Abstract
GATA-1 controls hematopoietic development by activating and repressing gene transcription, yet the in vivo mechanisms that specify these opposite activities are unknown. By examining the composition of GATA-1–associated protein complexes in a conditional erythroid rescue system as well as through the use of tiling arrays we detected the SCL/TAL1, LMO2, Ldb1, E2A complex at all positively acting GATA-1–bound elements examined. Similarly, the SCL complex is present at all activating GATA elements in megakaryocytes and mast cells. In striking contrast, at sites where GATA-1 functions as a repressor, the SCL complex is depleted. A DNA-binding defective form of SCL maintains association with a subset of active GATA elements indicating that GATA-1 is a key determinant for SCL recruitment. Knockdown of LMO2 selectively impairs activation but not repression by GATA-1. ETO-2, an SCL-associated protein with the potential for transcription repression, is also absent from GATA-1–repressed genes but, unlike SCL, fails to accumulate at GATA-1–activated genes. Together, these studies identify the SCL complex as a critical and consistent determinant of positive GATA-1 activity in multiple GATA-1–regulated hematopoietic cell lineages.
Introduction
Most transcription factors can function both as activators and repressors. The context that determines transcriptional activity is often unclear. This holds true for GATA transcription factors, a family of nuclear proteins that control the formation of diverse tissues. GATA-1, the founding member of the GATA family is essential for the normal development of erythroid cells, megakaryocytes, mast cells, and eosinophil granulocytes1-5 (for review see Ferreira et al,6 Crispino,7 and Cantor and Orkin8 ). GATA-1 has been best studied in erythroid cells where it activates all known erythroid-specific genes but also contributes to the repression of genes associated with the immature, proliferative state.9 Genes that are directly repressed by GATA-1 include Gata2 and the Kit cytokine receptor.10-12 The proper balance of GATA-1's activating and repressive functions is essential for normal hematopoietic cell maturation, and its disruption contributes to diseases with underlying GATA-1 mutations.13-15
Numerous proteins associate with GATA-1 to alter its activity. Among these, FOG-1 (Friend of GATA-1), a multitype zinc finger protein, is expressed in a tissue-specific pattern highly overlapping with that of GATA-1.16 Since FOG-1 does not bind DNA directly, its recruitment is entirely dependent on GATA factors.17 FOG-1 and GATA-1 display a highly similar genomic occupancy pattern. Although FOG-1 can be detected by chromatin immunoprecipitation (ChIP) at virtually all sites occupied by GATA-1, FOG-1 binding by GATA-1 is required for the regulation of most but not all GATA-1–activated and –repressed genes.18-21 FOG-1 interacts tightly with the histone deacetylase complex NuRD (nucleosome remodeling and deacetylase), providing a mechanism for GATA-1–dependent gene repression.22 GATA-1 also interacts directly with the histone acetyltransferase CBP,23 leading to a model in which coactivators and corepressors might be recruited in a distinct fashion to GATA-1–activated and –repressed genes, respectively. However, ChIP experiments surprisingly revealed that CBP is not only found at active genes11,24 but also persists at several repressive GATA-1 elements in vivo (Martowicz et al25 and this report). Conversely, NuRD proteins can be detected not only at repressed genes but also at most if not all genes activated by GATA-1 (Annarita Miccio, Wei Hong, Yuhuan Wang, and G.A.B., manuscript submitted January 20, 2009). Moreover, the ATP-dependent chromatin remodeler BRG1 is present at GATA-1–regulated genes prior to their activation by GATA-1.26 This unexpected pattern of cofactor occupancy suggests that the recruitment step of these chromatin modifying/remodeling complexes by GATA-1 is not the pivotal determinant of transcriptional activity. Another aspect of GATA-1–induced changes in chromatin organization, the formation of chromatin loops, occurs at both activated and repressed genes.27,28 This suggests that posttranslational modifications or additional GATA-1–associated proteins might regulate the activities of coactivator and corepressor proteins. We therefore explored whether other proteins that are known to associate with GATA-1 might consistently distinguish active from repressive GATA-1 complexes.
A protein complex composed of SCL/TAL1, LMO2, Ldb1/Nli1, E2A, and SSBP2 can physically and functionally associate with GATA-129-32 (for review see Lécuyer and Hoang et al33 ). SCL is a hematopoietic expressed basic helix-loop-helix (bHLH) transcription factor that binds to so-called E-box elements by forming heterodimers with widely expressed protein partners E2A, HEB, or E2-2. Cooperativity between GATA-1 and SCL occurs at regulatory regions containing juxtaposed GATA and E-box elements that assemble higher order protein networks that are anchored to DNA by GATA-1 and SCL/E2A, and are bridged by LMO2.29 Tandem GATA-E-box elements separated by 9 to 12 base pairs are found at several erythroid expressed genes, including the Klf1,34 Gata1,35,36 Epb42,37 and Gypa38 genes. Conserved tandem GATA-E-box elements are also present at HS2 of the human β-globin locus control region (LCR), although the spacing between them is slightly larger.39
SCL is expressed at both early and late stages of hematopoietic development and is essential for normal hematopoietic stem cell (HSC) formation and later for the full differentiation of erythroid cells, megakaryocytes (MKs), and mast cells.40-42 Like most transcriptional regulators, SCL can function as an activator or repressor. The latter function is controlled at least in part through interaction with the transcriptional corepressor ETO-2.31,32,43
Although a few GATA/E-box–containing erythroid genes have been identified that are regulated by SCL, there are several reasons to believe that SCL serves a broader role in transcriptional regulation in hematopoiesis, presumably through interaction with other hematopoietic transcription factors including GATA-1. First, SCL is coexpressed with GATA-1 in erythroid, MK, and mast cell lineages and, like GATA-1, is essential for the normal maturation of all 3 lineages.40-42 Second, DNA-binding activity of SCL is dispensable for specification of primitive and definitive hematopoiesis, but is required for complete erythroid maturation.44,45 Strikingly, a small fraction of mice homozygous for a DNA-binding–defective form of SCL (SCL-RER) survived to adulthood.45 Third, SCL can activate transcription in the absence of an E-box through association with other transcription factors. For example, SCL together with E47, LMO2, and Ldb1 stimulates the promoter of the Kit cytokine receptor gene in a manner requiring a Sp1-binding site but not an E-box or GATA element.46 Moreover, SCL and LMO2 can activate the promoter of the RALDH2 gene in T-cell acute lymphoblastic leukemia (T-ALL) cells through interaction with GATA-3 in the absence of an E-box.47 Fourth, forced expression of GATA-1, SCL, and LMO2 displayed strong synergy during induction of blood formation in developing Xenopus embryos.48 Fifth, it is noteworthy that at HS2 of the β-globin LCR, a GATA site mutation reduces SCL recruitment more strongly than does an E-box mutation.49 This suggests that GATA-1 plays a critical role in the recruitment of the SCL complex even in the absence of neighboring E-boxes.
