Abstract
Fanconi anemia (FA) is a heterogeneous genetic disorder characterized by bone marrow failure and complex congenital anomalies. Although mutations in FA genes result in a characteristic phenotype in the hematopoietic stem/progenitor cells (HSPCs), little is known about the consequences of a nonfunctional FA pathway in other stem/progenitor cell compartments. Given the intense functional interactions between HSPCs and the mesenchymalmicroenvironment, we investigated the FA pathway on the cellular functions of murine mesenchymal stem/progenitor cells (MSPCs) and their interactions with HSPCs in vitro and in vivo. Here, we show that loss of the murine homologue of FANCG (Fancg) results in a defect in MSPC proliferation and in their ability to support the adhesion and engraftment of murine syngeneic HSPCs in vitro or in vivo. Transplantation of wild-type (WT) but not Fancg−/− MSPCs into the tibiae of Fancg−/− recipient mice enhances the HSPC engraftment kinetics, the BM cellularity, and the number of progenitors per tibia of WT HSPCs injected into lethally irradiated Fancg−/− recipients. Collectively, these data show that FA proteins are required in the BM microenvironment to maintain normal hematopoiesis and provide genetic and quantitative evidence that adoptive transfer of WT MSPCs enhances hematopoietic stem cell engraftment.
Introduction
The microenvironment represents a defined anatomical compartment that provides regulatory signals to hematopoietic stem/progenitor cells (HSPCs) via interactions with the microenvironment as a 3-dimensional matrix of cells, extracellular matrix molecules, and cell-bound or soluble cytokines and chemokines.1-6 The essential cellular component of this microenvironment, also termed as the “niche,” is derived from a common progenitor of mesenchymal origin7 that gives rise to osteoblasts, self-renewing osteoprogenitors,3,8-12 and endothelial cells lining sinusoids.13 These mesenchymal stem/progenitor cells (MSPCs) express/secrete the cytokines, extracellular matrix proteins, and cell adhesion molecules that support the homing, migration, proliferation, and survival of HSPCs in vitro and in vivo.8,14-17 Given the functional importance of the microenvironment, there has been significant experimentation to test the hypothesis that transplantation of various stromal elements will enhance the engraftment of hematopoietic stem cells. However, despite the unambiguous role for stromal elements in supporting hematopoiesis, and the evidence that transplantation of mesenchymal cells enhances bone formation in patients with mesenchymal stem cell defects,18 cotransplantation of mesenchymal cells has yielded only modest and variable enhancement to the engraftment of hematopoietic stem cells (HSCs). The use of genetic models that have defects in hematopoietic and/or mesenchymal stem cells may facilitate characterizing the role of MSPCs in supporting hematopoiesis.
Fanconi anemia (FA) is a complex recessive inherited disorder caused by mutations in 13 genes whose products interact in a common pathway associated with the homologous recombination repair of DNA damage.19-22 Patients with FA are clinically characterized by congenital anomalies, a progressive bone marrow failure, and a high propensity to develop malignancies.19-21,23-26 Although it is established that FA has defects in the HSC compartment, the effect of loss of FA in other stem cell compartments has received relatively limited attention. In particular, other than variable cytokine production by FA fibroblasts and limited coculture experiments with variable results conducted more than 13 years ago,24,25,27,28 little information is available on the role of FA genes in influencing mesenchymal lineage functions and fates. However, the fact that patients with FA have characteristic anomalies in the skeletal, renal, and cutaneous tissues, all of which are mesenchymal derived, provides a strong rationale for exploring the role of FA genes in MSPC functions that provide good model systems in vitro and in vivo in syngeneic murine models of FA.
In the present study, we investigated the effect of MSPCs in modulating HSPC functions both in vivo and in vitro with the use of a murine model with targeted disruption of the Fancg gene (Fancg−/−), which has been shown to be hypersensitive in vivo to inflammatory cytokines and to have a 5- to 6-fold defect in the repopulating ability of hematopoietic stem cells.29-31 The results show that the FA pathway is critical for the BM microenvironment to maintain normal hematopoiesis, and they provide the first quantitative and genetic evidence that transplantation of mesenchymal cells enhances the engraftment of wild-type (WT) HSPCs.
Methods
Animals and reagents
The Fancg heterozygous (+/−) mice used in these studies have been previously described.29,30 Fancg+/− (C57Bl/6 × SV129) mice were backcrossed into a C57Bl/6J strain and then bred to generate Fancg−/− and WT mice. All protocols were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine. Six- to 8-week-old mice were used. Chemicals were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise stated.
Fibroblast colony-forming unit assay
To measure the frequency of MSPCs in BM, the fibroblast colony-forming unit (CFU-F) assay was performed as previously reported.32 Briefly, 2 × 106/mL BM mononuclear cells (BMMNCs) were plated into 6-well tissue-culture plates in triplicate for each condition in complete MesenCult medium (MesenCult basal media plus 20% of MesenCult Supplemental; StemCell Technologies, Vancouver, BC) and incubated at 37°C, 5% CO2. Selected experiments were conducted in hypoxic conditions under 1% O2. After 14 days of culture, medium was removed, and each well was washed with phosphate-buffered saline (PBS), stained with the HEMA-3 Quick Staining Kit (Fisher Scientific, Pittsburgh, PA) according to the manufacturer's instructions and photographed. A Fujifilm digital camera (FinePix 2400Zoom; Fujifilm, Tokyo, Japan) was used to take the photomicrographs of the CFU-Fs.
Isolation and expansion of MSPCs and purification of HSPCs
MSPCs were generated as previously described.32 Briefly, BM cells were collected from 6- to 8-week-old, age- and sex-matched WT and Fancg−/− mice by flushing the femurs and tibias with Iscove MEM (Invitrogen, Carlsbad, CA) containing 2% fetal bovine serum (FBS) with the use of a 21-gauge needle. BMMNCs were then separated by low-density gradient centrifugation and plated into a flask at 2 × 106/mL in 10 mL complete MesenCult medium. Once the culture reached 80% to 90% confluence, cells were trypsinized and replated at 5 × 105 cells/75 cm2. MSPCs at passage 7 to passage 10 were used for the following experiments. HSPCs were purified as described previously.33 Briefly, mature cell-lineage antigen-negative (Lin−) cells were enriched by immunomagnetic negative selection (Miltenyi Biotec, Auburn, CA), with the use of a mixture of purified rat anti–mouse mAbs specific for the mature cell-lineage antigens CD45R (B220, clone RA3-6B2), Gr-1 (Ly-6G, clone RB6-8C5), CD4 (L3T4, clone RM4-5), CD8a (Ly-2, clone 53-6.7), TER119 (TER119), and Mac-1 (CD11b, clone M1/70; BD PharMingen, San Diego, CA). The nonmagnetic Lin− fraction was collected, washed, and counted. The cells were then incubated with rat anti–mouse CD32/CD16 to avoid nonspecific antibody binding, after which they were stained with fluoresceinated rat anti–mouse CD117 (c-Kit; BD PharMingen). Negative control cells were stained with phycoerythrin-conjugated IgG2a and FITC-conjugated IgG2b. On the basis of these controls, Lin−c-Kit+ cells were isolated by sorting with a fluorescence-activated cell sorter (FACStar Plus; BD Biosciences, Franklin Lakes, NJ) under sterile conditions. The purity of Lin−c-Kit+ cells thus obtained was greater than 90%.
Cell-surface marker analysis
The phenotypic analyses of both MSPCs and HSPCs were performed by evaluating the expression of surface markers by flow cytometry on a FACSCalibur instrument (BD Biosciences). Antibodies for flow cytometry were purchased from BD PharMingen. MSPCs were stained with fluorescein isothiocyanate (FITC) anti–mouse CD44, R-phycoerythrin (R-PE)–conjugated anti–mouse CD49e, R-PE–conjugated anti–mouse CD29, purified rat anti–mouse CD105, anti–rat R-PE antibody, c-Kit, CD34, CD13, Mac-1, B220, and Gr-1 antibodies. HSPCs were analyzed by examining the expression of c-Kit. After incubation of cells with antibodies for 30 minutes at 4°C, cells were washed 3 times with PBS containing 0.1% bovine serum albumin and analyzed with a FACSCalibur instrument (BD Biosciences).
Clonogenic assays
Colony-forming units in culture (CFU-Cs) in the BM were assayed as previously described.34 Briefly, 5 × 104 BMMNCs were seeded onto a 35-mm gridded dish containing methylcellulose and murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), murine interleukin 3 (IL-3), murine stem cell factor (SCF; PeproTech, Rocky Hill, NJ), and M-CSF (R&D Research Laboratories, Minneapolis, MN) and were cultured at 37°C in a 5% CO2 incubator for 7 days.
Proliferation assays
Cell proliferation was examined by [3H]thymidine incorporation assay as previously described.32 Briefly, 104 MSPCs from WT or Fancg−/− mice were plated in 96-well flat-bottom plates in 200 μL α-MEM without supplements for 24 hours in a 37°C, 5% CO2, humidified incubator. The cells were then cultured for 48 hours in complete medium with MesenCult supplements, and [3H]thymidine was added to cultures 6 hours before harvest with an automated 96-well cell harvester (Brandel, Gaithersburg, MD). γ-Emission was measured with a microplate scintillation counter (PerkinElmer Life and Analytical Sciences, Boston, MA). Assays were performed in triplicate. For growth curves, MSPCs were plated into a flask at 2 × 106/mL in 10 mL complete MesenCult medium. Once the culture reached 80% to 90% confluence, cells were trypsinized and replated at 5 × 105 cells/75 cm.2 The fold increase in cell number was calculated over time, and the growth curve was plotted.
Determination of apoptosis
To evaluate whether loss of Fancg in MSPCs alters cell apoptosis, percentage of annexin V–FITC/propidium iodide (PI)–positive cells after serum deprivation was evaluated by flow cytometric analysis as described previously.35 Briefly, when MSPC cultures reached 70% to 80% confluence, the cultures were grown in DMEM without serum for 24 hours. The cells were detached from the culture plate with 0.05% trypsin-EDTA and then harvested and resuspended in 100 μL binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2). Cells were then stained with 5 μL annexin V–FITC (BD PharMingen) and 500 ng PI (Calbiochem, La Jolla, CA), incubated at room temperature for 15 minutes in the dark, and analyzed by flow cytometry. To assess whether intratibial MSPC injection decreases the cell apoptosis in vivo, after 5 months of BM cell and MSPC cotransplantation, BM cells were flushed from the tibia into IMDM containing 10% FBS. Low-density mononuclear cells were then stained with annexin V–FITC, PI, and c-Kit–APC per the manufacturer's instructions (BD PharMingen), and the percentage of apoptosis was evaluated by FACS analysis (BD Biosciences, San Jose, CA).
Senescence assay
Histochemical staining for β-galactosidase activity was used to measure the senescence of MSPCs.32,36 WT and Fancg−/− MSPCs were plated in chamber slides at 2 × 104/chamber and incubated at 37°C, 5% CO2 for 72 hours. Cells were then stained with a Senescence Staining Kit (Sigma-Aldrich) according to the manufacturer's instruction. Senescent cells displayed a blue color in the cytoplasm. Four thousand cells were counted for each chamber, and the percentage of positive cells/4000 cells was determined.
Generation of recombinant lentiviral plasmids and lentiviral transduction of MSPCs
The pCL1 vector is a derivative of the pGJ3 vector37 in which the lentiviral gag, pol, and rev genes were replaced by a truncated fragment of gag, a truncated envelope with the full-length rev responsive elements and the central polypurine track derived from the CSCG and plenty 6 vectors (Invitrogen).38 A multicloning site and an IRES-EGFP cassette were introduced from the vector S11IEG3 with the use of BstBI/BsrGI cuts, thereby creating the vector pCL1IEG. The FANCG cDNA was placed under the control of the SFFV U3 promotor by using the XhoI/EcoRI sites of pCL1IEG, thereby generating the vector pCL1FGIEG. Recombinant lentiviral vectors were produced in 293T cells with the use of the helper plasmid pCD/NL-BH (a kind gift by Jakob Reiser, Louisiana State University Health Sciences Center, New Orleans, LA) and the VSV-G envelope plasmid (kindly provided by Dirk Lindemann, Institution Virology, Technical University of Dresden, Dresden, Germany). Lentivirus containing supernatants were concentrated and titered on HT1080 cells as previously described,37 using the percentage of EGFP+ cells at serial dilutions as readout by flow cytometry. Titers of the 2 vectors on HT1080 cells were approximately 4.5 × 106 infectious particles/mL for pCL1IEG and 4.6 × 106 infectious agents/mL for pCL1FGIEG. MSPCs were split approximately 16 hours before transduction and then transduced overnight with concentrated supernatants resuspended in the appropriate fresh MSPC growth medium in the presence of 5 μg/mL polybrene. Medium was replaced the next day, and transduced cells were cultured and expanded. Some cultures were sorted for EGFP+ cells with the use of a FACSVantage flow cytometer.
Long-term culture of HSPCs on MSPC layers
To evaluate the effect of MSPCs in supporting proliferative hematopoietic cells, long-term coculture of MSPCs with Lin−c-Kit+ hematopoietic progenitors was established.39 Briefly, adherent MSPC layers were initiated with 1.5 × 105/well in 1 mL Iscove modified Dulbecco medium containing 10% horse serum, 10% FBS, 5 × 10−7 M hydrocortisone in a 24-well culture plate. The cultures were maintained at 33°C in a 5% CO2 incubator. When the single-cell layer of MSPC culture reached 90% confluence, the cultures were irradiated with 15 Gy and used as supporting cells. To initiate long-term cultures, the medium was completely removed from the irradiated MSPC cultures and replaced with fresh medium. Lin−c-Kit+ cells (5 × 104) were inoculated on the irradiated cell layers in triplicate wells per condition and incubated at 33°C in a 5% CO2 incubator. After 4 weeks of weekly half-media changes, the supporting effect of MSPCs to HSPCs was evaluated by counting cobblestone area-forming cells (CAFCs) as described previously.40,41 The areas that contained phase-dark hematopoietic clones having at least 5 cells beneath the MSPC layer were counted as CAFCs. The content of clonogenic progenitors of each well was assessed after 4 weeks in culture by plating all the cells in the culture in methylcellulose supplemented with G-CSF, M-CSF, IL-3, Epo, and SCF as described in “Clonogenic assays.”
Adhesion assay
MSPCs of WT or Fancg−/− were replated at 105 cells/well in 24-well plates, and after overnight incubation the cells became confluent and formed adherent single layers. Lin−c-kit+ cells (2 × 105) were added into each well containing WT or Fancg−/− MSPCs. After incubation for 1 hour at 37°C, the wells were washed with PBS 3 times and fixed with 4% paraformaldehyde at room temperature for 10 to 15 minutes. Adherent cells were counted under the phase contract microscope.
Cotransplantation of BMMNCs and MSPCs or Lin−c-Kit+ cells and MSPCs
For primary transplantation, 6- to 8-week-old WT or Fancg−/− mice were irradiated with 1100 cGy. Four hours after irradiation, the mice were anesthetized with Avertin at 375 μg/g body weight. A total of 106 MSPCs in 10-μL volume was injected directly into both tibiae of the WT and Fancg−/− mice by intra-BM transplantation (IBMT) using a Hamilton microinjection syringe equipped with a 31-gauge needle as described previously.17 The control group was given 10 μL PBS only. BMMNCs or Lin−c-Kit+ cells were administered intravenously by tail vein injection at different doses as required in different experiments. At 5 months after transplantation, the mice were killed, and the BM cells were collected separately from each tibia. The cells were counted and used for clonogenic cell assays.17 To verify that MSPCs were injected into the tibial marrow cavity, WT MSPCs transduced with luciferase by a SF91-oncoretro vector construct42 were injected into the tibia. Two weeks after the MSPC injection, mice were anesthetized with Avertin, luciferin was injected into the peritoneal cavity, and cell luminescence was measured with the use of a Night Owl instrument (LB 981; Berthold Technologies, Bad Wildbad, Germany). To confirm the MSPC reconstitution, CFU-F assays were performed with the use of the bone marrow flushed from the injected tibiae after 2 months of MSPC intratibial injection. To evaluate whether there is a cell-dosage effect of MSPCs on hematopoiesis, a range of dosages of WT and Fancg−/− MSPCs were injected into tibiae of lethally irradiated WT recipient mice, and 106 WT BMMNCs were given intravenously. Three months after cotransplantation, the BMMNCs were collected, and the GM-CFU assays were performed.
Homing studies
BMMNCs were prepared from whole BM of WT mice. After lethal irradiation (1100 cGy, split dose with 4 hours minimum between doses), Fancg−/− recipient mice that were 8 to 10 weeks old received PBS, 106 WT, or Fancg−/− MSPCs IBMT. Twenty-four hours after the IBMT, 300-μL cell suspension containing 106 cells was infused by intravenous tail vein injection. These mice were then killed 24 hours after tail vein injection. BM was harvested from the recipients, and CFU-C was performed as described in “Clonogenic assays.”
Statistical analysis
The Student t test was used to evaluate statistical differences between WT and Fancg−/− cells. Statistical significance was defined as P value less than .05.
Results
Fancg−/− mice have a lower frequency of CFU-F
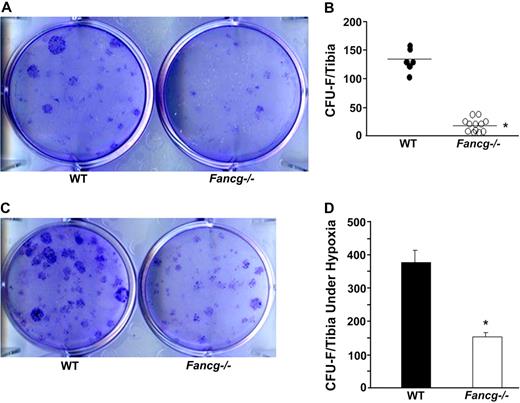
Work over the past few years has established that MSPC have a hierarchical system of multipotent and lineage-restricted progenitors that form clonogenic assays analogous to the hematopoietic system.43 In addition, previous work provided evidence that the number of CFU-Fs closely correlates with the in vivo frequency of MSPCs.44 To determine the role of Fancg on MSPC dynamics, CFU-F assays were performed in age- and sex-matched Fancg−/− and WT syngeneic mice. In 5 independent experiments, cultures of CFU-Fs isolated from Fancg−/− mice and maintained in ambient oxygen consistently yielded 5- to 6-fold fewer CFU-Fs compared with WT mice (Figure 1A,B, 1 representative experiment of 5 independent experiments). Some studies have suggested that the cellular defects in FA are exaggerated in ambient oxygen concentration.45,46 However, previous work has established that marrow from even WT mice form more CFU-Fs in 1% to 5% oxygen than with ambient oxygen tension.47 To assess these genotypic and physical variables, parallel CFU-F assays were established in 1% oxygen. Consistent with previous experiments in reduced oxygen, significantly more CFU-Fs were found from marrow of WT mice than from parallel WT cultures in ambient oxygen (Figure 1C,D). Importantly, Fancg−/− CFU-F formation was 2- to 3-fold lower than WT CFU-Fs in low-oxygen tension (Figure 1C,D). However, these genotypic differences were even more pronounced (5- to 6-fold) when the cultures were maintained in ambient oxygen. We conclude that there is a consistent reduction in the number of CFU-Fs in Fancg−/− mice that is accentuated by maintenance of cultures in ambient oxygen.
Evaluation of frequency of CFU-F from WT and Fancg−/− MSPCs. (A) A representative photomicrograph of CFU-Fs from WT and Fancg−/− BMMNCs, after staining with the HEMA-3 Quick Staining Kit. (B) Representative data of the quantitative frequency of CFU-Fs per tibia from WT and Fancg−/− mice are shown (*P < .001 for comparison of WT and Fancg−/− BMMNCs). (C) A representative photomicrograph of CFU-Fs from WT and Fancg−/− BMMNCs cultured in hypoxic conditions. (D) Total CFU-Fs per tibia in hypoxia culture condition are shown. *P < .001 comparing Fancg−/− with WT mice. Data are shown as mean ± SE of 5 independent experiments. The photomicrographs in panels A and C were taken (under 10× amplification) with a Nikon TE2000-S microscope (Nikon Instruments, Melville, NY), a QIMAGING camera, and QCapture Pro software (Fryer Company, Cincinnati, OH).
Evaluation of frequency of CFU-F from WT and Fancg−/− MSPCs. (A) A representative photomicrograph of CFU-Fs from WT and Fancg−/− BMMNCs, after staining with the HEMA-3 Quick Staining Kit. (B) Representative data of the quantitative frequency of CFU-Fs per tibia from WT and Fancg−/− mice are shown (*P < .001 for comparison of WT and Fancg−/− BMMNCs). (C) A representative photomicrograph of CFU-Fs from WT and Fancg−/− BMMNCs cultured in hypoxic conditions. (D) Total CFU-Fs per tibia in hypoxia culture condition are shown. *P < .001 comparing Fancg−/− with WT mice. Data are shown as mean ± SE of 5 independent experiments. The photomicrographs in panels A and C were taken (under 10× amplification) with a Nikon TE2000-S microscope (Nikon Instruments, Melville, NY), a QIMAGING camera, and QCapture Pro software (Fryer Company, Cincinnati, OH).
Fancg−/− MSPCs have diminished proliferating capability and increased apoptosis and introduction of human FANCG cDNA restores cellular functions of murine Fancg−/− MSPCs
Purified populations of phenotypically defined MSPCs from BM were established to conduct formal proliferation and apoptosis measurements with flow cytometry as previously described32 (Figure 2A). Consistent with previous studies by others and us,32,44 both WT and Fancg−/− MSPCs are negative for the expression of CD34, CD13, CD45, B220, Gr-1, and Mac-1 but positive for the expression of CD29, CD44, CD105, and Col I. WT and Fancg−/− MSPCs showed a similar level of expression of the panel of surface markers. Having isolated phenotypically equivalent populations of MSPCs, the proliferative ability of these MSPCs was scored with the use of [3H]thymidine incorporation in 2% to 10% FBS. In all FBS concentrations, WT MSPCs had increased proliferation compared with Fancg−/− MSPCs (Figure 2B).
Phenotypic analysis of MSPCs and introduction of WT FANCG gene restores normal MSPC proliferation and survival. (A) Representative expression of surface markers on WT and Fancg−/− MSPCs from 4 individual groups of cells was evaluated by flow cytometric analysis. Isotype controls are shown in dashed lines, and experimental samples are shown in gray area. (B) [3H]Thymidine incorporation in WT and Fancg−/− MSPCs was measured after stimulation with DMEM containing 0% to 10% FBS. Data are expressed as mean (± SE) from 4 individual experiments (*P < .001 for Fancg−/− vs WT cells). (C) At baseline and 24 and 48 hours after FBS depletion, the percentage of annexin/PI+ cells was analyzed by flow cytometry. Results of 4 independent experiments are shown. *P < .01 at 24 hours; **P < .001 at 48 hours for comparison of WT with Fancg−/− MSPCs. (D) Representative photographs of WT and Fancg−/− MSPC cultures after β-galactosidase staining are shown. Senescent cells were light blue cytoplasmic cells as pointed by red arrows. (E) Quantitative summary showed *P < .001 for Fancg−/− versus WT senescent cells from 3 independent experiments. (F) Flow cytometric analysis shows the percent enhanced green fluorescent protein (EGFP) expression on the MSPCs from WT mice (left) and Fancg−/− mice (right). (G) After transduction, cell proliferation was evaluated by counting the cell numbers in each culture condition, and data are shown as the fold change of cell numbers over the basal level in each genotype of cells after stimulation with DMEM containing 5% FBS (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence. (H) After transduction, cell apoptosis was evaluated by flow cytometry after deprivation of FBS up to 48 hours, and data are expressed as percentage of annexin V on different genotypes of MSPCs (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence.
Phenotypic analysis of MSPCs and introduction of WT FANCG gene restores normal MSPC proliferation and survival. (A) Representative expression of surface markers on WT and Fancg−/− MSPCs from 4 individual groups of cells was evaluated by flow cytometric analysis. Isotype controls are shown in dashed lines, and experimental samples are shown in gray area. (B) [3H]Thymidine incorporation in WT and Fancg−/− MSPCs was measured after stimulation with DMEM containing 0% to 10% FBS. Data are expressed as mean (± SE) from 4 individual experiments (*P < .001 for Fancg−/− vs WT cells). (C) At baseline and 24 and 48 hours after FBS depletion, the percentage of annexin/PI+ cells was analyzed by flow cytometry. Results of 4 independent experiments are shown. *P < .01 at 24 hours; **P < .001 at 48 hours for comparison of WT with Fancg−/− MSPCs. (D) Representative photographs of WT and Fancg−/− MSPC cultures after β-galactosidase staining are shown. Senescent cells were light blue cytoplasmic cells as pointed by red arrows. (E) Quantitative summary showed *P < .001 for Fancg−/− versus WT senescent cells from 3 independent experiments. (F) Flow cytometric analysis shows the percent enhanced green fluorescent protein (EGFP) expression on the MSPCs from WT mice (left) and Fancg−/− mice (right). (G) After transduction, cell proliferation was evaluated by counting the cell numbers in each culture condition, and data are shown as the fold change of cell numbers over the basal level in each genotype of cells after stimulation with DMEM containing 5% FBS (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence. (H) After transduction, cell apoptosis was evaluated by flow cytometry after deprivation of FBS up to 48 hours, and data are expressed as percentage of annexin V on different genotypes of MSPCs (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence.
Because FA HSPCs are predisposed to undergo apoptosis in vitro, even in the absence of genotoxic stimuli,48-50 we next addressed whether loss of Fancg alters MSPC apoptosis with annexin V expression as an indicator of apoptosis. Although at basal levels in the presence of serum genotypic survival was comparable, after 24 to 48 hours of serum deprivation, Fancg−/− MSPCs showed a significantly higher fraction of annexin V+ cells than did WT MSPCs (Figure 2C). Cellular senescence is associated with a loss of proliferative ability in response to mitogenic agents.51 To test whether the decrease in the proliferation of Fancg−/− MSPCs was also associated with a change in cellular senescence, a senescence assay was performed based on histochemical staining for β-galactosidase activity.32,36 A significant increase in the number of β-galactosidase–positive cells was observed in Fancg−/− MSPCs compared with WT MSPCs (Figure 2D,E). Collectively, these results suggest that the decreased proliferation in Fancg−/− MSPCs is associated with an increase in apoptosis and an increase in senescence activity of these cells compared with WT controls.
To formally test the hypothesis that the impaired cellular functions observed in Fancg−/− MSPCs are directly linked with the loss of Fancg gene, we transduced Fancg−/− MSPCs with a lentivirus vector containing either the Fancg and EGFP cDNAs or alternatively a control vector expressing EGFP only. Phenotypically defined populations of transduced MSPCs were sorted and subjected to proliferation and apoptosis assays.
Representative FACS analyses of the sorted cells are shown in Figure 2F. Consistent with our previous results, Fancg−/− MSPCs transduced with vector containing the EGFP reporter gene only have significantly reduced cell growth compared with that of WT cells (Figure 2G). Importantly, expression of the WT FANCG gene in Fancg−/− MSPCs restored normal proliferation. Furthermore, Fancg−/− MSPCs expressing the FANCG transgene normalize the induction of apoptosis mediated by serum depletion in Fancg−/− MSPCs, whereas the reporter transgene does not (Figure 2H). Collectively, these results indicate that the defect in the cellular functions of Fancg−/− MSPCs is a consequence of a specific defect in Fancg and that restoration of a functional FA pathway is sufficient to restore normal cellular function.
Fancg−/− MSPCs have impaired function in supporting HSPC proliferation and recruitment of HSPCs in vitro and in vivo
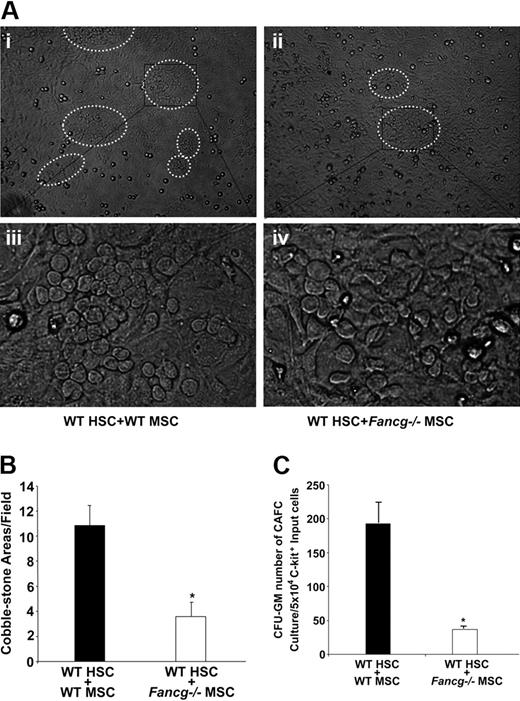
The CAFC assay is an established in vitro measurement to evaluated stem cell functions.39 The assay is conducted by overlaying HSPCs on stromal monolayers and scoring the number of CAFCs at day 28 of culture. To quantitatively examine the ability of Fancg−/− MSPCs to support HSPCs, MSPCs were seeded on tissue-culture plates, grown to confluence, and irradiated to prevent further cell division. WT and Fancg−/− MSPCs were then cocultured with the same pool of WT Lin−c-Kit+ cells that are highly enriched for HSPCs. On day 28 of culture, cocultures containing WT MSPCs formed multiple large CAFCs (Figure 3Ai,iii). In striking contrast, Fancg−/− MSPCs supported 3-fold less hypocellular CAFCs (Figure 3Aii,iv,B). When trypsinized cells from those cocultures were placed in semisolid medium to establish the growth of myeloid progenitors (CFU-GM), a 4-fold reduction of myeloid progenitors was observed from cocultures supported by Fancg−/− MSPCs compared with WT MSPCs (Figure 3C). Collectively, these data indicate that Fancg−/− MSPCs have a reduced ability to support HPSC proliferation and differentiation in vitro.
Coculture of WT HPSCs with MSPCs from WT or Fancg−/− mice. (A) Representative photomicroscopy of cobblestone-forming areas after 4-week coculture with WT (i,iii) or Fancg−/− MSPCs (ii,iv). Panels i and ii were taken under ×10, panels iii and iv under ×40 magnification. (B) Quantitative evaluation of the number of cobblestone-forming areas per low-power field. *P < .01 for comparison of cobblestone areas in Fancg−/− coculture versus WT coculture. (C) After 4-week coculture of WT HSPCs with WT or Fancg−/− MSPCs, the cells were harvested, and CFU-C assays were performed. The results shown are from 5 independent experiments. *P < .01 for comparison of WT HSPC/WT MSPC coculture with WT HSPCs-Fancg−/− MSPC coculture. Error bars represent mean (± SE).
Coculture of WT HPSCs with MSPCs from WT or Fancg−/− mice. (A) Representative photomicroscopy of cobblestone-forming areas after 4-week coculture with WT (i,iii) or Fancg−/− MSPCs (ii,iv). Panels i and ii were taken under ×10, panels iii and iv under ×40 magnification. (B) Quantitative evaluation of the number of cobblestone-forming areas per low-power field. *P < .01 for comparison of cobblestone areas in Fancg−/− coculture versus WT coculture. (C) After 4-week coculture of WT HSPCs with WT or Fancg−/− MSPCs, the cells were harvested, and CFU-C assays were performed. The results shown are from 5 independent experiments. *P < .01 for comparison of WT HSPC/WT MSPC coculture with WT HSPCs-Fancg−/− MSPC coculture. Error bars represent mean (± SE).
WT MSPCs promote the reconstitution of hematopoiesis in Fancg−/− mice
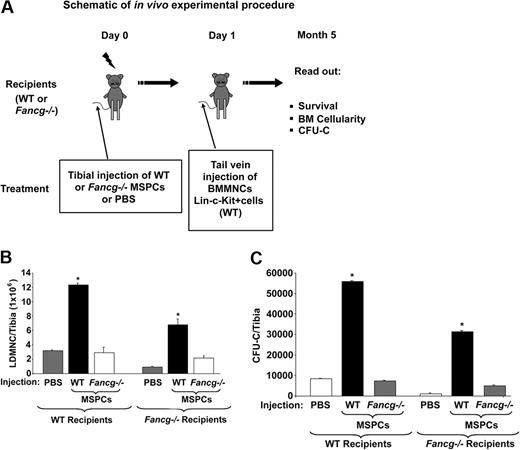
A limitation in consistent engraftment of MSPCs, and consequently their ability to affect hematopoiesis, is that the size of MSPCs is sufficiently large to be trapped in the lung. To obviate this problem, others have previously established that direct transplantation of MSPCs into the bone marrow cavity allows efficient MSPC engraftment.17 Using this method, we tested the hypothesis that genetic disruption of Fancg in MSPCs impairs the homing and engraftment of the hematopoietic stem/progenitor cells in vivo (Figure 4A). Fancg−/− and WT syngeneic mice received 1100 cGy irradiation. Four hours later, recipient mice received 106 WT or Fancg−/− MSPCs by intratibial injection as described in “Methods.” In initial experiments, 24 hours after the injection of MSPCs into the marrow, all recipients received WT 2 × 106 BMMNCs via tail vein injection. Two months after the intratibial injection of MSPCs, the reconstitution of MSPCs from the donor cells was confirmed by CFU-F assays after the intratibial injection of the MSPCs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and by a Night Owl Instrument (data not shown).
Effect of MSPCs on the reconstitution of hematopoiesis. (A) Schematic shows the in vivo experimental procedure. (B) Number of BMMNCs per tibia 5 months after transplantation; *P < .001 for comparison of mice injected with WT MSPCs versus PBS or Fancg−/− MSPCs. (C) CFU-Cs per tibia; *P < .001 for comparison of mice injected with WT MSPCs versus PBS or Fancg−/− MSPCs. *P < .01 for comparing recipients injected with WT MSPCs versus PBS or Fancg−/− MSPCs. Data represent mean (± SE) of CFU-GMs from 5 mice in each group.
Effect of MSPCs on the reconstitution of hematopoiesis. (A) Schematic shows the in vivo experimental procedure. (B) Number of BMMNCs per tibia 5 months after transplantation; *P < .001 for comparison of mice injected with WT MSPCs versus PBS or Fancg−/− MSPCs. (C) CFU-Cs per tibia; *P < .001 for comparison of mice injected with WT MSPCs versus PBS or Fancg−/− MSPCs. *P < .01 for comparing recipients injected with WT MSPCs versus PBS or Fancg−/− MSPCs. Data represent mean (± SE) of CFU-GMs from 5 mice in each group.
Five months later the total cellularity and the number of myeloid progenitors from the transplanted tibiae were scored. A 4.5-fold increase in cellularity per tibia was observed in WT recipients injected with WT MSPCs compared with the PBS-injected control group or Fancg−/− MSPCs (Figure 4B). In contrast, although tibia cellularity was lower in the Fancg−/− mice in general, Fancg−/− recipients that received intratibial WT MSPCs had a 2-fold increase in tibia cellularity compared with Fancg−/− hosts receiving PBS or Fancg−/− MSPCs (Figure 4B). Furthermore, this increase in total cellularity was associated with an increase in the number of myeloid progenitors per tibia of each group (Figure 4C). MSPCs exist in the bone marrow. To evaluate whether there is a cell-dosage effect of WT MSPCs on hematopoiesis, a range of MSPC concentrations was injected into lethally irradiated WT recipients followed by intravenous injection of 106 WT BMMNCs. Interestingly, a significant MSPC-dependent increase in CFU-GMs per tibia was observed in the recipients who received WT MSPCs, whereas no change in CFU-GM formation was observed in recipients who obtained Fancg−/− MSPCs (Figure S2). This result indicates that WT MSPCs enhance CFU-GMs in a dosage-dependent fashion.
To directly compare the whole marrow and myeloid progenitor cellularity in mice, we injected PBS (left tibia) or WT MSPCs (right tibia) into the same WT recipients, and the CFU-GMs were then scored 5 months after transplantation. Consistently, a significantly higher number of CFU-GMs per tibia were observed in the tibia that received WT MSPCs compared with that in the tibia that received PBS (Figure S3).
To avoid any contamination of MSPCs from donor WT HSPCs, we purified Lin−c-Kit+ WT cells and transplanted aliquots of the same purified HSPCs into Fancg−/− recipients after transplantation of WT or Fancg−/− MSPCs. Consistent with the previous studies, cotransplantation of WT MSPCs significantly enhanced the generation of CFU-GMs in the Fancg−/− recipient mice compared with CFU-GMs from recipients cotransplanted with Fancg−/− MSPCs (25 ± 1.6 × 103 vs 15 ± 1.0 × 103 CFU-GM/tibiae; n = 6; P < .01). Thus, cotransplantation of WT MSPCs and HSPCs in lethally irradiated Fancg−/− hosts enhances the hematopoietic cell content of transplanted cells.
WT but not Fancg−/− MSPCs increases the adhesion and homing of HSPCs
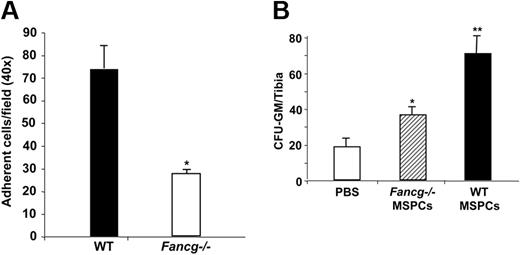
Recently, studies by Wagner et al provided direct evidence that cell-to-cell interactions of MSPCs and HSCs are beneficial for the maintenance of “stemness” of HSCs.52,53 Given the reduced support activity of Fancg−/− MSPCs to HSPCs, we hypothesized that loss of Fancg in MSPCs alters HSPC adhesion to these MSPCs which in turn leads to the defect in the supportive activity of MSPCs to HSPCs. To test the hypothesis, an in vitro adhesion assay was performed as described previously.33 In 6 independent experiments, a 2- to 3-fold reduction of adhesion of WT Lin−c-Kit+ cells on the Fancg−/− MSPCs was observed compared with that on WT MSPCs (Figure 5A).
Adhesion of WT HSPCs on MSPCs and homing of WT BMMNCs to the BM cavity. (A) WT Lin−c-kit+ cells (2 × 105) were added into each well preloaded with 105 cells/well WT or Fancg−/− MSPCs in 24-well plates. Adherent cells were counted under the phase contract microscope. Data are shown as mean (± SE) from 6 individual experiments (*P < .001 for Fancg−/− vs WT cells). (B) CFU-Cs from the tibia of Fancg−/− recipients after lethally irradiation and 106 BMMNCs injection with or without MSPC injection. □ indicates the PBS injection; ▨, Fancg−/− MSPC injection; and ■, the WT MSPC injection. *P < .05 for comparison of Fancg−/− MSPCs versus PBS injection, **P < .01 for comparison of WT MSPCs versus Fancg−/− MSPCs and PBS injection. The data show the mean (± SD) of 1 representative experiment of 3 independent experiments with similar results.
Adhesion of WT HSPCs on MSPCs and homing of WT BMMNCs to the BM cavity. (A) WT Lin−c-kit+ cells (2 × 105) were added into each well preloaded with 105 cells/well WT or Fancg−/− MSPCs in 24-well plates. Adherent cells were counted under the phase contract microscope. Data are shown as mean (± SE) from 6 individual experiments (*P < .001 for Fancg−/− vs WT cells). (B) CFU-Cs from the tibia of Fancg−/− recipients after lethally irradiation and 106 BMMNCs injection with or without MSPC injection. □ indicates the PBS injection; ▨, Fancg−/− MSPC injection; and ■, the WT MSPC injection. *P < .05 for comparison of Fancg−/− MSPCs versus PBS injection, **P < .01 for comparison of WT MSPCs versus Fancg−/− MSPCs and PBS injection. The data show the mean (± SD) of 1 representative experiment of 3 independent experiments with similar results.
It has been shown that a close functional correlation between in vitro adhesion defect and in vivo homing of hematopoietic stem cells exists.54,55 Given that engraftment of HSPCs in vivo is a multistep process that depends initially on appropriate homing of infused cells to the BM medullary cavity and adhesion in the hematopoietic microenvironment, specifically in the niche and that WT MSPCs enhance long-term HSPC reconstitution in Fancg−/− hosts, we next compared the homing capacity of WT HSPCs in Fancg−/− recipients in vivo. A significant increase in CFU-Cs was observed in the Fancg−/− recipients that received WT MSPCs and WT donor cells in the BM 24 hours after HSPC transplantation (Figure 5B). In addition, an increase in HSPC survival was also observed in the Fancg−/− recipients that obtained WT MSPCs compared with the Fancg−/− recipients that obtained PBS or Fancg−/− MSPCs (annexin V−, 92.5% ± 2.1% vs 83.5% ± 3.4%, respectively). These data suggest that direct intramarrow delivery of WT MSPCs in Fancg−/− recipients increases HSPC homing, adhesion, and survival that leads to an increase in the engraftment of long-term HSCs.
Discussion
FA is a heterogeneous genetic disorder with a high predisposition to bone marrow failure in childhood. Observations that transplantation of bone marrow–derived cells corrects the progressive aplastic anemia that is characteristic of FA have established that FA proteins are required for the long-term proliferation and survival of HSPCs. However, the numerous defects in mesenchymal-derived tissues, the observation that patients with FA have a high incidence of mesenchymal-derived cancers, and an emerging body of literature that illustrate the protean, complex interactions between hematopoietic cells and lineages in the microenvironment, prompted our functional examination of FA mesenchymal cells in supporting hematopoiesis. In this study we provide 3 independent lines of evidence indicating that FA proteins in MSPCs have an integral role in maintenance of hematopoiesis in FA-deficient mice.
First, we tested the hypothesis that FA proteins reduce clonogenic mesenchymal cell proliferation and survival analogous to observations that have been made in the hematopoietic system over 20 years.48,49,56-61 We found in multiple experiments that FANCG-deficient MSPCs have decreased clonogenic growth, as well as a decrease in proliferation and survival in vitro. Further, in murine in vitro systems, the microenvironment of FANCG-deficient cells support hematopoiesis less well than unaffected micro-environmental cells with the use of the same pool of hematopoietic cells. Complementary in vivo experiments were conducted in which we determined that Fancg−/−-recipient mice that received WT mesenchymal cells together with WT hematopoietic stem cells had greater bone marrow cellularity and higher numbers of progenitors than did Fancg−/− recipients that received Fancg−/− MSPCs and an equivalent number of WT HSCs from the same pool. This decrease in cellularity of mature and progenitor cells was associated with a reduction of adhesion, homing, and proliferation of long-term reconstituting WT hematopoietic stem cells. Third, introduction of the FANCG transgene into the MSPCs was sufficient to restore normal cell proliferation, differentiation, and survival of the mesenchymal precursors and to enhance the proliferation of hematopoietic precursors in vitro and in vivo, indicating that the intrinsic cell defects were causally related to the absence of the endogenous FANCG gene product in MSPCs.
Previous genetic proof-of-concept studies have provided direct molecular evidence that engraftment of exogenous mesenchymal cells enhances skeletal function. The most notable of these are studies focusing on osteogenesis imperfecta.62 Moreover, multiple studies of cotransplantation of MSPCs and HSPCs in animal models and in humans show that MSPC infusions are safe and may increase chimerism or accelerate hematopoietic recovery, suggesting a potential therapeutic role for MSPCs in the engraftment and repopulation of HSPCs.63-66 However, studies to show quantitatively the ability of MSPCs to enhance HSC engraftment have been hindered by the lack of a genetic model provided for the first time in these experiments. A common limitation in determining the role of MSPCs in enhancing HSC proliferation and engraftment is that a high proportion of MSPCs are trapped in the lung parenchymal tissue. Studies here avoided this potential pitfall in that we used a method published previously17 and conducted direct intrabone marrow transplantation of genetically corrected mesenchymal cells.
In the context of FA, the microenvironment could have a key role in enhancing engraftment of an allograft or genetically corrected autologous stem cells, particularly if limiting numbers of cells are available, such as in many cord blood transplantations or in the context of gene therapy. Several previous studies that used retroviral-mediated gene transfer in FANCA−/− and FANCC−/− patients have shown minimal short-term engraftment and no long-term engraftment of retroviral-marked stem cells.67-69 Because mesenchymal cells were excluded in these studies, it is possible that the lack of an appropriate microenvironment could have impaired the ability of transduced cells to home and proliferate in vivo.
Previous studies in human patients have had varied conclusions about the role of FA proteins in modulating the hematopoietic microenvironment.24,25,27,28 Those studies were limited by individual patient variability that was found in both FA and healthy donors, small numbers, and limiting reagents to conduct detailed experiments. A key advantage of the Fancg−/− murine model is that it is possible to conduct detailed in vivo experiments that cannot be accomplished in human patients, and, by backcrossing the knockouts for 10 generations onto a C57Bl/6 background, it is possible to make determinations on the role of the individual disrupted gene exclusively. Future studies confirming these observations in human FA cells in immunodeficient mice are now being initiated. If these results are confirmed, this study could have implications in enhancing the engraftment of genetically corrected autologous stem cells or allogeneic cord blood cells in patients with FA in phase 1 trials.
Finally, this genetic model may be useful for evaluating basic questions about the biology of mesenchymal stem cells and transplanted mesenchymal stem/progenitor cells. Although there are many tools to characterize the hierarchical and functional aspects of hematopoietic stem cells, there is a paucity of reagents for conducting comparable studies in mesenchymal stem/progenitor cells. It is established that mesenchymal precursors are able to differentiate into multiple progeny.70,71 However, the number of studies that have evaluated the long-term fates of individual mesenchymal precursors in a transplantation setting, analogous to those conducted by Lemischka et al72 and Dick et al73 in hematopoietic cells many years ago are lacking. Given the functional deficits in Fancg−/− mesenchymal cells found in these studies, it may be possible to use Fancg−/− mice as a model system to address some of these basic questions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Wade Clapp for critical reading of the manuscript, Deyu Zeng and Selina Estwick for technical support, and Paula L. Riggen and Marilyn Wales for administrative support. We also gratefully acknowledge Dr Alan D'Andrea at Harvard University for generating the Fancg−/− mice and making them available.
This work was supported by the Simmons Clinical Studies Fund (Indiana University, Indianapolis, IN; F.-C.Y.), the Department of Defense (NF073112; F.-C.Y.), the March of Dimes (6-FY08-246; F.-C.Y.), National Institutes of Health, (Bethesda, MD), the Showalter Trust Award (Indiana University, Indianapolis, IN; W.S.G.), and Deutsche Forschungsgemeinschaft (Bonn, Germany; K08 HL075253; W.S.G.; DFG SPP1230 HA2322/2-1; H.H.; and PPG-P01-HL533586; H.H. and K.P.).
Authorship
Contribution: Y.L. conducted experiments, analyzed data, and wrote the manuscript; S.C., K.P., and H.H. designed experiments and analyzed data; J.Y., Y.Y., J.M., and M.F. conducted experiments; J.L. and W.S.G. conducted experiments and analyzed data; X.W. analyzed data; and F.-C.Y. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feng-Chun Yang, Indiana University School of Medicine, Cancer Research Institute, 1044 West Walnut Street, R4/427, Indianapolis, IN 46202; e-mail: fyang@iupui.edu.

![Figure 2. Phenotypic analysis of MSPCs and introduction of WT FANCG gene restores normal MSPC proliferation and survival. (A) Representative expression of surface markers on WT and Fancg−/− MSPCs from 4 individual groups of cells was evaluated by flow cytometric analysis. Isotype controls are shown in dashed lines, and experimental samples are shown in gray area. (B) [3H]Thymidine incorporation in WT and Fancg−/− MSPCs was measured after stimulation with DMEM containing 0% to 10% FBS. Data are expressed as mean (± SE) from 4 individual experiments (*P < .001 for Fancg−/− vs WT cells). (C) At baseline and 24 and 48 hours after FBS depletion, the percentage of annexin/PI+ cells was analyzed by flow cytometry. Results of 4 independent experiments are shown. *P < .01 at 24 hours; **P < .001 at 48 hours for comparison of WT with Fancg−/− MSPCs. (D) Representative photographs of WT and Fancg−/− MSPC cultures after β-galactosidase staining are shown. Senescent cells were light blue cytoplasmic cells as pointed by red arrows. (E) Quantitative summary showed *P < .001 for Fancg−/− versus WT senescent cells from 3 independent experiments. (F) Flow cytometric analysis shows the percent enhanced green fluorescent protein (EGFP) expression on the MSPCs from WT mice (left) and Fancg−/− mice (right). (G) After transduction, cell proliferation was evaluated by counting the cell numbers in each culture condition, and data are shown as the fold change of cell numbers over the basal level in each genotype of cells after stimulation with DMEM containing 5% FBS (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence. (H) After transduction, cell apoptosis was evaluated by flow cytometry after deprivation of FBS up to 48 hours, and data are expressed as percentage of annexin V on different genotypes of MSPCs (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/10/10.1182_blood-2008-07-168138/4/m_zh8013093263002a.jpeg?Expires=1769948341&Signature=y4JabSYgfhk1vD8q5lsNScPHbwgNlw076k0IwI0w8G-tgsidmHOS6wiAbGiL-S6yj9tb-N~CIlfy2VRkf0kczr4lMdkSOOPkxYG~ZqCRkxa7OfDvbbmErx3xNkigISoc3iZ1T4a3q~DSM6wptH6HIYimyCDjWLCB8exjglelwzNpQiasPuGPFqfyu~QTVAFOjuwU7BwLLJm8vzz-n9btO7NvDWBTKmAylSIt71QyzSyQXGCsR8NEw9u7FE09paqOLjWvWsrXnkkq5aNywVT3ByK8wTWZ7mlVxSrvPrvxgET5dpmoM1n~busUuSIjM144qqeBjfjMH~kvXP3PyZSejA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Phenotypic analysis of MSPCs and introduction of WT FANCG gene restores normal MSPC proliferation and survival. (A) Representative expression of surface markers on WT and Fancg−/− MSPCs from 4 individual groups of cells was evaluated by flow cytometric analysis. Isotype controls are shown in dashed lines, and experimental samples are shown in gray area. (B) [3H]Thymidine incorporation in WT and Fancg−/− MSPCs was measured after stimulation with DMEM containing 0% to 10% FBS. Data are expressed as mean (± SE) from 4 individual experiments (*P < .001 for Fancg−/− vs WT cells). (C) At baseline and 24 and 48 hours after FBS depletion, the percentage of annexin/PI+ cells was analyzed by flow cytometry. Results of 4 independent experiments are shown. *P < .01 at 24 hours; **P < .001 at 48 hours for comparison of WT with Fancg−/− MSPCs. (D) Representative photographs of WT and Fancg−/− MSPC cultures after β-galactosidase staining are shown. Senescent cells were light blue cytoplasmic cells as pointed by red arrows. (E) Quantitative summary showed *P < .001 for Fancg−/− versus WT senescent cells from 3 independent experiments. (F) Flow cytometric analysis shows the percent enhanced green fluorescent protein (EGFP) expression on the MSPCs from WT mice (left) and Fancg−/− mice (right). (G) After transduction, cell proliferation was evaluated by counting the cell numbers in each culture condition, and data are shown as the fold change of cell numbers over the basal level in each genotype of cells after stimulation with DMEM containing 5% FBS (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence. (H) After transduction, cell apoptosis was evaluated by flow cytometry after deprivation of FBS up to 48 hours, and data are expressed as percentage of annexin V on different genotypes of MSPCs (mean ± SE) from 4 individual experiments. *P < .01 for Fancg−/− with vector control versus WT cells or Fancg−/− cells transduced with WT FANCG sequence.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/10/10.1182_blood-2008-07-168138/4/m_zh8013093263002f.jpeg?Expires=1769948341&Signature=BrUnZDIQOF-QSbBJ6M8PPq-ipoc-WptanyCX7n6NATvkInvrxgwmew~fwsjRduHtKt8RzmZOQ5hdxIkP0B2882OfIzt8EZDnZ8vdi1CnF2fLmh4CguGtyGGB0EabWbzr~0at5dStiBRVMGkRjaUmJ-6bUSMpKHG8dxHkkS4319tj8HYRzHR~ZIJRHpo05Zql89~ysqKiCQE2cwq7JB8EVOc~TbzeFBrmhtdXwoN9fxL6lu4emnNLIi5vBBx5YaQjIzbLIqRWoDeHmkFWEi8Yxk~oLYouY6tBFMo3MM~ZF9kQwOhzn2SzmX45qedYt8nOI7VF~RU-9r~CwFJYxHVP~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal