Abstract
Damage to the gastrointestinal tract during graft-versus-host disease (GVHD) from the conditioning regimen in conjunction with alloreactive donor T cells plays a pivotal role in the pathogenesis of this disease. In this study, we have identified secretion of interleukin-23 (IL-23) by donor antigen-presenting cells (APCs) as a critical event in the induction of GVHD of the colon linking conditioning regimen-induced mucosal injury and lipopoly-saccharide (LPS) translocation to subsequent proinflammatory cytokine production and GVHD-associated pathologic damage. In the absence of donor APC-derived IL-23 secretion, there is a selective and profound reduction in pathologic damage as well as a marked reduction in LPS and proinflammatory cytokine production in the colon microenvironment. The downstream proinflammatory effects of IL-23 are dependent upon donor-derived secretion of interferon-γ (IFN-γ), but are independent of donor IL-17 production. These findings define a novel organ-specific role for IL-23 in the pathophysiology of GVHD and demonstrate that IL-23 can direct tissue-specific pathology within the context of a systemic inflammatory disorder. Furthermore, these studies also identify IL-23 as a potential therapeutic target for the prevention of this life-threatening disorder.
Introduction
Host antigen-presenting cells (APCs) play a pivotal role in the initiation of graft-versus-host disease (GVHD) through their presentation of host peptides to alloreactive donor T cells.1,2 This results in the subsequent activation and expansion of donor T cells leading to cytokine production and acquisition of effector function, which together mediate target organ damage.2-5 Dendritic cells (DCs), in particular, are one, if not the most critical, APC population in GVHD and have been shown to be sufficient for the induction of the disease.6 Eventually, host APCs are eliminated and replaced by donor APCs, which become the predominant APC population7,8 and present host antigens to donor T cells via the indirect pathway of allorecognition.9,10 Recent studies have provided evidence that donor APCs may play a role in GVHD pathophysiology through their ability to propagate and maximize the severity of GVHD.11,12 The mechanism(s) by which these cells contribute to GVHD pathogenesis, however, remains poorly defined.
While DC interactions with T cells are critical for initiation of GVHD, DCs also elaborate cytokines that are able to shape and amplify the immune response.13 Interleukin-12 (IL-12) is a type I cytokine, comprising 2 subunits (p35 and p40), that is secreted by DCs and has an important role in the development of TH1 immune responses.14-16 More recently, another heterodimeric type I cytokine was discovered that possesses structural similarities to IL-12 and is also secreted by DCs, as well as other APCs such as macrophages. This cytokine, termed IL-23, shares the p40 subunit with IL-12, but has a novel p19 component that together binds to receptors present on memory/activated T cells, DCs, and macrophages.17-19 Secretion of IL-23 by APC populations has been shown to have proinflammatory effects that are mediated by both T cells and cells of the innate immune system.20-22 IL-23 is able to induce proliferation and interferon-γ (IFN-γ) secretion in memory, but not naive, CD4+ T cells17,23 due to the expression of the unique IL-23 receptor subunit on the former but not the latter cells.24 IL-23 also appears to be necessary for a fully functional TH17 response, which constitutes a CD4+ T-cell population that has a critical role in several inflammatory syndromes, including collagen-induced arthritis,25 experimental allergic encephalomyelitis (EAE),26 and the autoimmunity that occurs as a consequence of GVHD.27 A proinflammatory role for IL-23 independent of T cells is supported by studies using immunodeficient mice that lack T cells, which have shown that IL-23 plays a critical role in the induction of intestinal inflammation that occurs as a consequence of either bacterial infection or agonistic anti-CD40 antibody administration.20,21 This has been postulated to be due to direct effects of IL-23 on cells of the innate immune system such as monocytes and macrophages that result in their secretion of proinflammatory cytokines (IL-1, IL-6, and tumor necrosis factor-α [TNF-α]).28,29 Given the emerging role of donor APCs in GVHD biology coupled with the proinflammatory profile that is conferred by secretion of IL-23, the purpose of the current study was to examine the role of donor APC-derived IL-23 in the pathophysiology of GVHD.
Methods
Mice
C57BL/6 (B6) (H-2b), Balb/cJ (H-2d), FVB (H-2q), IFNγ−/− (B6 background), C57BL/10ScSnJ (B10) (H-2b), and C57BL/10ScNJ TLR4−/− mice were bred in the Animal Resource Center at the Medical College of Wisconsin (MCW) or purchased from The Jackson Laboratory (Bar Harbor, ME). IL-23p19 knockout mice (IL-23−/−)30 on a B6 background were kindly provided by Dr Nico Ghilardi (Genentech, South San Francisco, CA). IL-17 knockout mice (IL-17−/−) on a B6 background were obtained from Dr Yoichiro Iwakura (University of Tokyo, Tokyo, Japan). All animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)–accredited Animal Resource Center of the Medical College of Wisconsin. Experiments were all carried out under protocols approved by the MCW Institutional Animal Care and Use Committee.
Bone marrow transplantation
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco modified Eagle medium (DMEM; Gibco-BRL Life Technologies, Grand Island, NY) and passed through sterile mesh filters to obtain single-cell suspensions. Host mice were conditioned with total body irradiation administered as a single exposure at a dose rate of 84 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). Irradiated recipients received a single intravenous injection in the lateral tail vein of BM with or without added spleen cells.
Cell sorting and flow cytometry
Cells were sorted on a FACSVantage flow sorter with a DIVA option (Becton Dickinson, Mountain View, CA). Monoclonal antibodies (mAbs) conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin, peridinin-chlorophyll protein complex (Per-CP) Cy5.5, or PE-Cy5.5 were used to assess cell populations and were purchased from BD Biosciences Pharmingen, Caltag (San Francisco, CA) or eBiosciences (San Diego, CA). Cells were analyzed on a FACScan flow cytometer with Cellquest software (Becton Dickinson). Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Histologic analysis
Representative samples of liver, colon, and lung were obtained from transplanted recipients and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm-thick sections and stained with hematoxylin and eosin. A semiquantitative scoring system was used to account for histologic changes in the colon and liver as previously described.27 Histologic changes compatible with GVHD of the lung were interstitial/alveolar inflammation along with periluminal bronchial and vascular infiltrates. Interstital/alveolar inflammation was graded as none, mild (< 25%), moderate (25%-50%), and severe (> 50%). The extent of periluminal disease was determined using the scoring system of Cooke et al,31 then dividing the cumulative score by 4 to obtain an average periluminal index. Interstitial/alveolar inflammation and periluminal disease were then combined to achieve a total GVHD lung score. Images were visualized with a Nikon Eclipse E400 microscope and a Nikon Plan APO 10×/0.45 lens (Nikon, Tokyo, Japan). Image acquisition was performed with a Zeiss Axiom camera and Axiovision 3.0.6 SPZ software (Zeiss, Berlin, Germany).
Cytokine analysis
Serum was collected from mice by retroorbital bleeds and analyzed on a Bioplex System (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. To assess cytokine concentration in colonic supernatant, small pieces of colon (approximately 5 mm of mid-colon) were isolated and weighed under aseptic conditions.21 Colon explants were cultured overnight in complete RPMI 1640 (Gibco-BRL Life Technologies) at 37°C. After centrifugation to pellet debris, culture supernatants were analyzed for IL-23p19 using a specific sandwich enzyme-linked immunosorbent assay (ELISA; eBiosciences) or all proinflammatory cytokines (IL-1β, TNF-α, IL-6, IL-17, granulocyte colony-stimulating factor [GCSF], IL-12, and IFN-γ) using the multiplex cytokine Bio-Rad Laboratories' assay system. Cytokine levels were normalized to the weight of the colon explants. All samples were run in duplicate.
Serum lipopolysaccharide determination
For determination of endotoxin concentration, the Limulus Amebocyte Lysate (LAL) assay (Lonza Walkersville, Walkersville, MD) was performed according to the manufacturer's protocol. The absorbance of the assay plate was read at 405 nm (Bio-Tek Instruments, Winooski, VT). Samples and standards were run in duplicate, and the lower limit of detection was 0.10 U/mL. Lipopolysaccharide (LPS) levels were normalized to the weight of the colon explants.
Cell isolation
Splenic CD4+ and CD8+ T cells were isolated by positive selection using the MACS magnetic bead cell-separation system (Miltenyi Biotec, Auburn, CA). To isolate lamina propria mononuclear cells (LPMCs), pooled colons were incubated in Hank balanced salt solution (HBSS) buffer (Gibco-BRL Life Technologies) supplemented with 5% fetal bovine serum (FBS), 0.05 mM ethylenediaminetetraacetic acid (EDTA), 0.6 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer, 100 U/mL penicillin/100 μg/mL streptomycin (Gibco-BRL Life Technologies), and 15 μg/mL dithiothreitol (Invitrogen, Carlsbad, CA) at 37°C for 30 minutes and subsequently digested in a solution of 0.2 mg/mL collagenase D (Roche Diagnostics, Indianapolis, IN) in RPMI medium for 1 hour at 37°C. To isolate lamina propria lymphocytes (LPLs), the resulting cell suspension was layered on a 44%/67% Percoll gradient (Sigma-Aldrich, St Louis, MO). Liver lymphocytes were isolated by collagenase D digestion followed by layering on a Percoll gradient as described above.