To test a general role for the SCL complex in GATA-1–dependent transcription activation, we determined the occupancy of SCL, LMO-2, E2A, and Ldb1 at numerous regulatory sites where GATA-1 functions as an activator or repressor. Using an erythroid cell line in which GATA-1 can be activated conditionally (G1E-ER4), we found that the entire SCL complex is invariably recruited to all sites where GATA-1 activates transcription. In contrast, GATA-1 fails to recruit the SCL complex at sites where it functions as a repressor. Prior to gene repression by GATA-1, the SCL complex is frequently found to correlate with the presence of GATA-2. The positive correlation of the SCL complex with active GATA elements in erythroid cells was extended through the use of ChIP-on-chip using high-density tiling arrays that cover 66 megabases of mouse chromosome 7. Moreover, SCL and GATA-1 co-occupy active genes in megakaryocytes and mast cells, further demonstrating the general nature of SCL as a GATA-1 coactivator. The corepressor ETO-2 was absent from all GATA-1/SCL–activated genes examined. Together, this work demonstrates that in contrast to several tissue-specific or general GATA-1 cofactors studied previously, the SCL complex is a clear indicator of positive transcriptional activity of GATA-1 in several hematopoietic lineages. We speculate that the SCL complex modulates the activity of other GATA-1 cofactors to specify transcriptional activation.
Methods
Detailed descriptions of ChIP-on-chip constructs, antibodies, cell-culture conditions, and all ChIP primers can be found on the Blood website; see the Supplemental Materials link at the top of the online article. This study received Institutional Review Board approval for the use of mice from the Children's Hospital of Philadelphia.
Results and discussion
GATA-1 recruits the SCL complex to active erythroid genes
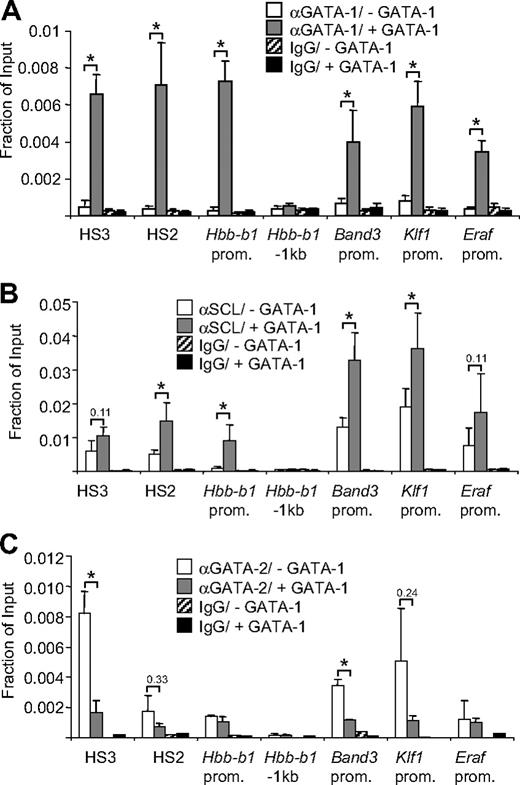
A working model for GATA-1 function predicts that coactivator and corepressor molecules are recruited to active and repressed genes, respectively. Surprisingly, neither the classic coactivators such as BRG1 and the histone acetyltransferase CBP (Figure S1) nor the corepressor complex NuRD (Annarita Miccio, Wei Hong, Yuhuan Wang, and G.A.B., manuscript submitted January 20, 2009)50 or several other GATA-1–binding factors such as FOG-1 are reliable predictors of active versus repressive transcription factors complexes (see “Introduction”). Since the SCL complex contains multiple subunits with transcription activating function, we examined whether it might associate selectively with GATA-1–activated genes in G1E-ER4 cells. G1E cells are erythroblasts that lack GATA-1 and are arrested in their maturation.51 G1E-ER4 cells were derived from G1E cells by stable introduction of GATA-1 fused to the ligand-binding domain of the estrogen receptor (GATA-1-ER). Treatment with estradiol or tamoxifen leads to synchronous erythroid maturation and cell cycle arrest as reflected in the activation and repression of GATA-1–dependent gene expression. A great advantage of G1E-ER4 cells for this study is that genomic occupancy of GATA-1 and its coregulators can be examined under dynamic conditions. We performed ChIP assays to compare SCL and GATA-1 occupancy at GATA-1–activated genes in parental G1E cells and G1E-ER4 cells treated with estradiol for 24 hours. The reason for choosing parental G1E cells for comparison instead of uninduced G1E-ER4 cells is that the latter display detectable GATA-1-ER occupancy at a few select sites in the absence of estradiol.28,52 Increases in GATA-1 occupancy were accompanied by increases in SCL recruitment at positive acting GATA sites including DNase1 hypersensitive site 2 (HS2) and HS3 of the LCR and the Hbb-b1 promoter (Figure 1A,B). Notably, the Hbb-b1 promoter lacks conserved E-boxes, suggesting that SCL recruitment is mediated by GATA-1 independently of SCL DNA binding. Control experiments showed that neither GATA-1 nor SCL was detected in a region approximately 1 kb upstream of the Hbb-b1 transcription start site that lacks GATA sites (Figure 1A). Moreover, SCL recruitment was stimulated by GATA-1 at additional GATA-1–dependent genes, including Band3, Klf1 (EKLF), and Eraf (AHSP). These results suggest that SCL recruitment is a general feature of GATA-1–activated genes regardless of the presence of E-box elements. This agrees with observations at regulatory regions of the Gata1 gene where SCL complexes correlated with the presence of GATA-1 even in the absence of E-boxes.53 In the absence of GATA-1, SCL occupancy is detected at some but not all of the examined sites (Figure 1B) possibly due to the presence of E-boxes (at Band3 and Klf1) or GATA-2, which is highly expressed prior to its repression by GATA-1.10 Indeed, compared with controls, we found substantial enrichment for GATA-2 at HS3, Band3, Klf1, and Eraf prior to activation by GATA-1 (Figure 1C). Moreover, at sites of initially high GATA-2 occupancy, GATA-2 was depleted upon GATA-1 activation.
GATA-1 recruits SCL to active erythroid genes. ChIP analysis using GATA-1 (A), SCL (B), and GATA-2 (C) antibodies or isotype-matched control antibodies (IgG) and primers for indicated sites. Primers for 1 kb upstream of the Hbb-b1 promoter (−1 kb) served as negative control. ChIP experiments were performed in G1E cells (−GATA-1) and G1E-ER4 cells after E2 treatment for 21 to 24 hours (+GATA-1). The data are the averages of 3 or more independent experiments. Error bars represent SDs. *Statistical significance (P ≤ .05) based on a 2-tailed t test. Numbers indicate P values.
GATA-1 recruits SCL to active erythroid genes. ChIP analysis using GATA-1 (A), SCL (B), and GATA-2 (C) antibodies or isotype-matched control antibodies (IgG) and primers for indicated sites. Primers for 1 kb upstream of the Hbb-b1 promoter (−1 kb) served as negative control. ChIP experiments were performed in G1E cells (−GATA-1) and G1E-ER4 cells after E2 treatment for 21 to 24 hours (+GATA-1). The data are the averages of 3 or more independent experiments. Error bars represent SDs. *Statistical significance (P ≤ .05) based on a 2-tailed t test. Numbers indicate P values.
Since SCL is part of a multimeric protein complex, we investigated by ChIP whether Ldb1, LMO2, and E2A follow a similar pattern. The results in Figure S2 show that all 3 molecules are similarly enriched upon GATA-1 activation. Western blots of nuclear extracts demonstrated that protein levels of all 4 proteins were essentially unchanged following GATA-1-ER activation (Figure S3), indicating that recruitment rather than elevated expression accounts for the increased occupancy of the SCL complex. Thus, at all sites examined so far, SCL, LMO2, Ldb-1, and E2A recruitment increases as a result of increased GATA-1 occupancy.
To further establish that SCL recruitment is critically determined by GATA-1 occupancy, we performed time course ChIP experiments at the Hbb-b1 promoter in G1E-ER4 cells treated with estradiol for 4, 8, 12, 16, and 20 hours. We found that the levels of SCL occupancy paralleled those of GATA-1, suggesting that association with GATA-1 rather than direct DNA binding is essential for SCL recruitment at this site (Figure S4).
We also investigated SCL occupancy at the α-globin locus before and after GATA-1-ER activation, focusing on the distal regulatory sites HS-31, HS-12, and the Hba-a1 promoter. Notably, prior to GATA-1 activation, high levels of SCL, E2A, Ldb1, and LMO2 were found at HS-31 and HS-12 (Figure S5). In addition, low levels of the SCL complex were detected at the Hba-a1 promoter (Figure S5). This generally agrees with previous observations in which SCL and Ldb1 were found at the α-globin locus in immature cells before the onset of transcription54 and could be due to the presence of GATA-2 or E-boxes or a combination of the two. Following activation of GATA-1-ER, the levels of SCL, E2A, Ldb1, and LMO2 decreased slightly but remained very high compared with other GATA-1–activated genes (Figure 1; Figure S5). This finding is consistent with a model in which the SCL complex functions as coactivator for GATA-1. However, the function of the SCL complex at the inactive α-globin locus is unclear. It is possible that the SCL complex is insufficient for transcription or that it actually keeps the α-globin locus in its repressed state, perhaps via ETO-2.31,32,43 Our ChIP experiments using antibodies against ETO-2 support the latter possibility (below).
One observation that remains unclear is that the relative amounts of SCL and GATA-1 occupancy vary. For example, at the Klf1, Eraf, and Band3 genes, SCL occupancy is higher than at the β-globin locus, whereas GATA-1 occupancy is somewhat higher at the β-globin locus (Figure 1). Moreover, at the Hba-a1 promoter the ratio of GATA-1 to SCL is higher than at HS-31 and HS-12. It is likely that additional transcription factors or cofactors influence SCL recruitment. Nevertheless, the SCL complex is present at all active GATA sites examined.
To confirm these results in primary cells, we performed ChIP experiments using erythroid cells from E14.5 fetal livers. SCL, LMO2, and Ldb1 were detected at active GATA-1 target genes at levels very similar to those found in induced G1E-ER4 cells (Figure S6).
SCL and GATA-1 co-occupy active genes in megakaryocytes and mast cells
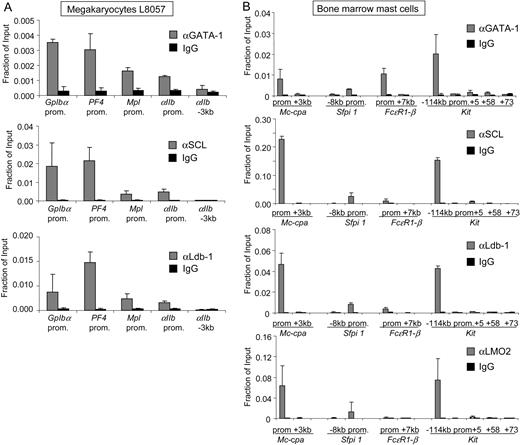
The normal development of megakaryocytes and mast cells depends on both GATA-1 and SCL.4,40-42,55 To determine the relationship between GATA-1 and SCL occupancy at MK-specific promoters, we performed ChIP experiments with the murine megakaryocytic cell line L8057. Primer pairs were directed toward the proximal promoter sequences of the early expressed αIIb and Mpl genes and the late expressed GpIbα and Pf4 genes, all of which harbor well-characterized GATA elements.56 We found high levels of GATA-1 at the proximal promoters of GpIbα and Pf4 and somewhat lower levels at the αIIb and Mpl genes (Figure 2A). GATA-1 was undetectable at a control region 3 kb upstream of the αIIb transcription start site that is devoid of GATA elements (Figure 2A). Consistent with our findings in erythroid cells, occupancy levels of SCL and Ldb1 correlated with that of GATA-1 at megakaryocytic target genes (Figure 2A).
GATA-1, SCL, and Ldb-1 co-occupy active genes in megakaryocytes and mast cells. ChIP analysis using GATA-1, SCL, Ldb-1, or isotype-matched control antibodies in megakaryocytic L8057 cells (A) and primary bone marrow–derived mast cells (B). The −3-kb region upstream of αIIb served as negative control. The data shown are the averages of 3 or more independent experiments in panel A and 2 independent experiments in panel B. Error bars represent SDs.
GATA-1, SCL, and Ldb-1 co-occupy active genes in megakaryocytes and mast cells. ChIP analysis using GATA-1, SCL, Ldb-1, or isotype-matched control antibodies in megakaryocytic L8057 cells (A) and primary bone marrow–derived mast cells (B). The −3-kb region upstream of αIIb served as negative control. The data shown are the averages of 3 or more independent experiments in panel A and 2 independent experiments in panel B. Error bars represent SDs.
To examine whether the tight correlation between GATA-1 and SCL proteins extends to mast cells, we performed ChIP experiments in primary cultured bone marrow–derived mast cells. We examined regions with known GATA-binding sites near the genes encoding the mast cell carboxypeptidase A (Mc-Cpa),57 the mast cell–specific IgE receptor (FcϵRI) β-chain,58 the transcription factor PU.1 (Sfpi1) (S. Chou, M. J. Weiss, manuscript in preparation), and the Kit gene.12,28 As expected, GATA-1 was present at the relevant sites but not control regions of all 4 genes (Figure 2B). The occupancy of SCL, Ldb1, and LMO2 followed a very similar pattern with regard to both spatial distribution and amount of detectable protein (Figure 2B). The −114-kb region of Kit and the FcϵRI-β promoter do not harbor any E-boxes within at least 100 base pairs of the GATA elements, consistent with GATA-1 likely accounting for most of the SCL recruitment at these sites.
At the promoter of the Mc-Cpa gene, the ratio of GATA-1 to SCL proteins was lower than that found at other sites. In contrast, this ratio was higher at the FcϵRI-β promoter. The reason for this is unclear but in contrast to mature erythroid cells, mast cells express high levels of GATA-2, leading us to speculate that the degree of SCL recruitment might reflect combined levels of GATA-1 and GATA-2.
Immunoprecipitation experiments suggested that GATA-1 associates with distinct complexes containing SCL or FOG-1 but not both.59 However, FOG-1 is recruited to all known activating GATA sites in a manner virtually indistinguishable from SCL proteins19,20 . In trying to reconcile these observations, it is possible that the composition of GATA-1–associated protein complexes changes over time such that FOG-1 is substituted by SCL or vice versa. Although preliminary time course ChIP studies in G1E-ER4 cells do not support this model (not shown), very closely spaced time points might be required to reveal differences in the kinetics of cofactor assembly. It is also possible that distinct FOG-1– and SCL-containing GATA-1 complexes associate in a variegated manner with alleles of the same genes. For example, alternate/combinatorial use of transcription cofactors has been described at estrogen-receptor regulated genes.60 Whereas conventional ChIP experiments reflect the sum of protein interactions at all alleles, sequential ChIP (double ChIP) experiments might address the issue of variegated protein occupancy. Nevertheless, our results demonstrate that the SCL complex is present at all active GATA sites examined in erythroid cells, megakaryocytes, and mast cells even in the absence of conserved E-box sequences, strongly suggesting that the SCL complex represents a tissue-specific coactivator complex for GATA-1.
The SCL complex fails to assemble at GATA-1–repressed genes
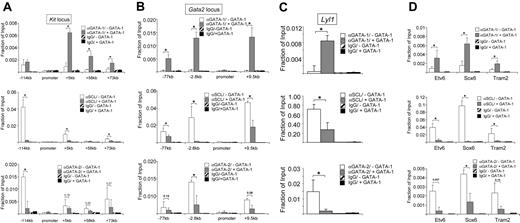
If the SCL complex directs positive activity of GATA-1, it would be expected that it is reduced or absent from genes where GATA-1 functions as repressor. To test this hypothesis, we interrogated the occupancy of the SCL complex at genes directly repressed by GATA-1, comparing G1E cells to induced G1E-ER4 cells. We examined several GATA elements at the Gata2 and Kit genes that were previously shown to be occupied by GATA-1 during their repression.11,12,19,28 In induced G1E-ER4 cells, GATA-1 occupied GATA elements positioned 114 kb upstream and 5 kb, 58 kb, and 73 kb downstream of the transcription start site but not at the promoter of the Kit gene (Figure 3A) in agreement with previous results.28 Strikingly, all SCL components were depleted from all 4 GATA-1–occupied elements at the repressed Kit locus (Figure 3A; Figure S7). Similar results were obtained at the Gata2 locus where high levels of GATA-1 were associated with reduced or lost SCL occupancy 77 kb and 2.8 kb upstream and 9.5 kb downstream of the transcription start site (Figure 3B). Ldb-1, LMO2, and E2A followed the same trend (not shown). At the active Kit and Gata2 loci, SCL levels correlated well with the presence of GATA-2 (Figure 3A,B),28,61 suggesting that GATA-2 might function via SCL to activate Kit and Gata2 expression in immature erythroid cells.
Depletion of SCL from GATA-1–repressed genes. ChIP analysis using GATA-1, SCL, GATA-2, or isotype-matched control antibodies at Kit (A), Gata2 (B), the FOG-1–independent Lyl1 gene (C), and the GATA-E-box–containing genes Etv6, Sox6, and Tram2 (D). ChIP experiments were performed in parental G1E cells and estradiol-treated G1E-ER4 cells as in Figure 1. Error bars represent SDs. *Statistical significance (P ≤ .05) based on a 2-tailed t test. Numbers indicate P values. Please note that the difference in absolute signal intensity for the SCL ChIP between panel C and the other panels is due to the use of a different batch of SCL antibodies.
Depletion of SCL from GATA-1–repressed genes. ChIP analysis using GATA-1, SCL, GATA-2, or isotype-matched control antibodies at Kit (A), Gata2 (B), the FOG-1–independent Lyl1 gene (C), and the GATA-E-box–containing genes Etv6, Sox6, and Tram2 (D). ChIP experiments were performed in parental G1E cells and estradiol-treated G1E-ER4 cells as in Figure 1. Error bars represent SDs. *Statistical significance (P ≤ .05) based on a 2-tailed t test. Numbers indicate P values. Please note that the difference in absolute signal intensity for the SCL ChIP between panel C and the other panels is due to the use of a different batch of SCL antibodies.
Repression of Kit and Gata2 by GATA-1 requires FOG-1. To investigate whether FOG-1 might be required for the depletion of SCL, we examined the Lyl1 gene, which is repressed directly by GATA-1 in a FOG-1–independent manner.21 We found that SCL levels correlated inversely with GATA-1 occupancy (Figure 3C) but correlated positively with GATA-2 protein levels (Figure 3C). We also examined 3 more GATA-1–repressed genes, Etv6, Sox6, and Tram2, that contain composite GATA-E-box elements in their regulatory regions.62 In the absence of GATA-1, we found high levels of GATA-2 and SCL at all 3 promoters (Figure 3D). In contrast, upon GATA-1–induced gene repression, SCL was dramatically reduced (Figure 3D), again showing that despite the presence of conserved E-boxes in these genes, a repressive GATA-1–containing complex is incompatible with the presence of SCL. Together, our results show that at all examined GATA-1–dependent enhancer elements, the SCL complex coexists with GATA-1 in erythroid cells, mast cells, and megakaryocytes (Table 1). In contrast, at all 12 sites where GATA-1 functions as a repressor, the SCL complex is reduced or absent (Table 2). Despite the strong correlation between GATA-1 and SCL at active genes but not repressed genes, we cannot rule out the existence of GATA-1–regulated genes where this correlation breaks down and other factors control the overall gene activity. Future studies spanning the entire genome will address this issue.
GATA-1–activated genes
| GATA elements . | SCL complex occupancy . |
|---|---|
| G1E cells: −GATA-1 vs +GATA-1 | |
| β-globin HS3 | ↑ |
| β-globin HS2 | ↑ |
| Hbb-b1 promoter | ↑ |
| Band3 promoter | ↑ |
| Klf1 promoter | ↑ |
| Eraf promoter | ↑ |
| α-globin −31 kb | + |
| α-globin −12 kb | + |
| Fetal liver E14.5 | |
| β-globin HS3 | + |
| β-globin HS2 | + |
| Hbb-b1 promoter | + |
| Band3 promoter | + |
| Klf1 promoter | + |
| Eraf promoter | + |
| α-globin −31 kb | + |
| α-globin −12 kb | + |
| Megakaryocytes | |
| Gplbα promoter | + |
| Pf4 promoter | + |
| mpl promoter | + |
| αllb promoter | + |
| Primary mast cells | |
| Mc-Cpa promoter | + |
| Sfpi1 promoter | + |
| FcϵR1-β promoter | + |
| Kit −114 kb | + |
| GATA elements . | SCL complex occupancy . |
|---|---|
| G1E cells: −GATA-1 vs +GATA-1 | |
| β-globin HS3 | ↑ |
| β-globin HS2 | ↑ |
| Hbb-b1 promoter | ↑ |
| Band3 promoter | ↑ |
| Klf1 promoter | ↑ |
| Eraf promoter | ↑ |
| α-globin −31 kb | + |
| α-globin −12 kb | + |
| Fetal liver E14.5 | |
| β-globin HS3 | + |
| β-globin HS2 | + |
| Hbb-b1 promoter | + |
| Band3 promoter | + |
| Klf1 promoter | + |
| Eraf promoter | + |
| α-globin −31 kb | + |
| α-globin −12 kb | + |
| Megakaryocytes | |
| Gplbα promoter | + |
| Pf4 promoter | + |
| mpl promoter | + |
| αllb promoter | + |
| Primary mast cells | |
| Mc-Cpa promoter | + |
| Sfpi1 promoter | + |
| FcϵR1-β promoter | + |
| Kit −114 kb | + |
GATA-1–repressed genes
| GATA elements . | SCL complex occupancy . |
|---|---|
| G1E cells: −GATA-1 versus +GATA-1 | |
| Kit −114 kb | ↓ |
| Kit +5 kb | ↓ |
| Kit +58 kb | − |
| Kit +73 kb | ↓ |
| Gata2 −77 kb | ↓ |
| Gata2 −2.8 kb | ↓ |
| Gata2 +9.5 kb | ↓ |
| Lyl1 | ↓ |
| Rgs18* | ↓ |
| Etv6 | ↓ |
| Sox6 | ↓ |
| Tram2 | ↓ |
| GATA elements . | SCL complex occupancy . |
|---|---|
| G1E cells: −GATA-1 versus +GATA-1 | |
| Kit −114 kb | ↓ |
| Kit +5 kb | ↓ |
| Kit +58 kb | − |
| Kit +73 kb | ↓ |
| Gata2 −77 kb | ↓ |
| Gata2 −2.8 kb | ↓ |
| Gata2 +9.5 kb | ↓ |
| Lyl1 | ↓ |
| Rgs18* | ↓ |
| Etv6 | ↓ |
| Sox6 | ↓ |
| Tram2 | ↓ |
Summary of ChIP experiments (not including ChIP-on-chip data) measuring SCL recruitment at genes activated (A) or repressed (B) by GATA-1.
↓ indicates dynamic changes in SCL occupancy following increases in GATA-1 occupancy in G1E cells; +, significant enrichment compared with controls; and −, no significant signal.
HA-tagged SCL.
ETO-2 occupancy at GATA-1–regulated genes
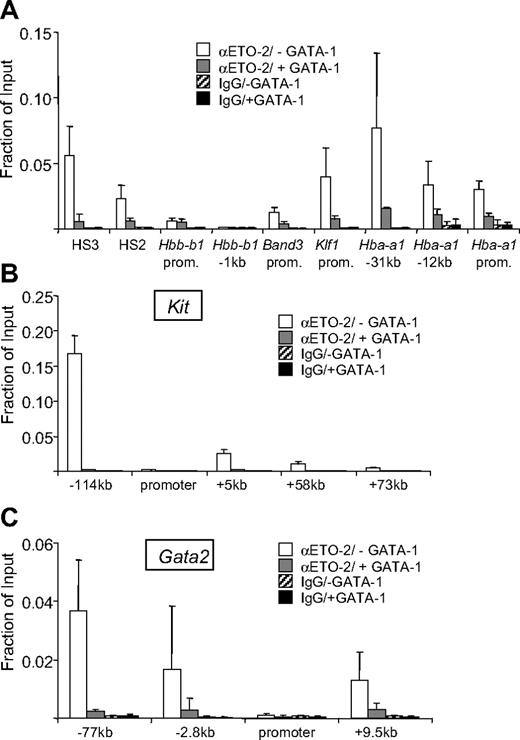
Since SCL is largely displaced from genes at which GATA-1 represses transcription, SCL is unlikely to play a direct role in their repression. However, at genes where SCL is present prior to their activation, it is possible that it helps to maintain them in an inactive state. For example, at the α-globin locus SCL has been detected prior to its transcriptional activation.54 The SCL complex harbors ETO-2, a member of ETO/MTG family of corepressor proteins that can bind histone deacetylases.31,32,43 Since ETO-2 is a candidate for SCL-mediated gene repression, we examined its occupancy at GATA-1 target genes before and after their activation by GATA-1. We found significant levels of ETO-2 at the α-globin gene in the absence of GATA-1 that was dramatically reduced upon GATA-1-ER activation (Figure 4A). Loss of ETO-2 association with the α-globin gene was not a result of reduced ETO-2 protein levels as determined by Western blotting (Figure S3). ETO-2 was also present at other GATA-1 target genes including Band3, Hbb-b1, and Klf1 prior to their activation. Notably, the amounts of ETO-2 correlated well with GATA-2 levels (compare with Figure 1C). This suggests that ETO-2 might indeed participate in repressing these genes in immature erythroid cells probably via GATA-2 and SCL. Consistent with this interpretation, forced expression of ETO-2 in the erythroid cell line MEL reduced α-globin expression, and a dominant interfering form of SCL raised α-globin levels in erythroid progenitors.31 However, to our surprise, at the active Gata2 and Kit genes we also found high levels of ETO-2, which was lost upon repression (Figure 4B,C). ETO-2 levels largely mirrored those of SCL and GATA-2 (compare with Figure 3A,B) except for the −77-kb region of the Gata2 gene where ETO-2 levels were significantly higher than would have been predicted based on the amounts GATA-2. Thus, the ratio of ETO-2 to SCL appears to vary not only during erythroid maturation31,32,43 but also among regulatory elements. From these data, we conclude that the mere presence of ETO-2 does not predict whether a target gene is active or repressed. This raises the possibility that the activity of ETO-2 is regulated such that it “permits” transcription of certain active genes while contributing to the repression of others. Moreover, ETO-2 does not appear to contribute to the silencing of Gata2 and Kit expression in G1E-ER4 cells since it is removed from these genes along with the SCL core complex. Therefore, it is surprising that expression of a dominant-negative form of E2A that is defective for ETO-2 binding can increase Kit expression in differentiating MEL cells.31 Perhaps mature MEL cells maintain residual levels of SCL at the Kit gene to allow E2A recruitment or the effects of mutant E2A were indirect.
ETO-2 occupancy at GATA-1–regulated genes. ChIP analysis using ETO-2 or isotype-matched control antibodies at indicated GATA-1–activated (A) and GATA-1–repressed Kit (B) and Gata2 (C) genes. Prom indicates promoter. ChIP experiments were performed as in Figure 1. The data are the averages of 3 or more independent experiments. Error bars represent SDs.
ETO-2 occupancy at GATA-1–regulated genes. ChIP analysis using ETO-2 or isotype-matched control antibodies at indicated GATA-1–activated (A) and GATA-1–repressed Kit (B) and Gata2 (C) genes. Prom indicates promoter. ChIP experiments were performed as in Figure 1. The data are the averages of 3 or more independent experiments. Error bars represent SDs.
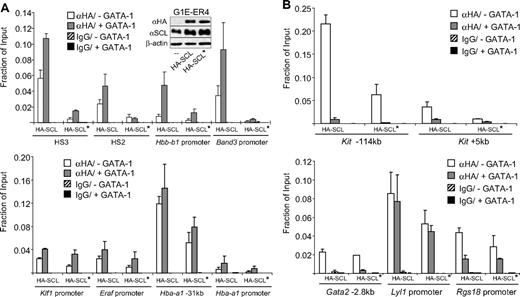
SCL recruitment in the absence of DNA binding
Our results presented thus far support a general role for the SCL complex as a GATA factor coactivator consistent with its ability to perform broad functions during erythropoiesis even in the absence of direct DNA binding.44-47 To evaluate the extent to which DNA binding by SCL is dispensable for occupancy at GATA-1 target genes, we infected G1E-ER4 cells with retrovirus expressing HA-tagged SCL or a version bearing 3 point mutations in the SCL basic domain that abrogates DNA binding (SCL-RER44 ). Western blots showed comparable expression of HA-SCL and HA-SCL-RER (insert in Figure 5A). After 24 hours of estradiol treatment, ChIP assays were performed with anti-HA antibodies. Wild-type SCL displayed a pattern of occupancy resembling that of endogenous SCL with increasing occupancy at GATA-1–activated genes (Figure 5A). SCL-RER occupancy was reduced at HS3, HS2, Hbb-b1, and Band3, but occurred at virtually normal levels at the Klf1, Eraf, and Hba-a1 genes (Figure 5A). Among the genes repressed by GATA-1, 2 sites at the Kit gene (−114 kb and +5 kb) displayed reduced occupancy of SCL-RER, whereas at the Gata2, Lyl1, and Rgs18 genes wild-type SCL and SCL-RER were recruited similarly (Figure 5B). These results demonstrate that although direct contacts with DNA contribute to SCL occupancy, GATA-1–induced SCL recruitment can occur at numerous sites independently of DNA binding. Since G1E-ER4 cells are definitive erythroid precursor cells, our results are also consistent with the observation that SCL DNA binding is required for normal definitive erythropoiesis.44,45 Based on previous work,44 it is predicted that in primitive erythroid cells GATA-1 or perhaps additional transcription factors might be entirely sufficient for tethering the SCL complex to its target genes.
SCL recruitment in the absence of DNA binding. Anti-HA or control IgG ChIP of GATA-1–activated (A) and GATA-1–repressed (B) genes in G1E-ER4 cells expressing HA-tagged SCL (HA-SCL) or HA-SCL-RER (HA-SCL*), before and after estradiol (E2) treatment for 21 to 24 hours. The data shown are the averages of 2 (Klf1, Gata2 −2.8), 3 (HS3, Hba-a1, −31, Hba-a1 promoter, Lyl1 and Rgs18 promoters), and 4 (HS2, Hbb-b1, Hbb-b1 promoter, Band3, Band3 promoter, Kit −114, Kit +5) independent experiments. Error bars represent SDs. Note that the decrease in HA-SCL occupancy at the Lyl1 gene is less pronounced than that observed with endogenous SCL (Figure 3C). This is likely the result of leakiness with regard to GATA-1-ER activity in this experiment.
SCL recruitment in the absence of DNA binding. Anti-HA or control IgG ChIP of GATA-1–activated (A) and GATA-1–repressed (B) genes in G1E-ER4 cells expressing HA-tagged SCL (HA-SCL) or HA-SCL-RER (HA-SCL*), before and after estradiol (E2) treatment for 21 to 24 hours. The data shown are the averages of 2 (Klf1, Gata2 −2.8), 3 (HS3, Hba-a1, −31, Hba-a1 promoter, Lyl1 and Rgs18 promoters), and 4 (HS2, Hbb-b1, Hbb-b1 promoter, Band3, Band3 promoter, Kit −114, Kit +5) independent experiments. Error bars represent SDs. Note that the decrease in HA-SCL occupancy at the Lyl1 gene is less pronounced than that observed with endogenous SCL (Figure 3C). This is likely the result of leakiness with regard to GATA-1-ER activity in this experiment.
Widespread association of GATA-1 with SCL at enhancers
To examine the generality of the association between GATA-1 and SCL, we investigated the co-occupancy of DNA segments by GATA-1 and the SCL complex over a 66-Mb region of mouse chromosome 7. ChIP was performed with antibodies against SCL and Ldb1 in G1E-ER4 cells treated with estradiol for 24 hours and parental G1E cells lacking GATA-1. ChIP material was amplified and hybridized to high-density NimbleGen custom arrays (Roche NimbleGen, Madison, WI) spanning 66 Mb chromosome 7. Data were analyzed and compared with GATA-1 ChIP-on-chip.63 Raw results can be viewed on a custom browser at http://www.bx.psu.edu/∼yong/gerd. The peak-calling program Tamalpais64 identified 97 GATA-1–bound sites in estradiol-treated G1E-ER4 cells (Table S1A) within regions containing few repetitive sequences (“chipable regions”). Sixty-three DNA segments were unequivocally found to be occupied by GATA-1 as confirmed by quantitative polymerase chain reaction (PCR).63 Hybridization with SCL and Ldb1 ChIP DNA revealed a strikingly similar pattern of occupancy for these 2 proteins. Overall, the signal intensity for SCL was highly correlated with that for Ldb1 (r = 0.66), and 86% of the SCL peaks overlap with Ldb1 peaks. This suggests that in the majority of cases Ldb1 and SCL are associated.
SCL and Ldb1 proteins also showed a strong tendency for co-occupancy with GATA-1. Of the 63 DNA segments validated as occupied by GATA-1, 48 (76%) were also positive for SCL and Ldb1 occupancy. Almost all of these are associated with enhancer activity (below). In addition, considering individual probes in the 63 GATA-1–occupied DNA segments, the hybridization signals between SCL and Ldb1 ChIPs are tightly correlated (r = 0.93), even higher than the correlation for the entire 66-Mb target region. This higher correlation suggests that all 3 proteins, GATA-1, SCL and Ldb1, tend to bind DNA in a complex.
We identified 247 SCL-occupied sites (Table S1A), which exceeded the number of GATA-1 peaks. Although the great majority of the validated GATA-1–occupied DNA segments were co-occupied by SCL and Ldb1, only 20% of the SCL-occupied segments are co-occupied by GATA-1. This is consistent with SCL having functions that are independent of GATA-1.
We next examined whether the DNA segments co-occupied by GATA-1, SCL, and Ldb1 are active enhancers in vivo. Sixty-one of the 63 GATA-1–bound DNA segments were added to a luciferase expression plasmid with a HBG1 gene promoter and transfected into K562 cells. Based on their activities, the DNA segments were partitioned into a group of 27 that have enhancer activity (increased luciferase activity more than 2-fold compared with the parental plasmid), a group of 21 with no positive activity (< 1.5-fold effect), and a group of 13 with threshold activities (1.5- to 2-fold effects). All but one of the active enhancers were also occupied by SCL and Ldb1 by the ChIP-on-chip data (Figure 6). The sole exception (Figure 6; activity indicated by the gray box) was in a repeat-rich region that is problematic for ChIP-on-chip analysis, and indeed we found that it also was occupied by SCL and Ldb1 by quantitative PCR (not shown). Thus, all GATA-1–occupied DNA segments with enhancer activity were associated with SCL and Ldb1. In contrast, the GATA-1–bound DNA segments with no positive activity were co-occupied by SCL and Ldb1 much less frequently (10 of 21). The correlation of co-occupancy with enhancer activity was highly significant (P < .001 by Fisher exact test).
Erythroid enhancers are co-occupied by GATA-1, SCL, and Ldb1. Sixty-one DNA segments occupied in vivo by GATA-1, whose enhancer activities were determined by Cheng et al,63 were evaluated for co-occupancy by SCL and Ldb1, based on the ChIP-on-chip results. The distribution of results after transient transfection of K562 cells (range of 8 to 24 determinations for each GATA-1–occupied DNA segment) is shown as a box plot, with the internal line indicating the median, the box extending to the first and third quartiles, and the whiskers extending to the most extreme data point that is no more than 1.5 times the interquartile range. Black boxes represent DNA segments co-occupied by GATA-1, SCL, and Ldb1; white boxes represent sequences that are occupied only by GATA-1. The gray box represents a segment that was not called as an SCL peak in ChIP-on-chip but was shown by quantitative PCR (qPCR) to be occupied by SCL. The horizontal line demarcates the threshold for enhancer activity. The results were partitioned into DNA segments with no enhancer activity (class I), activity clustering around the threshold (class II), and more than 2-fold activity (class III; see bottom panel for a summary of the results). Class I and class III are statistically different, tested by both χ2 test and Fisher exact test (P < .001).
Erythroid enhancers are co-occupied by GATA-1, SCL, and Ldb1. Sixty-one DNA segments occupied in vivo by GATA-1, whose enhancer activities were determined by Cheng et al,63 were evaluated for co-occupancy by SCL and Ldb1, based on the ChIP-on-chip results. The distribution of results after transient transfection of K562 cells (range of 8 to 24 determinations for each GATA-1–occupied DNA segment) is shown as a box plot, with the internal line indicating the median, the box extending to the first and third quartiles, and the whiskers extending to the most extreme data point that is no more than 1.5 times the interquartile range. Black boxes represent DNA segments co-occupied by GATA-1, SCL, and Ldb1; white boxes represent sequences that are occupied only by GATA-1. The gray box represents a segment that was not called as an SCL peak in ChIP-on-chip but was shown by quantitative PCR (qPCR) to be occupied by SCL. The horizontal line demarcates the threshold for enhancer activity. The results were partitioned into DNA segments with no enhancer activity (class I), activity clustering around the threshold (class II), and more than 2-fold activity (class III; see bottom panel for a summary of the results). Class I and class III are statistically different, tested by both χ2 test and Fisher exact test (P < .001).
Another important conclusion is that a GATA element (WGATAR) is a better predictor of SCL occupancy than is the E-box (CANNTG). We measured the enrichment of motifs in bound segments as the proportion of protein-bound DNA segments that contain a specified motif divided by the proportion of background DNA segments that contain the same motif. The segments occupied by SCL showed very little enrichment for the E-box (1.03) but substantial enrichment for the GATA element (1.25; Table S1A).
A notable result is that an E-box might not be essential for SCL recruitment. Of the 48 sites co-occupied by GATA-1, SCL, and Ldb1, 47 had a perfect WGATAR motif, as expected, but only 25 had an E-box within 20 base pairs of the GATA element (Table S1B). This suggests that at the remaining sites, SCL recruitment occurred via GATA-1 and not via direct DNA binding of SCL. However, given the frequent occurrence of E-boxes it remains impossible to discern whether E-boxes that reside further away from the GATA-1–bound site contribute to SCL recruitment. Nevertheless, our observation of the highly dynamic nature of SCL recruitment in response to changes in GATA factor occupancy (Figure 1) is consistent with a model in which levels of SCL recruitment are controlled by GATA-1 at GATA-1–occupied sites.
Many of the elements occupied by GATA-1 and/or SCL are found at substantial distances from the nearest genes, making it difficult to assess which of these genes if any they control. However, it is notable that 27 of 48 elements occupied by GATA-1, SCL, and Ldb1 reside closer to activated genes than to repressed genes. Conversely, the majority of elements (11 of 15) occupied solely by GATA-1 are found closer to genes that are repressed. Together, these results are consistent with the notion that the SCL complex functions as cofactor for GATA-1 during activation but not repression of transcription.
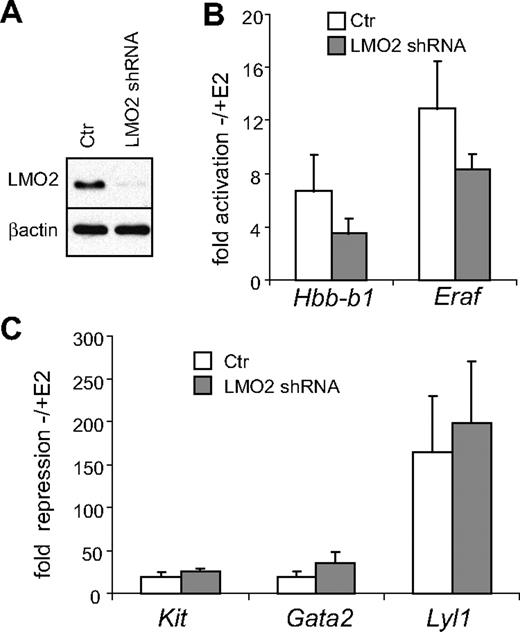
LMO2 is required for activation but not repression of GATA-1–dependent genes
The selective presence of the SCL complex at active GATA-1–bound regions is consistent with its role as a coactivator. Consequently, loss of SCL or its associated proteins is expected to impair GATA-1–mediated activation but not repression. To test this prediction, we knocked down LMO2, which bridges GATA-1 and the SCL complex. An LMO2 shRNA was introduced into G1E-ER4 cells with the retroviral vector pMSCV/LTRmiR30-PIG containing GFP. Since we observed significant cell death upon infection, we used a derivative of G1E-ER4 cells expressing the antiapoptotic protein Bcl-XL, which improved viability.9 fluorescence-activated cell sorting (FACS) was used to generate pools of GFP-positive cells. LMO2 expression was diminished as determined by Western blotting (Figure 7A). SCL levels were unaffected by the LMO2 knockdown (not shown). Cells were treated with estradiol or were left untreated for 24 hours, and mRNA levels of GATA-1–activated and –repressed genes were analyzed by quantitative reverse-transcription (RT)–PCR using GAPDH as internal standard. We observed a 47% and 36% reduction in the fold increase of Hbb-b1 and Eraf, respectively, in the LMO2 knockdown cells compared with controls (Figure 7B; for absolute values see Figure S8A,B). In contrast, the LMO2 knockdown did not diminish the GATA-1–induced repression of the Kit, Gata2, and Lyl1 genes (Figure 7C). In the case of Gata2, the fold repression was even increased from 17-fold in control cells to 26-fold in the LMO2 knockdown cells. These results are consistent with the SCL complex serving selectively as coactivator but not corepressor of GATA-1. There was a trend in LMO2 knockdown cells toward a slight reduction in the expression of the Kit, Gata2, and Lyl1 genes prior to their repression by GATA-1, although the results are not statistically significant (Figure S8D-F). This might reflect a requirement of SCL for the expression of these genes through interaction with GATA-2.
LMO2 is required for activation but not repression of GATA-1–regulated genes. (A) Anti-LMO2 Western blot of nuclear extracts from Bcl-XL–expressing G1E-ER4 cells transduced with vector producing an shRNA against LMO2 or empty vector (ctr). β-Actin served as loading control. (B,C) Cells were treated with estradiol (E2) for 24 hours and mRNA levels of indicated genes were determined by real-time RT-PCR. Results were normalized to Gapdh mRNA and plotted as fold change compared with untreated cells. The data are the averages of 4 independent experiments. Error bars represent SD.
LMO2 is required for activation but not repression of GATA-1–regulated genes. (A) Anti-LMO2 Western blot of nuclear extracts from Bcl-XL–expressing G1E-ER4 cells transduced with vector producing an shRNA against LMO2 or empty vector (ctr). β-Actin served as loading control. (B,C) Cells were treated with estradiol (E2) for 24 hours and mRNA levels of indicated genes were determined by real-time RT-PCR. Results were normalized to Gapdh mRNA and plotted as fold change compared with untreated cells. The data are the averages of 4 independent experiments. Error bars represent SD.
Our work indicates that SCL and associated proteins represent a tissue-specific GATA-1–coactivator complex that is present at activating but not repressive GATA-1–bound regulatory elements. In addition, SCL recruitment by GATA-1 can occur in the absence of adjacent E-boxes. The next challenge will be to determine what mechanism accounts for the selective presence of SCL proteins at active GATA elements. It is possible that selectivity is achieved at the level of recruitment. How such specificity would be achieved is unclear but might involve posttranslational modifications of GATA-1. Alternatively, the SCL complex might be recruited to both active and repressed GATA-1–bound genes but removed selectively at sites where GATA-1 represses transcription. This might involve proteasome-mediated turnover of LMO2 or Ldb1.65,66 Indeed, treatment of G1E-ER4 cells with the proteasome inhibitors MG132 or lactacystein blocks removal of SCL from the Kit and Gata2 genes during GATA-1–induced repression (not shown). However, since the proteasome is also required for the exchange of GATA-1 for GATA-2,67,68 further work is needed to assess the role, if any, of proteasome-mediated turnover of the SCL complex at GATA-1–repressed genes. One prediction from our results is that forced constitutive interaction between SCL and GATA-1 might impair the ability of GATA-1 to function as transcriptional repressor. To test this idea, we fused GATA-1 in frame to SCL or LMO2 and introduced fusion constructs into G1E cells via retroviral transduction. We found that the GATA-1-SCL and GATA-1-LMO2 fusion proteins functioned almost indistinguishably from wild-type GATA-1 with regard to both activating and repressive functions (not shown). This result might be explained if one or more components of the SCL complex are proteolyzed at repressed elements, thus allowing repression to proceed unimpaired.
If the presence of the SCL complex specifies activating GATA-1, are there tissue-specific or general GATA-1 cofactors that consistently specify repression by GATA-1? As mentioned before, the presence of neither FOG-1 nor NuRD is diagnostic for repressive GATA-1 complexes as they are also found at GATA-1–activated genes. Candidate GATA-1–corepressor proteins include Gfi-1b, which can physically associate with GATA-1. However Gfi-1b is detected only at a subset of GATA-1–repressed genes.59 Gfi-1b also binds to SCL presumably via ETO-2, but its functional role within this complex remains unknown.31 PU.1 binds directly to GATA-1 and is thought to antagonize its activity in progenitor cells to inhibit erythroid development.69-71 However, PU.1 expression is extinguished during erythroid differentiation and might not function during the repression of GATA-1–dependent genes in late maturing erythroid cells. Considering the available evidence, it is possible that repression by GATA-1 involves distinct corepressor molecules depending on the cell lineage, promoter context, and maturation stage.
An important question remains as to how SCL augments GATA-1 transcriptional activity. SCL associates with histone acetyltransferases p300 and P/CAF and is itself acetylated, similar to GATA-1.23,72-74 Increasing the levels of p300 and/or P/CAF might increase the ratio of histone acetylases to deacetylases to facilitate transcriptional activation. However, we and others have found that the p300 paralog CBP remains associated with some GATA-1–repressed genes. Whether the same is true for P/CAF and p300 remains to be examined. Given that GATA-1–initiated gene activation and repression are accompanied by histone acetylation and deacetylation, respectively,11,24,26,28,75 this suggests that SCL might augment the specific activity or substrate recognition of histone acetyltransferases. An example for the latter possibility was provided by studies of the hematopoietic transcription factor NF-E2, which enhanced the nucleosome directed acetyltransferase activity of CBP.76 In any case, the SCL complex fulfills the criteria of a bona fide coactivator in that it can stimulate GATA-1 activity and is associated with most if not all active but not repressive GATA factor complexes in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Catherine Porcher for the SCL-RER construct, and Mitchell Weiss and Mortimer Poncz for critical comments on the paper.
This work was supported by National Institutes of Health (NIH, Bethesda, MD) grants DK54937 (G.A.B.) and DK65806, (R.C.H.) and by Tobacco Settlement Funds from the Pennsylvania Department of Health (Harrisburg, PA; R.C.H.). C.R.V. was supported by NIH training grant T32 HL007150.
National Institutes of Health
Authorship
Contribution: T.T., W.D., C.R.V., R.C.H., and G.A.B. designed the research; T.T., W.D., R.C.H., and G.A.B. wrote the paper; and T.T., W.D., C.R.V., Y.C., G.D.G., and Y.Z. performed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerd A. Blobel, Children's Hospital of Philadelphia, ARC 316H, 3615 Civic Center Boulevard, Philadelphia, PA 19104; e-mail: blobel@email.chop.edu.
References
Author notes
*T.T. and W.D. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal