotted with rabbit anti-ILK polyclonal Ab, mouse antitalin mAb 8D4, and rabbit anti-GAPDH polyclonal Ab. (D) Adhesion of siRNA transfected cells or ILK-transfected cells to immobilized fibrinogen. (i) Parental cells were transiently transfected with scrambled Ilk1255 or Ilk1255 at 100 nM. After 72 hours, cell adhesion to microtiter wells coated with the indicated concentrations of fibrinogen was tested in triplicate. (ii) Mutant cells were transfected with an empty plasmid (2 μg) or a plasmid encoding rat ILK (2 μg), and cell adhesion was tested. Results are representative of 3 independent experiments. Data are mean plus or minus SD of triplicate measurements of optical density at 405 nm.
Effects of ILK siRNA on activated αIIbα6Bβ3 in parental cells. (A) Flow cytometric analysis of PAC-1 binding to siRNA transfectants. Parental cells were transiently transfected with scrambled siRNAs, ILK-specific siRNAs (Ilk467 and Ilk1255), or talin-specific siRNA (Tln5465) at a final concentration of 100 nM. After 72 hours, PAC-1 binding to transfectants was analyzed by flow cytometry in either DTT-treated or nontreated condition. The thin line histogram represents cells incubated with a PE-conjugated secondary Ab alone. Results are representative of 3 independent experiments. (B) The activation index of transfectants. The activation index was determined by the formula shown in “Flow cytometry.” Data are mean plus or minus SD of 3 experiments. (C) Immunoblotting of ILK and talin showing knockdown effects of siRNA transfection. Whole-cell lysates from the indicated transfectants were electrophoresed on SDS-PAGE and immunoblotted with rabbit anti-ILK polyclonal Ab, mouse antitalin mAb 8D4, and rabbit anti-GAPDH polyclonal Ab. (D) Adhesion of siRNA transfected cells or ILK-transfected cells to immobilized fibrinogen. (i) Parental cells were transiently transfected with scrambled Ilk1255 or Ilk1255 at 100 nM. After 72 hours, cell adhesion to microtiter wells coated with the indicated concentrations of fibrinogen was tested in triplicate. (ii) Mutant cells were transfected with an empty plasmid (2 μg) or a plasmid encoding rat ILK (2 μg), and cell adhesion was tested. Results are representative of 3 independent experiments. Data are mean plus or minus SD of triplicate measurements of optical density at 405 nm.
Because immobilized fibrinogen is a higher affinity and an activation-independent ligand for αIIbβ3,37 we further studied cell adhesion to immobilized fibrinogen to assess the αIIbα6Bβ3-affinity state in ILK knocked-down parental cells and mutant cells deficient in ILK. ILK siRNA transfectants showed a clear reduction in adhesion to immobilized fibrinogen relative to that of the scrambled siRNA transfectants (Figure 6Di). Mutant cells transfected with an empty plasmid also displayed decreased adhesion to immobilized fibrinogen compared with mutant cells transfected with rat ILK cDNA (Figure 6Dii).
ILK overexpression in talin knocked-down parental cells
We next evaluated whether overexpression of ILK could induce integrin activation in the talin knocked-down parental cells (Figure 7). It has been described that the F3 subdomain of the talin FERM domain binds to the β3 cytoplasmic domain and leads to integrin activation.12 When talin-F3 cDNA was cotransfected with control shRNA into parental cells, PAC-1 binding was further increased. In contrast, cotransfection of rat ILK cDNA with control shRNA into the parental cells had no effects on PAC-1 binding. In addition, cotransfection of talin-F3 cDNA with talin shRNA showed no significant reduction of PAC-1 binding compared with empty plasmid cotransfection with control shRNA; however, rat ILK cDNA or empty plasmid cotransfection with talin shRNA resulted in a significant decrease of PAC-1 binding in the transfected cells. Furthermore, transfection of talin-F3 or talin-F23 cDNA into mutant cells induced PAC-1 binding (data not shown). These results indicate that ILK is necessary, but not sufficient for integrin activation and that ILK may support talin-mediated integrin activation.
Effects of ILK overexpression on impaired PAC-1 binding in talin knocked-down parental cells. (A) Dot plot analysis of PAC-1 binding to cotransfectants of empty plasmid, talin-F3, or ILK with shRNA. GFP was used as a transfection marker. An empty plasmid (2 μg), a plasmid encoding a talin-F3 domain (2 μg), or rat ILK (2 μg) was cotransfected with plasmids encoding EGFP (0.5 μg) and shRNA (4 μg) to parental cells. Cells were stained with PAC-1 for flow cytometry. (B) Quantitative estimates of PAC-1 binding to the indicated transfectants. PAC-1 binding was measured in cells that showed high levels of GFP fluorescence (boxed regions in panel A) using flow cytometry. Nonspecific PAC-1 binding was measured in the presence of Ro44-9883, and specific PAC-1 binding was determined by subtracting the MFI of nonspecific binding from the MFI of PAC-1 binding to the indicated transfectants. Data are mean ± SD of 2 experiments.
Effects of ILK overexpression on impaired PAC-1 binding in talin knocked-down parental cells. (A) Dot plot analysis of PAC-1 binding to cotransfectants of empty plasmid, talin-F3, or ILK with shRNA. GFP was used as a transfection marker. An empty plasmid (2 μg), a plasmid encoding a talin-F3 domain (2 μg), or rat ILK (2 μg) was cotransfected with plasmids encoding EGFP (0.5 μg) and shRNA (4 μg) to parental cells. Cells were stained with PAC-1 for flow cytometry. (B) Quantitative estimates of PAC-1 binding to the indicated transfectants. PAC-1 binding was measured in cells that showed high levels of GFP fluorescence (boxed regions in panel A) using flow cytometry. Nonspecific PAC-1 binding was measured in the presence of Ro44-9883, and specific PAC-1 binding was determined by subtracting the MFI of nonspecific binding from the MFI of PAC-1 binding to the indicated transfectants. Data are mean ± SD of 2 experiments.
Discussion
We performed expression cloning using signaling-defective mutant cells expressing inactivated αIIbα6Bβ3 established by EMS-based random mutagenesis, and successfully isolated an ILK cDNA as a molecule that restored the activated state of integrin. This strategy is useful for exploring cellular molecules modifying integrin function, and it may make it possible to identify novel molecules or new functions of already-known molecules. EMS is particularly effective for introducing point mutations into genes. CHO cells show functional hemizygosity at several genes.38 These genes may easily exhibit functional damage resulting from a single mutation. However, we found that ILK mRNA in the mutant contained 2 nonsense mutations for compound heterozygosity. Our data suggest that the ILK gene in CHO cells has 2 functional alleles and that these may be mutational hot spots in CHO cells.
ILK is a nonreceptor serine/threonine protein kinase that directly binds to the cytoplasmic domains of integrin β1 and β3 subunits.39 ILK knockout mice were shown to die during the peri-implantation stage of embryonic development. ILK-deficient cells showed impaired adhesion, defective cell spreading, and diminished proliferation,40,41 indicating that ILK contributed to outside-in signaling. We carried out cell adhesion experiments and found that the adhesion of ILK knocked-down parental cells and ILK-deficient mutant cells to immobilized fibrinogen was clearly reduced. Furthermore, complementation of ILK in ILK-deficient mutant cells induced the activated conformation of integrin rather than its clustering, and ILK knockdown in parental cells suppressed integrin activation. These observations suggest that ILK is associated with integrin inside-out signaling and also at least in part with outside-in signaling.
We assessed the effects of the kinase domain, the ankyrin repeat domain, the ankyrin repeat/PH domain, or the PH/kinase domain of ILK on integrin activation. We found that the mutant cells or the parental cells transfected with each of these domains failed to show significant effects on PAC-1 binding, suggesting that a truncated domain is not sufficient and a linear organization of domains is essential for integrin activation.
ILK kinase activity has been shown in many in vitro substrates.42 We generated kinase-deficient ILK mutants, K220M and S343A, to evaluate whether kinase activity is necessary for integrin activation. Transfection of these kinase-dead ILKs into mutant cells moderately rescued PAC-1 binding. This suggests that kinase activity is not essential for integrin activation in the mutant cells. Similar findings were reported in studies in which kinase-dead ILK rescued the function in ILK-deficient fibroblasts40 and restored the phenotype of loss-of-function mutations of ILK in Drosophila.43 Although there is one recent report in which ILK-K220M retained the weak kinase activity showing a 80% reduction of ILK-WT,44 taken together, our data suggest that kinase activity is not directly involved in integrin activation in the mutant cells.
Importantly, overexpression of the talin head domain induces further activation of integrin in CHO cells (Figure 7).19 However, ILK overexpression in parental cells had no further activation on the high-affinity state of integrin, and ILK overexpression in talin knocked-down parental cells did not rescue an inactivated integrin phenotype, indicating that ILK is necessary but not sufficient for integrin activation.
It has been shown that ILK forms a ternary complex with adaptor proteins PINCH and parvin in a signal-dependent manner,45 and the recruitment of the PINCH-ILK-parvin ternary complex to integrin adhesion sites is required for the further binding of ILK to other proteins, such as Mig-2/kindlin-2.46 Thus, the PINCH-ILK-parvin complex may support kindlin-2-mediated integrin activation.14 Interestingly, the interaction of each component of the ternary complex was necessary to prevent its degradation. Indeed, ILK-deficient mutant cells in this study showed a significant decrease of parvin expression, whereas kindlin-2 was similarly expressed in both parental and mutant cells (data not shown). Further experiments are needed to clarify whether ILK is involved in kindlin-2-mediated integrin activation.
In human platelets, ILK is expressed, forms the PINCH-ILK-parvin ternary complex, and interacts with the β integrin cytoplasmic domains of αIIbβ3 and collagen receptor α2β1 in response to platelet agonists, such as adenosine diphosphate, phorbol 12-myristrate 13-acetate, thrombin, and collagen.47-49 The kinase activity of ILK was also elevated in parallel to the attachment to the integrin cytoplasmic domains and induced phosphorylation of them. Platelet ILK appears to be activated under both aggregation-dependent and -independent conditions followed by agonist stimulation and regulates the function of platelet integrins αIIbβ3 and α2β1, suggesting that ILK may be involved in inside-out as well as outside-in signaling pathways.48,49 Under the revision process of our paper, the study on the phenotype of ILK-deficient platelets using conditional ILK-knockout mice was published. It provided evidence that ILK-deficient platelets displayed reduced levels of fibrinogen binding, aggregation, thrombus formation, and α-granule secretion.50 The data demonstrated that ILK is involved in platelet integrin αIIbβ3 activation. Thus, our present data are compatible with their findings.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Koichi Kokame, Kyoko Hidaka (National Cardiovascular Center Research Institute), Hirokazu Kashiwagi, and Masamichi Shiraga (Department of Hematology and Oncology, Osaka University Graduate School of Medicine) for helpful discussion.
This work was supported by grants from the Ministry of Health, Labor, and Welfare of Japan; the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation of Japan; and Academic Frontier Project.
Authorship
Contribution: Y.M. and T.K. provided essential reagents; S.H., H.S.-I., and S.T. performed research; S.H., H.S.-I., S.T., Y.M., Y.T., and T.M. analyzed data; and S.H., Y.T., and T.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shigenori Honda, National Cardiovascular Center Research Institute, 5-7-1 Fujishirodai, Suita, Osaka 565-8565, Japan; e-mail: shige@ri.ncvc.go.jp.