Intracellular cytokine staining
Lymphocytes isolated from spleen, liver, and colon were stimulated with phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and ionomycin (Calbiochem, La Jolla, CA) as previously described.27 Cells were surface-stained with PE Cy5.5 anti-CD4 (Caltag) and then intracellularly stained with PE-labeled antibody to IL-17 or FITC-labeled antibody to IFN-γ (BD Biosciences Pharmingen).
Real-time quantitative polymerase chain reaction
Real-time quantitative polymerase chain reaction (PCR) was performed using Rotor-Gene 3000 (Corbett Research, Morelake, Australia), QuantumRNA 18S Internal Standards (Ambion, Austin, TX), IL-23 p19 primers (5′-AGCGGGACATATGAATCTACTAAGAGA-3′, 5′-GTCCTAGTAGGGAGGTGTGAAGTTG-3′),20 IL-23R primers (5′-TGAAAGAGACCCTACATCCCTTGA-3′, 5′-CAGAAAATTGGAAGTTGGGATATGTT-3′),24 IL-17A primers (5′-GCTCCAGAAGGCCCTCAG-3′, 5′-CTTTCCCTCGCATTGACA-3′),20 Toll-like receptor 4 (TLR4) primers (5′-AGTGGGTCAAGGAACAGAAGCA-3′, 5′-CTTTACCAGCTCATTTCTCACC-3′),32 and QuantiTect SYBR Green PCR Master Mix (QIAGEN, Valencia, CA) according to the manufacturers' instructions. Primers were purchased from Integrated DNA Technologies (Coralville, IA). Specificity for all quantitative PCRs was verified by melting curve analysis. Data were analyzed with the Rotor-Gene 3000 software using the cycle threshold for quantification. Relative gene expression data (fold change) between samples was accomplished using the mathematical model described by Pfaffl.33
Statistics
Group comparisons of T-cell and B-cell populations, cytokine levels, LPS levels, pathology scores, mean weights, and gene expression data were performed using the Mann-Whitney U test. Survival curves were constructed using the Kaplan-Meier product limit estimator and compared using the log rank rest. A P value less than or equal to .05 was deemed to be significant in all experiments.
Results
Transplantation of IL-23–deficient marrow grafts significantly reduces the severity of acute GVHD
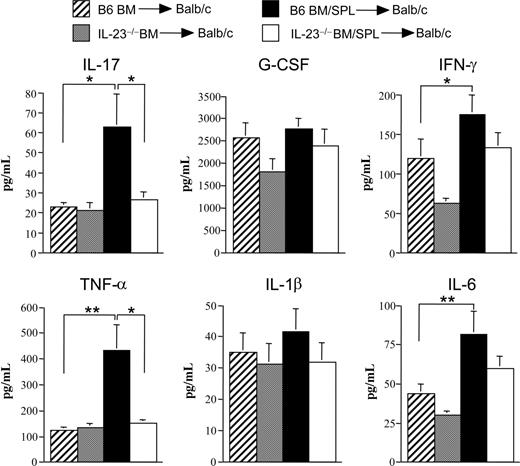
Lethally irradiated Balb/c mice received transplants of BM and spleen cells from either IL-23−/− or wild-type B6 animals, while control mice received either B6 or IL-23−/− BM cells alone. Mice transplanted with IL-23−/− BM and spleen cells had significantly increased survival compared with recipients of marrow grafts from B6 mice (93% vs 33% at day 35, P < .001; Figure 1A). To understand the mechanism by which mice transplanted with IL-23−/− marrow grafts were protected from GVHD lethality, we repeated these studies with a reduced donor T-cell dose so that mice in all groups would survive and be able to be examined for immunologic reconstitution and pathologic analysis. In these studies, animals transplanted with IL-23−/− BM and spleen cells had significantly less weight loss (P ≤ .05) beginning 2 weeks posttransplantation compared with recipients of B6 BM and spleen cells (Figure 1B). Because GVHD deleteriously affects immune reconstitution, mice in all cohorts were killed 3 to 4 weeks after BM transplantation (BMT) and examined for engraftment as well as the extent of splenic and thymic recovery. Complete donor engraftment was observed in the spleen of all 4 groups of mice (Figure 1C). There was no difference in splenic cellularity, overall number of splenic B cells, thymus size, or absolute number of double positive thymocytes in control mice transplanted with B6 or IL-23−/− BM alone (Figure 1D-G). There was, however, a significant reduction in all 4 parameters in recipients of wild-type BM and spleen cells indicative of ongoing GVHD. In contrast, transplantation with IL-23−/− BM and spleen cells resulted in significant increases in thymus and spleen size, as well as in the absolute number of splenic B cells and double positive thymocytes, although mean values were less than that observed in control mice. The total number of splenic CD4+ (0.69 ± 0.21 × 106 vs 0.09 ± 0.02 × 106; P < .001) and CD8+ T cells (1.36 ± 0.46 × 106 vs 0.18 ± 0.07 × 106; P = .004) were also higher in mice transplanted with IL-23−/− as opposed to B6 BM and spleen cells. Similar results were observed when these studies were repeated using a different major histocompatibility complex (MHC)–incompatible murine strain combination (B6 [H-2b] → FVB [H-2q]; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Because GVHD induces an inflammatory environment, we also examined serum proinflammatory cytokine levels to determine whether transplantation with IL-23−/− marrow grafts affected other systemic manifestations of GVHD. Mice transplanted with B6 BM plus spleen cells had significantly elevated levels of IL-17, IFN-γ, TNF-α, and IL-6 compared with B6 BM control animals (Figure 2). Notably, we observed that both IL-23 (p19) and IL-12 (p70) levels in the serum were below the level of detection in these same animals (data not shown). The transplantation of BM and spleen cells from IL-23−/− donors resulted in a significant reduction in serum IL-17 and TNF-α levels, while there was also a trend toward reduced levels of IL-1β and IL-6 (P = .07). Collectively, these data indicated that systemic manifestations of GVHD were substantially attenuated after transplantation of marrow grafts from IL-23−/− donors.
Transplantation with IL-23−/− marrow grafts is associated with reduced GVHD severity. (A) Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM (10 × 106) alone from B6 (■, n = 6) or IL-23−/− (□, n = 6) donors or BM and spleen cells (1.0-1.6 × 106) from B6 (●, n = 15) or IL-23−/− animals (○, n = 15). Because the percentage of αβ+ T cells in the spleens of donor IL-23−/− mice was observed to be higher in some instances (by approximately 5%-10%), the spleen cell dose was adjusted in these experiments so that the administered number of T cells was equivalent. Overall survival is depicted. Data are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM (10 × 106) alone from B6 (■, n = 8) or IL-23−/− (□, n = 11) donors or BM and a reduced dose of spleen cells (0.4-0.6 × 106) from B6 (●, n = 20) or IL-23−/− animals (○, n = 20). The percentage of weight change over time of mice from all 4 groups is depicted. Data are cumulative results from 5 experiments. (C-G) Percentage of donor cells in the spleen (actual values ± standard error of the mean [SEM] are 99 ± 1, 99 ± 0, 96 ± 1, and 99 ± 0 for all 4 groups, respectively) (C), spleen cellularity (D), total number of splenic B cells (E), thymus size (F), and absolute number of double positive (CD4+ CD8+) thymocytes (G) in mice 3 to 4 weeks posttransplantation with B6 BM alone (n = 4, ▫), IL-23−/− BM alone (n = 3,  ), B6 BM plus spleen cells (n = 14-15, ■), or IL-23−/− BM and spleen cells (n = 12-14, □). Data are presented as the mean plus or minus SEM. *P ≤ .05; **P < .01.
), B6 BM plus spleen cells (n = 14-15, ■), or IL-23−/− BM and spleen cells (n = 12-14, □). Data are presented as the mean plus or minus SEM. *P ≤ .05; **P < .01.
Transplantation with IL-23−/− marrow grafts is associated with reduced GVHD severity. (A) Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM (10 × 106) alone from B6 (■, n = 6) or IL-23−/− (□, n = 6) donors or BM and spleen cells (1.0-1.6 × 106) from B6 (●, n = 15) or IL-23−/− animals (○, n = 15). Because the percentage of αβ+ T cells in the spleens of donor IL-23−/− mice was observed to be higher in some instances (by approximately 5%-10%), the spleen cell dose was adjusted in these experiments so that the administered number of T cells was equivalent. Overall survival is depicted. Data are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM (10 × 106) alone from B6 (■, n = 8) or IL-23−/− (□, n = 11) donors or BM and a reduced dose of spleen cells (0.4-0.6 × 106) from B6 (●, n = 20) or IL-23−/− animals (○, n = 20). The percentage of weight change over time of mice from all 4 groups is depicted. Data are cumulative results from 5 experiments. (C-G) Percentage of donor cells in the spleen (actual values ± standard error of the mean [SEM] are 99 ± 1, 99 ± 0, 96 ± 1, and 99 ± 0 for all 4 groups, respectively) (C), spleen cellularity (D), total number of splenic B cells (E), thymus size (F), and absolute number of double positive (CD4+ CD8+) thymocytes (G) in mice 3 to 4 weeks posttransplantation with B6 BM alone (n = 4, ▫), IL-23−/− BM alone (n = 3,  ), B6 BM plus spleen cells (n = 14-15, ■), or IL-23−/− BM and spleen cells (n = 12-14, □). Data are presented as the mean plus or minus SEM. *P ≤ .05; **P < .01.
), B6 BM plus spleen cells (n = 14-15, ■), or IL-23−/− BM and spleen cells (n = 12-14, □). Data are presented as the mean plus or minus SEM. *P ≤ .05; **P < .01.
Transplantation with IL-23−/− marrow grafts reduces systemic proinflammatory cytokine production. Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM alone from B6 (n = 21, ▫) or IL-23−/− (n = 20,  ) animals or BM and spleen cells (0.4-0.6 × 106) from B6 (n = 23, ■) or IL-23−/− animals (n = 22, □). Mice were bled 7 days after transplantation, and serum was assayed for the indicated cytokines using the multiplex cytokine Bio-Rad Laboratories assay system. Data are presented as the mean plus or minus SEM and are derived from 6 independent experiments. *P ≤ .05; **P < .01.
) animals or BM and spleen cells (0.4-0.6 × 106) from B6 (n = 23, ■) or IL-23−/− animals (n = 22, □). Mice were bled 7 days after transplantation, and serum was assayed for the indicated cytokines using the multiplex cytokine Bio-Rad Laboratories assay system. Data are presented as the mean plus or minus SEM and are derived from 6 independent experiments. *P ≤ .05; **P < .01.
Transplantation with IL-23−/− marrow grafts reduces systemic proinflammatory cytokine production. Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM alone from B6 (n = 21, ▫) or IL-23−/− (n = 20,  ) animals or BM and spleen cells (0.4-0.6 × 106) from B6 (n = 23, ■) or IL-23−/− animals (n = 22, □). Mice were bled 7 days after transplantation, and serum was assayed for the indicated cytokines using the multiplex cytokine Bio-Rad Laboratories assay system. Data are presented as the mean plus or minus SEM and are derived from 6 independent experiments. *P ≤ .05; **P < .01.
) animals or BM and spleen cells (0.4-0.6 × 106) from B6 (n = 23, ■) or IL-23−/− animals (n = 22, □). Mice were bled 7 days after transplantation, and serum was assayed for the indicated cytokines using the multiplex cytokine Bio-Rad Laboratories assay system. Data are presented as the mean plus or minus SEM and are derived from 6 independent experiments. *P ≤ .05; **P < .01.
Attenuation of GVHD is attributable to the preferential reduction in colonic GVHD
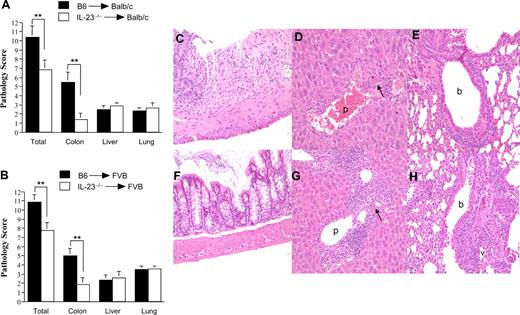
We then examined whether the reduction in systemic manifestations of GVHD was associated with any amelioration of GVHD pathology. The composite pathology score derived from histologic examination of lung, liver, and colon tissues was significantly lower in mice transplanted with IL-23−/− BM and spleen cells versus similarly comprised wild-type marrow grafts (10.4 ± 1.2 vs 6.9 ± 1.0; P = .018; Figure 3A). Individual GVHD target organ scores, however, revealed that the protective effect derived from the transplantation of IL-23−/− grafts was manifested only in the colon, as there was no difference in pathology scores in either lung or liver. Similar findings were observed when these studies were repeated using a B6 → FVB murine GVHD model (Figure 3B). In these experiments, the composite pathology score was also significantly reduced in animals that received IL-23−/− marrow grafts (10.9 ± 0.9 vs 7.8 ± 0.8; P = .01); however, the colon was the only target organ with a significant reduction in pathologic damage (5 ± 0.8 vs 1.9 ± 0.7; P = .004; Figure 3C-H). To determine the source of IL-23 production responsible for the development of GVHD in the colon, lethally irradiated Balb/c mice were transplanted with either B6 or IL-23−/− BM and purified T cells from B6 mice. Protection in the colon was observed only in mice transplanted with IL-23−/− BM, indicating that BM-derived donor APCs were the source of IL-23 in the colon (Figure S2).
Attenuation of GVHD severity is attributable to the preferential reduction of pathologic damage in the colon. Lethally irradiated Balb/c (900 cGy) (A) or FVB (1000 cGy) (B) mice were transplanted with B6 BM (10 × 106) plus spleen cells (■) or IL-23−/− BM (10 × 106) and spleen cells (□) adjusted to yield an equivalent number of mature T cells. Mice were killed 20 to 29 days after transplantation, and GVHD target tissues (colon, liver, and lung) were examined for pathologic damage using a semiquantitative scoring system as detailed in “Histologic analysis.” Data are presented as the mean (± SEM) and are the cumulative results from 3 (n = 14-15/group for Balb/c) or 4 (n = 16-19 for FVB) experiments. **P < .01. (C-H) Histology of colon (C,F), liver (D,G), and lung (E,H) from representative FVB recipients 20 to 29 days after transplantation with B6 BM and spleen cells (C-E) or IL-23 BM and spleen cells (F-H). Colon in panel C shows ulceration of the mucosal surface, extensive crypt cell destruction, and goblet cell depletion, while colon in panel F has normal appearing mucosa with no inflammatory infiltration and preserved goblet cell content. Livers in panels D and G both show infiltration in the portal triads with mononuclear and granulocytic cells (p), endothelialitis and hepatocellular apoptosis (arrows). Lungs in panels E and H demonstrate peribronchial (b) and perivascular (v) cuffing attributable to mononuclear and granulocytic cells along with associated interstitial inflammation.
Attenuation of GVHD severity is attributable to the preferential reduction of pathologic damage in the colon. Lethally irradiated Balb/c (900 cGy) (A) or FVB (1000 cGy) (B) mice were transplanted with B6 BM (10 × 106) plus spleen cells (■) or IL-23−/− BM (10 × 106) and spleen cells (□) adjusted to yield an equivalent number of mature T cells. Mice were killed 20 to 29 days after transplantation, and GVHD target tissues (colon, liver, and lung) were examined for pathologic damage using a semiquantitative scoring system as detailed in “Histologic analysis.” Data are presented as the mean (± SEM) and are the cumulative results from 3 (n = 14-15/group for Balb/c) or 4 (n = 16-19 for FVB) experiments. **P < .01. (C-H) Histology of colon (C,F), liver (D,G), and lung (E,H) from representative FVB recipients 20 to 29 days after transplantation with B6 BM and spleen cells (C-E) or IL-23 BM and spleen cells (F-H). Colon in panel C shows ulceration of the mucosal surface, extensive crypt cell destruction, and goblet cell depletion, while colon in panel F has normal appearing mucosa with no inflammatory infiltration and preserved goblet cell content. Livers in panels D and G both show infiltration in the portal triads with mononuclear and granulocytic cells (p), endothelialitis and hepatocellular apoptosis (arrows). Lungs in panels E and H demonstrate peribronchial (b) and perivascular (v) cuffing attributable to mononuclear and granulocytic cells along with associated interstitial inflammation.
Absence of donor APC-derived secretion of IL-23 reduces proinflammatory cytokine production in the colon
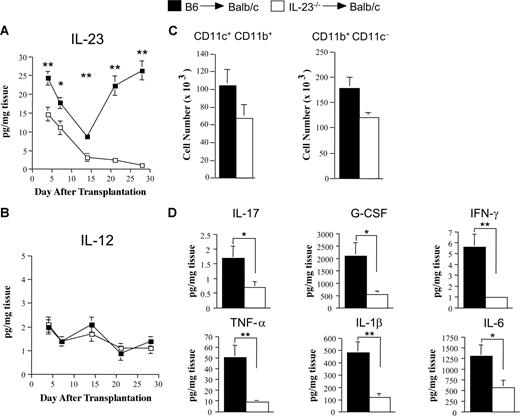
The preferential reduction in colonic GVHD led us to examine the colon microenvironment to determine the kinetics of IL-23 secretion and the role of this cytokine in local proinflammatory cytokine production. In contrast to the serum where IL-23 levels were below the limit of detection in GVHD control mice, we observed that IL-23 levels in colon explant tissues had a measurable biphasic time course reaching a nadir at 2 weeks after transplantation before increasing to levels approximating those observed on day 4 (Figure 4A). In mice transplanted with marrow grafts containing IL-23−/− BM and spleen cells, IL-23 levels paralleled those observed in recipients of wild-type grafts for the first 2 weeks, although they were significantly lower at all time points. Beginning on day 14, however, in contrast to GVHD control mice, levels continued to decline in recipients whose donor APCs were incapable of IL-23 production. Because IL-12 is also secreted by APCs, shares the p40 subunit with IL-23, and has been implicated in the pathogenesis of GVHD,34 we also quantitated IL-12 levels in colonic explants. In contrast to IL-23, IL-12 levels were negligible throughout the course of GVHD in both cohorts of mice (Figure 4B). Proinflammatory cytokine levels were then measured 21 to 28 days after transplantation at a time when histologic analysis confirmed the differential severity of colonic GVHD in animals transplanted with wild-type versus IL-23−/− marrow grafts. At this time point, examination of LPMCs in the colon revealed that CD11c+CD11b+ and CD11b+CD11c− cells were all of donor origin (data not shown) and present in equivalent numbers in recipients of B6 versus IL-23−/− marrow grafts (Figure 4C). In the absence of donor APC-derived secretion of IL-23, there was a significant reduction in proinflammatory cytokines compared with GVHD control mice (Figure 4D and Figure S3), indicating that IL-23 has a pivotal proinflammatory role in colonic GVHD.
Absence of donor APC-derived IL-23 significantly attenuates proinflammatory cytokine production in the colon. (A,B) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106) (■) or IL-23−/− BM (10 × 106) and spleen cells (□) adjusted to yield an equivalent number of mature T cells. Cohorts of mice (7-9/group) were killed at the indicated time points (days 4, 7, 14, 21, and 28), and segments of colon tissue from individual mice were cultured overnight in media. Colonic tissue supernatants were collected and analyzed for the amount of IL-23p19 (A) and IL-12p70 (B) by ELISA and multiplex, respectively. Data are presented as mean amount of cytokine divided by the weight of cultured colon tissue (± SEM) and are cumulative results from 2 experiments. (C) Groups of mice were transplanted as in panels A and B and killed 28 days posttransplantation. Colons (n = 4-5/group per experiment) were pooled and digested with collagenase D. Total number of isolated LPMCs that were CD11c+ CD11b+ or CD11b+ CD11c− in Balb/c recipients of B6 BM and spleen cells (■) or IL-23−/− BM and spleen cells (□) is depicted. Data are derived from 3 independent experiments and are presented as the mean cell number (×1000) per mouse (± SEM) (D). Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (n = 10, ■) or IL-23−/− BM and spleen cells adjusted to yield equivalent numbers of T cells (n = 9, □). Mice were killed 21-29 days posttransplantation, and colon explant tissue was assayed for levels of proinflammatory cytokines by multiplex. Data are derived from cumulative results from 2 experiments and are presented as the mean amount of cytokine divided by the weight of cultured colon tissue (± SEM). Statistics: *P ≤ .05; **P < .01.
Absence of donor APC-derived IL-23 significantly attenuates proinflammatory cytokine production in the colon. (A,B) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106) (■) or IL-23−/− BM (10 × 106) and spleen cells (□) adjusted to yield an equivalent number of mature T cells. Cohorts of mice (7-9/group) were killed at the indicated time points (days 4, 7, 14, 21, and 28), and segments of colon tissue from individual mice were cultured overnight in media. Colonic tissue supernatants were collected and analyzed for the amount of IL-23p19 (A) and IL-12p70 (B) by ELISA and multiplex, respectively. Data are presented as mean amount of cytokine divided by the weight of cultured colon tissue (± SEM) and are cumulative results from 2 experiments. (C) Groups of mice were transplanted as in panels A and B and killed 28 days posttransplantation. Colons (n = 4-5/group per experiment) were pooled and digested with collagenase D. Total number of isolated LPMCs that were CD11c+ CD11b+ or CD11b+ CD11c− in Balb/c recipients of B6 BM and spleen cells (■) or IL-23−/− BM and spleen cells (□) is depicted. Data are derived from 3 independent experiments and are presented as the mean cell number (×1000) per mouse (± SEM) (D). Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (n = 10, ■) or IL-23−/− BM and spleen cells adjusted to yield equivalent numbers of T cells (n = 9, □). Mice were killed 21-29 days posttransplantation, and colon explant tissue was assayed for levels of proinflammatory cytokines by multiplex. Data are derived from cumulative results from 2 experiments and are presented as the mean amount of cytokine divided by the weight of cultured colon tissue (± SEM). Statistics: *P ≤ .05; **P < .01.
IL-23 and IL-23 receptor gene expression is increased in colon-derived APCs and CD4+ T cells during GVHD
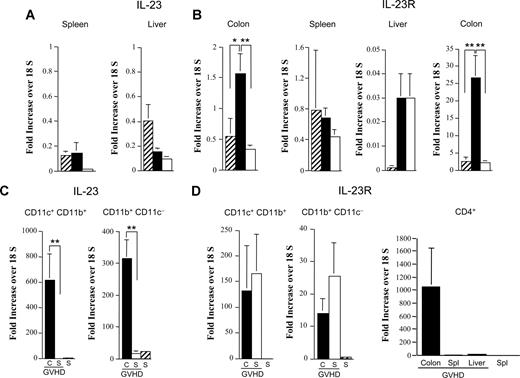
To further investigate the preferential protection observed in the colon, we examined IL-23 mRNA levels in the spleen, liver, and colon to determine whether there was differential gene expression in these organs during GVHD. We observed that, in comparison to normal nontransplanted Balb/c mice, there was a selective increase (3-fold) in IL-23 mRNA levels in the colon of GVHD animals, as no differences between normal and GVHD mice were seen in the spleen or liver (Figure 5A). Notably, mice that were protected from colonic GVHD after transplantation with IL-23−/− BM and spleen cells had a significant reduction (4.5-fold) in IL-23 gene expression in the colon. Examination of IL-23 receptor (IL-23R) mRNA levels in these same tissues again revealed a marked increase only in the colon (Figure 5B). To define the donor APC populations in the colon responsible for secretion of IL-23, CD11c+CD11b+ and CD11b+CD11c− cells were sorted and examined for expression of IL-23 mRNA. IL-23 mRNA levels were significantly increased (600-fold) in CD11c+CD11b+ cells derived from the colon as opposed to the spleen of GVHD mice in which IL-23 expression was undetectable (Figure 5C). Similarly, gene expression of IL-23 was 20-fold higher in colon versus splenic-derived CD11b+CD11c− cells. Analysis of IL-23R mRNA levels was then conducted to determine which cells in the colon were responsive to the actions of IL-23. We observed that gene expression for the IL-23R was increased in both CD11c+CD11b+ and CD11b+CD11c− cells in the colon, as well as the spleen of GVHD animals compared with normal spleen cells (Figure 5D). The most striking finding, however, was that there was a dramatic increase (approximately 1000-fold) in IL-23R expression in purified colon-derived CD4+ T cells, whereas CD4+ T cells isolated from the spleen or liver of these same mice had negligible expression.
Increased gene expression of IL-23 and IL-23R in the colon during GVHD. (A,B) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106; n = 9-14, ■) or IL-23−/− BM (10 × 106) and spleen cells adjusted to yield an equivalent number of mature T cells (n = 9-14, □). Mice were killed 28 days after transplantation. RNA was extracted from spleen, colon, and liver tissues obtained from these animals as well as from normal nontransplanted Balb/c mice (n = 4-6, ▫). Gene expression of IL-23 (A) or IL-23R (B) was analyzed by real-time quantitative PCR as described in “Methods.” Data are cumulative results from 3 independent experiments for mice transplanted with B6 or IL-23−/− marrow grafts. (C,D) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106) and killed 28 days posttransplantation. LPMCs and spleen cells were isolated from pooled colons and spleens (n = 4-5/group per experiment), respectively. Spleen cells from normal nontransplanted B6 mice served as controls. CD11c+/CD11b+ and CD11b+/CD11c− cells were sorted from LPMCs (■) and spleen cells (□) of GVHD mice and from normal splenocytes (▫). CD4+ T cells were sorted from splenocytes, liver lymphocytes, and colon LPMCs of GVHD animals and from spleen cells of normal mice. RNA was extracted from these cells, and gene expression of IL-23 (C) and IL-23R (D) was analyzed by real-time quantitative PCR. Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA (± SEM). Cumulative results from 3 independent experiments are shown. Statistics: *P ≤ .05; **P < .01.
Increased gene expression of IL-23 and IL-23R in the colon during GVHD. (A,B) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106; n = 9-14, ■) or IL-23−/− BM (10 × 106) and spleen cells adjusted to yield an equivalent number of mature T cells (n = 9-14, □). Mice were killed 28 days after transplantation. RNA was extracted from spleen, colon, and liver tissues obtained from these animals as well as from normal nontransplanted Balb/c mice (n = 4-6, ▫). Gene expression of IL-23 (A) or IL-23R (B) was analyzed by real-time quantitative PCR as described in “Methods.” Data are cumulative results from 3 independent experiments for mice transplanted with B6 or IL-23−/− marrow grafts. (C,D) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106) and killed 28 days posttransplantation. LPMCs and spleen cells were isolated from pooled colons and spleens (n = 4-5/group per experiment), respectively. Spleen cells from normal nontransplanted B6 mice served as controls. CD11c+/CD11b+ and CD11b+/CD11c− cells were sorted from LPMCs (■) and spleen cells (□) of GVHD mice and from normal splenocytes (▫). CD4+ T cells were sorted from splenocytes, liver lymphocytes, and colon LPMCs of GVHD animals and from spleen cells of normal mice. RNA was extracted from these cells, and gene expression of IL-23 (C) and IL-23R (D) was analyzed by real-time quantitative PCR. Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA (± SEM). Cumulative results from 3 independent experiments are shown. Statistics: *P ≤ .05; **P < .01.
The proinflammatory effects of IL-23 in the colon are dependent upon IFN-γ, but not IL-17
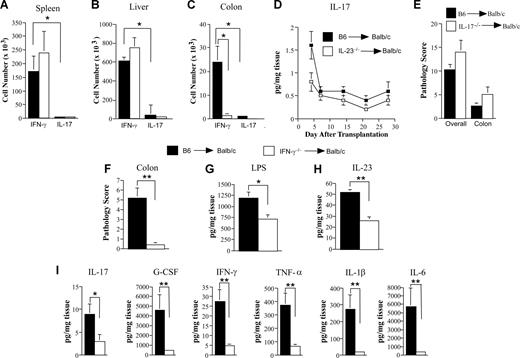
The actions of IL-23 have been shown to be mediated by memory CD4+ T cells that express the unique component of the IL-23R complex.17,23 Given the increased expression of IL-23R that was observed in CD4+ T cells derived from the colon, we examined whether these cells had an activated/memory phenotype. We observed that transplantation of wild-type as opposed to IL-23−/− marrow grafts was associated with a significant increase in the absolute number of CD4+ T cells in the colon, but not the spleen or liver (Figure S4). Notably, there was no difference in the absolute number of CD8+ T cells in the colon between recipients of B6 (168.6 × 103) versus IL-23−/− (135.2 × 103) grafts (P = .40). Approximately half of CD4+ T cells in the colon were noted to be CCR7−CD44hi consistent with an activated/memory phenotype (Figure S4). Because IL-23 induces secretion of IL-17 and IFN-γ by memory CD4+ T cells, we then performed studies to determine whether there was a requirement for either cytokine in the induction of pathologic damage. We observed that CD4+ IL-17+ cells were present in negligible numbers in the spleen, liver, and colon of GVHD animals (Figure 6A-C). Levels of IL-17 in the colon were also found to be significantly depressed throughout the posttransplantation course (Figure 6D), and IL-17 mRNA levels were undetectable in the colons of these same animals (data not shown). Furthermore, transplantation with marrow grafts from IL-17−/− donors had no protective effect on either overall (P = .16) or colon-specific (P = .30) GVHD-associated pathology (Figure 6E), providing evidence that the proinflammatory effects of IL-23 were independent of IL-17. Conversely, we observed that the absolute number of CD4+ IFN-γ+ cells in the colon was significantly increased relative to CD4+ IL-17+ cells (Figure 6C). Moreover, transplantation with IL-23−/− marrow grafts resulted in a profound reduction in CD4+ IFN-γ+ T cells compared with recipients of B6 grafts, further implicating a role for these cells in colonic GVHD. To confirm a functional role for IFN-γ, lethally irradiated mice were transplanted with marrow grafts from wild-type or IFN-γ−/− donors. Transplantation with IFN-γ−/− marrow grafts resulted in a significant reduction in colonic pathology compared with recipients of wild-type grafts (Figure 6F). This was associated with a marked reduction in LPS and IL-23 levels as well as proinflammatory cytokine production (Figure 6G-I), demonstrating that the inflammatory effects of IL-23 in the colon were markedly attenuated in the absence of donor-derived IFN-γ secretion.
Interleukin 23-mediated colonic damage is dependent upon donor-derived IFN-γ, but not IL-17. (A-C) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM and spleen cells (0.4-0.6 × 106; ■) or IL-23−/− BM and spleen cells adjusted to yield an equivalent number of mature T cells (□). Mice in each cohort were killed 28-30 days after transplantation. Lymphocytes were isolated from the spleen (A), liver (B), or colon (C) of mice (n = 4-5/group per experiment) and pooled suspensions of these cells were stimulated with PMA and ionomycin in the presence of GolgiStop and then intracellularly stained with IFN-γ and IL-17–specific antibodies. Data are presented as the mean absolute number of CD4+ IFN-γ+ or CD4+ IL-17+ cells (± SEM) and are cumulative results from 4 independent experiments. (D) Lethally irradiated Balb/c mice were transplanted with marrow grafts from either B6 or IL-23−/− donor mice as in panels A through C. Cohorts of mice (n = 8/group) were killed at the indicated time points (days 4, 7, 14, 21, and 28), and colonic tissue supernatants from individual mice were collected and analyzed for IL-17A by multiplex. Data are presented as mean amount of cytokine divided by the weight of cultured colon tissue (± SEM) and are cumulative results from 2 experiments. (E) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106; ■, n = 9) or IL-17−/− BM (10 × 106) and spleen cells adjusted to yield the same T-cell dose (□, n = 9). Mice were killed 35 days posttransplantation and examined for overall (lung, liver, and colon) and colon-specific pathology using the semiquantitative scoring system detailed in “Histologic analysis.” Data are cumulative results from 2 independent experiments. (F-I) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (adjusted to yield a T-cell dose of 0.225 × 106 per mouse; ■, n = 6) or IFN-γ−/− BM (10 × 106) and spleen cells adjusted to yield the same T-cell dose (□, n = 5). Mice were killed 30 to 33 days posttransplantation, and the colon was examined for pathologic damage (F). Data are presented as the mean plus or minus SEM and are the cumulative results from 2 experiments. Colon tissue from the same mice was also cultured overnight in medium as described in “Methods” and assayed for levels of LPS (G), IL-23p19 (H), and proinflammatory cytokines (I). Data are presented as the mean amount of cytokine or LPS divided by the weight of cultured colon tissue (± SEM). *P ≤ .05; **P < .01.
Interleukin 23-mediated colonic damage is dependent upon donor-derived IFN-γ, but not IL-17. (A-C) Lethally irradiated (900 cGy) Balb/c mice were transplanted with B6 BM and spleen cells (0.4-0.6 × 106; ■) or IL-23−/− BM and spleen cells adjusted to yield an equivalent number of mature T cells (□). Mice in each cohort were killed 28-30 days after transplantation. Lymphocytes were isolated from the spleen (A), liver (B), or colon (C) of mice (n = 4-5/group per experiment) and pooled suspensions of these cells were stimulated with PMA and ionomycin in the presence of GolgiStop and then intracellularly stained with IFN-γ and IL-17–specific antibodies. Data are presented as the mean absolute number of CD4+ IFN-γ+ or CD4+ IL-17+ cells (± SEM) and are cumulative results from 4 independent experiments. (D) Lethally irradiated Balb/c mice were transplanted with marrow grafts from either B6 or IL-23−/− donor mice as in panels A through C. Cohorts of mice (n = 8/group) were killed at the indicated time points (days 4, 7, 14, 21, and 28), and colonic tissue supernatants from individual mice were collected and analyzed for IL-17A by multiplex. Data are presented as mean amount of cytokine divided by the weight of cultured colon tissue (± SEM) and are cumulative results from 2 experiments. (E) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (0.4-0.6 × 106; ■, n = 9) or IL-17−/− BM (10 × 106) and spleen cells adjusted to yield the same T-cell dose (□, n = 9). Mice were killed 35 days posttransplantation and examined for overall (lung, liver, and colon) and colon-specific pathology using the semiquantitative scoring system detailed in “Histologic analysis.” Data are cumulative results from 2 independent experiments. (F-I) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (adjusted to yield a T-cell dose of 0.225 × 106 per mouse; ■, n = 6) or IFN-γ−/− BM (10 × 106) and spleen cells adjusted to yield the same T-cell dose (□, n = 5). Mice were killed 30 to 33 days posttransplantation, and the colon was examined for pathologic damage (F). Data are presented as the mean plus or minus SEM and are the cumulative results from 2 experiments. Colon tissue from the same mice was also cultured overnight in medium as described in “Methods” and assayed for levels of LPS (G), IL-23p19 (H), and proinflammatory cytokines (I). Data are presented as the mean amount of cytokine or LPS divided by the weight of cultured colon tissue (± SEM). *P ≤ .05; **P < .01.
Absence of donor APC-derived IL-23 secretion significantly reduces local and systemic LPS levels as well as gene expression of TLR4 in the colon
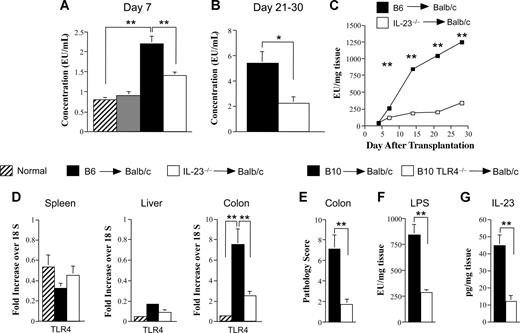
Translocation of LPS across a damaged mucosal barrier has been shown to play an important role in the pathophysiology of GVHD.35,36 LPS is able to trigger cytokine release from mononuclear cells through engagement of TLR4, and the resulting “cytokine storm” contributes to local damage as well as systemic toxicity. We observed that animals transplanted with IL-23−/− marrow grafts had a significant reduction in serum LPS levels as early as day 7 compared with GVHD controls (Figure 7A). This disparity was even more pronounced when similarly transplanted mice were examined 3 to 4 weeks after BMT, indicative of an increase in systemic LPS levels with ongoing GVHD (Figure 7B). A kinetic examination of colonic explant supernatants in GVHD control mice revealed a progressive increase in LPS levels over the first 4 weeks (Figure 7C). In contrast, transplantation with IL-23−/− marrow grafts resulted in a 70% to 80% reduction in LPS levels beginning 2 weeks after transplantation. Notably, the sharp rise in LPS levels in GVHD control animals occurred approximately 1 week earlier than the increase in IL-23 levels (Figure 4A), supporting the premise that LPS translocation was a proximate trigger for subsequent secretion of IL-23 by donor APCs. LPS signaling has been shown to occur through the binding to a LPS receptor complex composed of TLR4, MD2, and CD14 on the surface of macrophages and DCs.37-39 We therefore examined TLR4 mRNA levels in the spleen, liver, and colon to determine whether there was differential gene expression in these organs during GVHD. We observed that there was a selective increase in TLR4 (14-fold) mRNA levels in the colon of GVHD animals (Figure 7D). Moreover, mice that were protected from colonic GVHD after transplantation with IL-23−/− marrow grafts had a significant reduction (3-fold) in TLR4 gene expression in this target organ. To confirm a direct link between LPS/TLR4 signaling and IL-23–mediated pathology, lethally irradiated Balb/c mice were transplanted with either normal C57BL/10 or TLR4−/− BM and spleen cells to induce GVHD. Recipients of TLR4−/− marrow grafts had significantly less GVHD-induced pathologic damage in the colon (Figure 7E). This was associated with substantially reduced LPS and IL-23 levels in the colon microenvironment (Figure 7F,G), demonstrating a direct linkage between an intact LPS/TLR4 signaling pathway and IL-23 production.
Donor APC secretion of IL-23 in the colon is dependent upon an intact LPS/TLR4 signaling pathway. (A) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) alone (n = 11, ▫), IL-23−/− BM alone (10 × 106; n = 10,  ), B6 BM plus spleen cells (0.4-0.6 × 106; n = 18, ■), or BM and spleen cells from IL-23−/− animals adjusted to yield the same dose of mature T cells (n = 18, □). Mice were bled 7 days after transplantation, and serum was assayed for endotoxin. Data are presented as the mean plus or minus SEM and are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (n = 18, ■) or IL-23−/− BM and spleen cells (n = 18, □). Serum was obtained from mice 21 to 30 days after BMT and assayed for endotoxin. Data are cumulative results from 3 experiments and are presented as the mean plus or minus SEM. (C) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (■) or IL-23−/− BM and spleen cells adjusted to yield the same T-cell dose (□). Groups of mice (n = 7-9/time point) were killed at the indicated times (days 4, 7, 14, 21, and 28), and colon tissue from each animal was cultured overnight in media. Supernatant was then analyzed for the amount of endotoxin. Data are derived from 2 experiments and are presented as the mean amount of endotoxin divided by the weight of cultured colon tissue (± SEM). (D) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (n = 9-14, ■) or IL-23−/− BM (10 × 106) and spleen cells adjusted to yield the same dose of T cells (n = 9-14, □). Mice were killed 28 days posttransplantation. RNA was extracted from spleen, colon, and liver tissues obtained from these animals as well as from normal nontransplanted Balb/c mice (n = 4-6, ▫). Gene expression of TLR4 was analyzed as described in “Real-time quantitative polymerase chain reaction.” Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA plus or minus SEM. Data are cumulative results from 3 independent experiments for mice transplanted with B6 or IL-23−/− marrow grafts. (E-G) Lethally irradiated Balb/c mice were transplanted with B10 BM (10 × 106) and spleen cells adjusted to yield 0.4-0.5 × 106 T cells (n = 9, ■) or TLR4−/− BM (10 × 106) and an equivalent number of splenic T cells (n = 10, □). Mice were killed 32 days after transplantation and examined for pathologic damage in the colon (E). Colon explant tissues from these same mice were cultured overnight in medium and assayed for LPS (F) and IL-23p19 (G) levels. Data are cumulative results from 2 experiments. *P ≤ .05; **P < .01.
), B6 BM plus spleen cells (0.4-0.6 × 106; n = 18, ■), or BM and spleen cells from IL-23−/− animals adjusted to yield the same dose of mature T cells (n = 18, □). Mice were bled 7 days after transplantation, and serum was assayed for endotoxin. Data are presented as the mean plus or minus SEM and are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (n = 18, ■) or IL-23−/− BM and spleen cells (n = 18, □). Serum was obtained from mice 21 to 30 days after BMT and assayed for endotoxin. Data are cumulative results from 3 experiments and are presented as the mean plus or minus SEM. (C) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (■) or IL-23−/− BM and spleen cells adjusted to yield the same T-cell dose (□). Groups of mice (n = 7-9/time point) were killed at the indicated times (days 4, 7, 14, 21, and 28), and colon tissue from each animal was cultured overnight in media. Supernatant was then analyzed for the amount of endotoxin. Data are derived from 2 experiments and are presented as the mean amount of endotoxin divided by the weight of cultured colon tissue (± SEM). (D) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (n = 9-14, ■) or IL-23−/− BM (10 × 106) and spleen cells adjusted to yield the same dose of T cells (n = 9-14, □). Mice were killed 28 days posttransplantation. RNA was extracted from spleen, colon, and liver tissues obtained from these animals as well as from normal nontransplanted Balb/c mice (n = 4-6, ▫). Gene expression of TLR4 was analyzed as described in “Real-time quantitative polymerase chain reaction.” Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA plus or minus SEM. Data are cumulative results from 3 independent experiments for mice transplanted with B6 or IL-23−/− marrow grafts. (E-G) Lethally irradiated Balb/c mice were transplanted with B10 BM (10 × 106) and spleen cells adjusted to yield 0.4-0.5 × 106 T cells (n = 9, ■) or TLR4−/− BM (10 × 106) and an equivalent number of splenic T cells (n = 10, □). Mice were killed 32 days after transplantation and examined for pathologic damage in the colon (E). Colon explant tissues from these same mice were cultured overnight in medium and assayed for LPS (F) and IL-23p19 (G) levels. Data are cumulative results from 2 experiments. *P ≤ .05; **P < .01.
Donor APC secretion of IL-23 in the colon is dependent upon an intact LPS/TLR4 signaling pathway. (A) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) alone (n = 11, ▫), IL-23−/− BM alone (10 × 106; n = 10,  ), B6 BM plus spleen cells (0.4-0.6 × 106; n = 18, ■), or BM and spleen cells from IL-23−/− animals adjusted to yield the same dose of mature T cells (n = 18, □). Mice were bled 7 days after transplantation, and serum was assayed for endotoxin. Data are presented as the mean plus or minus SEM and are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (n = 18, ■) or IL-23−/− BM and spleen cells (n = 18, □). Serum was obtained from mice 21 to 30 days after BMT and assayed for endotoxin. Data are cumulative results from 3 experiments and are presented as the mean plus or minus SEM. (C) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (■) or IL-23−/− BM and spleen cells adjusted to yield the same T-cell dose (□). Groups of mice (n = 7-9/time point) were killed at the indicated times (days 4, 7, 14, 21, and 28), and colon tissue from each animal was cultured overnight in media. Supernatant was then analyzed for the amount of endotoxin. Data are derived from 2 experiments and are presented as the mean amount of endotoxin divided by the weight of cultured colon tissue (± SEM). (D) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (n = 9-14, ■) or IL-23−/− BM (10 × 106) and spleen cells adjusted to yield the same dose of T cells (n = 9-14, □). Mice were killed 28 days posttransplantation. RNA was extracted from spleen, colon, and liver tissues obtained from these animals as well as from normal nontransplanted Balb/c mice (n = 4-6, ▫). Gene expression of TLR4 was analyzed as described in “Real-time quantitative polymerase chain reaction.” Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA plus or minus SEM. Data are cumulative results from 3 independent experiments for mice transplanted with B6 or IL-23−/− marrow grafts. (E-G) Lethally irradiated Balb/c mice were transplanted with B10 BM (10 × 106) and spleen cells adjusted to yield 0.4-0.5 × 106 T cells (n = 9, ■) or TLR4−/− BM (10 × 106) and an equivalent number of splenic T cells (n = 10, □). Mice were killed 32 days after transplantation and examined for pathologic damage in the colon (E). Colon explant tissues from these same mice were cultured overnight in medium and assayed for LPS (F) and IL-23p19 (G) levels. Data are cumulative results from 2 experiments. *P ≤ .05; **P < .01.
), B6 BM plus spleen cells (0.4-0.6 × 106; n = 18, ■), or BM and spleen cells from IL-23−/− animals adjusted to yield the same dose of mature T cells (n = 18, □). Mice were bled 7 days after transplantation, and serum was assayed for endotoxin. Data are presented as the mean plus or minus SEM and are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (n = 18, ■) or IL-23−/− BM and spleen cells (n = 18, □). Serum was obtained from mice 21 to 30 days after BMT and assayed for endotoxin. Data are cumulative results from 3 experiments and are presented as the mean plus or minus SEM. (C) Lethally irradiated Balb/c mice were transplanted with B6 BM plus spleen cells (■) or IL-23−/− BM and spleen cells adjusted to yield the same T-cell dose (□). Groups of mice (n = 7-9/time point) were killed at the indicated times (days 4, 7, 14, 21, and 28), and colon tissue from each animal was cultured overnight in media. Supernatant was then analyzed for the amount of endotoxin. Data are derived from 2 experiments and are presented as the mean amount of endotoxin divided by the weight of cultured colon tissue (± SEM). (D) Lethally irradiated Balb/c mice were transplanted with B6 BM (10 × 106) and spleen cells (n = 9-14, ■) or IL-23−/− BM (10 × 106) and spleen cells adjusted to yield the same dose of T cells (n = 9-14, □). Mice were killed 28 days posttransplantation. RNA was extracted from spleen, colon, and liver tissues obtained from these animals as well as from normal nontransplanted Balb/c mice (n = 4-6, ▫). Gene expression of TLR4 was analyzed as described in “Real-time quantitative polymerase chain reaction.” Data are normalized for 18S ribosomal RNA and presented as fold increase over 18S RNA plus or minus SEM. Data are cumulative results from 3 independent experiments for mice transplanted with B6 or IL-23−/− marrow grafts. (E-G) Lethally irradiated Balb/c mice were transplanted with B10 BM (10 × 106) and spleen cells adjusted to yield 0.4-0.5 × 106 T cells (n = 9, ■) or TLR4−/− BM (10 × 106) and an equivalent number of splenic T cells (n = 10, □). Mice were killed 32 days after transplantation and examined for pathologic damage in the colon (E). Colon explant tissues from these same mice were cultured overnight in medium and assayed for LPS (F) and IL-23p19 (G) levels. Data are cumulative results from 2 experiments. *P ≤ .05; **P < .01.
Discussion
In this study, we demonstrate that IL-23 has a unique and selective role in the induction of colonic inflammation during acute GVHD and serves as a critical mediator linking conditioning regimen-induced mucosal injury and LPS translocation to subsequent proinflammatory cytokine production and GVHD-associated pathologic damage. The major finding was that GVHD target organ protection in the absence of donor APC-derived IL-23 was restricted to the colon (Figure 3), indicating that absence of a single cytokine can have a selective tissue-protective effect within the context of a systemic inflammatory disorder. A potential explanation for this selectivity derives from our observations that IL-23, IL-23R, and TLR4 gene expression were selectively augmented in the colon compared with other organs, such as the spleen and liver. This was accompanied by a progressive increase in actual IL-23 levels in the colon microenvironment beginning 2 weeks after transplantation. Notably, the increase in IL-23 in the colon occurred approximately 1 week after the sharp rise in colonic LPS levels. These findings strongly suggest that the proximity of donor APCs in the colon, as opposed to APCs in other GVHD target organs, to high concentrations of LPS provides a strong stimulus for IL-23 secretion and downstream proinflammatory cytokine production. The importance of the LPS/TLR4 signaling pathway was confirmed by studies demonstrating that mice transplanted with TLR4-deficient donor cells had significantly reduced IL-23 levels and pathologic damage in the colon (Figure 7). An important aspect of our studies was that we also observed a modest degree of systemic protection in mice transplanted with IL-23−/− grafts. These animals had significantly less weight loss and overall improved thymic and splenic reconstitution (Figure 1). Moreover, we observed a reduction in some (ie, TNF-α and IL-17), but not all, inflammatory cytokines (Figure 2) as well a marked reduction in serum endotoxin levels in these mice (Figure 7). Our data appear to have clinical relevance based on a recent report40 demonstrating that patients, who receive stem cell grafts from donors who possess a mutation in their IL-23 receptor gene that blocks IL-23 signaling, have a reduced incidence of severe acute GVHD.
The effects of IL-23 have been shown to be mediated through both T cell–dependent and T cell–independent pathways.20-22 The primary T-cell target populations of IL-23 are memory CD4+ T cells with TH1 and TH17 cytokine phenotypes. Specifically, IL-23 appears to be necessary for survival, expansion, and full functionality of TH17 cells41,42 and has been shown to enhance proliferation and IFN-γ secretion in TH1 cells.17 We observed that donor-derived CD4+ T cells with a memory/activated phenotype were present in the colon of GVHD animals transplanted with wild-type marrow grafts (Figure S4). Moreover, there was a 1000-fold increase in expression of the IL-23R in purified CD4+ T cells obtained from these same mice (Figure 5), providing evidence that these cells were responsive to the actions of IL-23. We then examined whether either of the TH1 (ie, IFN-γ) or TH17 (ie, IL-17) signature cytokines played a role in mediating the downstream effects of IL-23. While the majority of experimental colitis models have implicated TH17 cells as the downstream effectors of IL-23–induced mucosal pathology,20-22 our studies did not reveal a role for IL-17 in the pathophysiology of GVHD in the colon. We observed that elevated levels of IL-23 were not accompanied by corresponding increases in IL-17 in the colon microenvironment nor were CD4+ IL-17+ cells found in appreciable numbers in this target organ (Figure 6). Moreover, gene expression of IL-17 was virtually undetectable in the colons of GVHD animals, which would also appear to exclude non–T-cell sources of IL-17 (eg, neutrophils) that might be present in the absence of TH17 cells and have been implicated as playing a role in other inflammatory disease models.21,22,43 Finally, to provide a more functional assessment of a putative role for IL-17, studies were performed using IL-17−/− mice as donors and demonstrated that absence of donor-derived IL-17 production did not result in any protective effect in the colon.
Our studies did, however, identify a crucial role for IFN-γ in IL-23–mediated pathologic damage during GVHD. In the absence of donor-derived IFN-γ secretion, there was a significant reduction in pathology and proinflammatory cytokine production in the colon (Figure 6). LPS and IL-23 levels were also substantially reduced consistent with a feedback loop mechanism whereby reduced pathologic damage leads to decreased translocation of LPS across mucosal surfaces and reduced IL-23 secretion. A role for IFN-γ in GVHD-induced colonic damage has precedence from studies that have shown that IFN-γ can prime macrophages, which license them to release significant quantities of proinflammatory cytokines after exposure to LPS.44 IFN-γ can also mediate direct effects on colonic enterocytes inducing crypt hyperplasia and villous atrophy,45 and recent data suggest that these actions may play a dominant role in GVHD of the colon.46 Another important result from these studies is the confirmation that mucosal pathology mediated by IL-23 can occur in a TH17-independent manner. Although 2 prior studies47,48 have also made this observation, the mechanism by which this occurred was not elucidated. Our studies now clearly establish the existence of an alternative pathway whereby the proinflammatory effects of IL-23 in the colon are mediated through secretion of IFN-γ.
Several studies have shown that IL-23 can also have direct effects on the innate immune system independent of T cells.20,21 Our results are consistent with this being an additional mechanism by which IL-23 mediates pathologic damage in the colon during GVHD. We observed increased expression of both IL-23 and IL-23R mRNA in APCs in the colon microenvironment (Figure 5), which supports a model whereby autocrine and/or paracrine interactions occur between IL-23 and IL-23R on the same or neighboring cells. Binding of IL-23 to IL-23R on APCs has been shown to induce the release of proinflammatory cytokines, such as IL-1β, TNF-α, IL-6, and IFN-γ,19,21 which were all significantly elevated in the colon microenvironment and have been found to be proximate mediators of pathologic damage in GVHD.49 A somewhat unexpected result was the observation that IL-23R mRNA levels were also increased in splenic APCs to an extent commensurate with that observed in the colon. A potential explanation for this finding derives from studies that have shown that expression of the IL-23R can be induced by IFN-γ24 and IL-6,50 both of which were increased in the circulation of GVHD mice and therefore able to disseminate to other tissues sites. It is noteworthy, however, that increased expression of both IL-23 and IL-23R was found only in the colon, as this provides a likely explanation for why the absence of donor APC-derived IL-23 resulted in such dramatic tissue-protective effects in this organ.
We would note that our studies do not exclude the possibility that IL-23 may also be important for the homing of donor T cells into the colon. Prior characterization of these mice has demonstrated no difference in the expression of cell surface markers, such as CD62L, that are associated with T-cell migration.30 Thus, we do not believe that GVHD protection resulting from the absence of donor APC-derived IL-23 was the consequence of a generalized trafficking defect since, under these conditions, one would have expected to see reduced numbers of CD4+ T cells in all target organs. However, this was not observed in the spleen or liver (Figure S4). Nonetheless, it is possible that IL-23 may have selective effects and be important for the regulation of gut-specific homing markers that allow donor T cells to traffic into the colon and thereby mediate GVHD-associated pathologic damage. Further studies will be needed to more definitively address this issue.
Another important conclusion from these studies is confirmation that donor APCs play a critical role in the pathogenesis of GVHD. While a role for host APCs in the induction of GVHD has been supported by several reports,1,2,6 the role of donor APCs has been much less clear.51 Matte and colleagues11 showed that donor APCs appear to increase the severity of GVHD compared with murine transplant recipients whose donor APCs were disabled due to lack of MHC molecules. Recently, we also demonstrated that GVHD induced by donor T-cell allorecognition of host alloantigens presented in the context of host APCs could be propagated by adoptive transfer into animals where alloreactivity occurred through indirect presentation,12 further supporting a role for donor APCs in the propagation of GVHD. The current study, however, for the first time provides conclusive evidence that donor APCs play a direct role in the pathophysiology of GVHD and that secretion of IL-23 is a primary mechanism by which propagation of the disease occurs. Thus, with respect to the colon, the presence of functional host APCs with the capacity to secrete IL-23 is insufficient for the full expression of GVHD-associated pathologic damage that is dependent upon the presence of fully competent donor APCs.
The results of this study may have clinical implications beyond the direct application to GVHD. Separation of graft-versus-leukemia (GVL) and GVH reactivity has been a longstanding but elusive goal in clinical allogeneic BMT. The inability to dissociate these 2 responses stems in part from the fact that alloreactive donor T cells are equally capable of trafficking to sites of disease as well as target organs such as the colon, liver, and skin. The ability to selectively inhibit the ability of donor T cells to mediate pathologic damage in a specific target organ without interfering with the ability of these same cells to traffic to sites of underlying disease is a potential strategy that might allow for the separation of GVL and GVH effects. Although further experimentation is clearly required to validate this premise, our data demonstrating the unique role that IL-23 has in the pathophysiology of GVHD of the colon raises the possibility that in vivo blockade of IL-23 might be a clinically applicable approach that would permit the dissociation of GVHD-induced gut toxicity from an otherwise systemically directed GVL response. Finally, our finding that a single cytokine can have profound effects in mediating pathologic damage in a specific GVHD target organ raises the possibility that other, as of yet unidentified, cytokines may be responsible for directing pathology in selected tissue sites, not only in GVHD, but more generally in other systemic inflammatory disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Biomedical Research Center for excellent animal care. We also thank Dr Nico Ghilardi from Genentech for provision of breeding pairs of IL-23p19–deficient mice and Dr Yoichiro Iwakura from the University of Tokyo for providing IL-17–deficient animals.
This research was supported by grants from the National Institutes of Health (Bethesda, MD; HL64603 and 081650) and by awards from the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI) and the Ridin for Research Foundation (Milwaukee, WI).
National Institutes of Health
Authorship
Contribution: R.D. designed experiments, performed animal and colon explant studies, conducted real-time quantitative PCR analyses, analyzed data, and wrote the manuscript; X.C. assisted with Bioplex cytokine measurement studies; R.K. examined and graded all pathologic tissues; M.H. assisted with the design and interpretation of real-time quantitative PCR data; and W.R.D. designed experiments, analyzed data, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William R. Drobyski, Bone Marrow Transplant Program, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; e-mail: wdrobysk@mcw.edu.

![Figure 1. Transplantation with IL-23−/− marrow grafts is associated with reduced GVHD severity. (A) Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM (10 × 106) alone from B6 (■, n = 6) or IL-23−/− (□, n = 6) donors or BM and spleen cells (1.0-1.6 × 106) from B6 (●, n = 15) or IL-23−/− animals (○, n = 15). Because the percentage of αβ+ T cells in the spleens of donor IL-23−/− mice was observed to be higher in some instances (by approximately 5%-10%), the spleen cell dose was adjusted in these experiments so that the administered number of T cells was equivalent. Overall survival is depicted. Data are cumulative results from 3 experiments. (B) Lethally irradiated Balb/c mice (900 cGy) were transplanted with either BM (10 × 106) alone from B6 (■, n = 8) or IL-23−/− (□, n = 11) donors or BM and a reduced dose of spleen cells (0.4-0.6 × 106) from B6 (●, n = 20) or IL-23−/− animals (○, n = 20). The percentage of weight change over time of mice from all 4 groups is depicted. Data are cumulative results from 5 experiments. (C-G) Percentage of donor cells in the spleen (actual values ± standard error of the mean [SEM] are 99 ± 1, 99 ± 0, 96 ± 1, and 99 ± 0 for all 4 groups, respectively) (C), spleen cellularity (D), total number of splenic B cells (E), thymus size (F), and absolute number of double positive (CD4+ CD8+) thymocytes (G) in mice 3 to 4 weeks posttransplantation with B6 BM alone (n = 4, ▫), IL-23−/− BM alone (n = 3, ), B6 BM plus spleen cells (n = 14-15, ■), or IL-23−/− BM and spleen cells (n = 12-14, □). Data are presented as the mean plus or minus SEM. *P ≤ .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/10/10.1182_blood-2008-08-175448/4/m_zh80080931480001.jpeg?Expires=1769131839&Signature=036x4HlRK6jvu9MENpU51jgCMIcRXAxzWO9rjyyvGq8Eaq6bGmpnV0AU36JzlyJGWTmGsJcNJ1f6-ciZQIOW4nOfH2dMWWJUUgUxneX3sqJUiZrdO7Gf5y~-Xvs2P6idP4Lte8iJCpjkyjJALfTwlEWlbKyZI6KWac0nDOCVThPlBIsJ1QThVpCF4JBNHPl1r38PU2NgaZDzI6OPnOGdj51lDnRaheK3fa1UFLzX2p4C1PA0K6anlCTLelH0wnh~72TzGZeKZLFHwb2-u5V3I605MQXLAKUO5TgFajsc2~p~JmPbwZr5uglMmf4lQU30-JbBfSELIjjqI4dqg4n88g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal