Bortezomib reversibly inhibits 26S proteasomal degradation, interferes with NF-κB, and exhibits antitumor activity in human malignancies. Zinc finger protein Sp1 transactivates DNMT1 gene in mice and is functionally regulated through protein abundance, posttranslational modifications (ie, ubiquitination), or interaction with other transcription factors (ie, NF-κB). We hypothesize that inhibition of proteasomal degradation and Sp1/NF-κB–mediated transactivation may impair aberrant DNA methyltransferase activity. We show here that, in addition to inducing accumulation of polyubiquitinated proteins and abolishment of NF-κB activities, bortezomib decreases Sp1 protein levels, disrupts the physical interaction of Sp1/NF-κB, and prevents binding of the Sp1/NF-κB complex to the DNMT1 gene promoter. Abrogation of Sp1/NF-κB complex by bortezomib causes transcriptional repression of DNMT1 gene and down-regulation of DNMT1 protein, which in turn induces global DNA hypomethylation in vitro and in vivo and re-expression of epigenetically silenced genes in human cancer cells. The involvement of Sp1/NF-κB in DNMT1 regulation is further demonstrated by the observation that Sp1 knockdown using mithramycin A or shRNA decreases DNMT1 protein levels, which instead are increased by Sp1 or NF-κB overexpression. Our results unveil the Sp1/NF-κB pathway as a modulator of DNA methyltransferase activity in human cancer and identify bortezomib as a novel epigenetic-targeting drug.
Introduction
Methylation of CpG islands in promoter region of genes is due to enzymatic addition of a methyl (CH3) group at the carbon 5 position of cytosine and has been shown to inhibit gene transcription.1 This enzymatic reaction is mediated by DNA methyltransferases (DNMTs: DNMT1, 3a, and 3b) that use s-adenosyl-methionine (SAM) as a methyl donor. While DNMT3a and 3b are important to establish novel methylation sites on nascent DNA, DNMT1 plays a critical housekeeping role in maintaining established patterns of DNA methylation in dividing cells.2 DNMTs have recently been found to be overexpressed in human acute myeloid leukemia (AML) and solid tumors, thereby supporting a role of these enzymes in the development and maintenance of the neoplastic phenotype.3,4 Inhibition of DNMT1 by antisense or shRNA oligonucleotides or nucleoside analogs (eg, 5-aza-2′-deoxycytidine [decitabine]) induces DNA hypomethylation and reactivation of hypermethylated tumor suppressor genes in leukemia cells.5,,,,,–11 This ultimately restores normal patterns of cell proliferation, differentiation, and apoptosis, which in turn leads to a significant antitumor activity.
To date, 2 hypomethylating nucleoside analogs (decitabine and 5-azacitidine, referred to hereafter as azanucleosides) have been approved by the FDA for the treatment of myelodysplastic syndromes (MDSs) and are currently in clinical trials for other types of cancers.12,13 An accepted mechanism for the antitumor activity of these agents is their incorporation into newly synthesized DNA strands followed by covalent binding, sequestration, and depletion of the DNMT enzymes.12,13 Clinical responses to azanucleosides, however, appear to be restricted to a minority of hematopoietic malignancies, which are characterized by a relatively high proliferative cell fraction.14 Thus, development of novel hypomethylating compounds with mechanisms of action distinct from azanucleosides may broaden the therapeutic toolbox targeting epigenetic aberrations in human cancer.
Recent studies suggest that DNMT1 expression is tightly regulated during normal cell growth, and its transcription is modulated by the Sp1 protein in mice.15 Sp1 is a ubiquitous zinc finger transcription factor that binds GC-rich cis-acting elements ((G/A)(G/A)GGCC(G/T)(G/A)(G/A)) in the promoter region of inducible genes.16 The complexity of the regulatory functions mediated by Sp1 can be explained by a variety of posttranslational modifications (ie, ubiquitination, glycosylation, phosphorylation) of this protein16,17 and/or its physical interaction with other transcription factors, such as those of the NF-κB family18,19 that are constitutively activated in AML and controlled by the proteasomal degradation.20,21
The 26S ubiquitin-proteasome, formed by the 20S core complex and the 19S regulatory particle, is present in both the cytoplasm and the nucleus of all eukaryotic cells22,,,,–27 and modulates both the levels and functions of many proteins involved in cell cycle progression, differentiation, apoptosis, and adhesion to the microenvironment.22,27,,,–31 The proteolytic activity of 26S proteasome also plays a key role in regulating gene transcription by modulating coactivator recruitment, RNA transcriptional elongation, and protein posttranslational modifications.32,33 Disorders in ubiquitin-dependent proteolysis appear to be integral to the neoplastic phenotype, and newly developed inhibitors of the proteasome system have been demonstrated to effectively induce apoptosis in malignant cells.34,,,–38
Bortezomib, the first proteasome inhibitor approved by the FDA for clinical use in multiple myeloma, is a dipeptidyl boronic acid that reversibly inhibits proteasome activity via formation of a pseudotetrahedral complex between the threonine amino-terminal group and the boronic acid pharmacophore.38 It has been shown to inhibit NF-κB activation by blocking the ubiquitination and proteasomal degradation of the endogenous NF-κB inhibitor I-κB.35,39 We postulated that exposure to bortezomib may cause disruption of the NF-κB and Sp1 interplay, leading in turn to DNMT1 down-regulation and DNA hypomethylation. Consistent with our hypothesis, we demonstrated here, for the first time, that bortezomib is a potent inhibitor of DNA methylation in malignant cells by interfering with Sp1/NF-κB DNA–binding activity, which in turn results in decreased DNMT1 expression, DNA hypomethylation, and transcription of methylation-silenced genes. These findings support bortezomib as a novel, nonazanucleoside therapeutic agent to target aberrant DNA hypermethylation in cancer.
Methods
Plasmid and cell lines
Construction of the human Sp1 in Epstein-Barr virus (EBV)/retroviral hybrid vector (Pinco-Sp1) was established as previously described.40 Retroviral infection to obtain Pinco-Sp1 or Pinco alone stably expressed in 293T cells was performed as previously reported.41,42 Two Sp1 shRNA constructs were obtained by cloning the 2 correspondent annealed oligos into a pSuper.retro.neo + GFP vector (OligoEngine, Seattle, WA).43,44 The sequences of the 2 oligos for Sp1 shRNA-1 were 5′-GATCCCCAAGTGTTTCGTGAGGAGTGTTCAAGAGACACTCCTCATGAAGCGCTTTTTTTA-3′ and 5′-AGCTTAAAAAAAGCGCTTCATGAGGAGTGTCTCTTGAACACTCCTCACGAAACACTTGGG-3′. The sequences of the 2 oligos for Sp1 shRNA-2 were 5′-GATCCCCTCATTCTATGGGTGAAATGTTCAAGAGACATTTCATCCATGGAGTGATTTTTA-3′ and 5′-AGCTTAAAAATCACTCCATGGATGAAATGTCTCTTGAACATTTCACCCATAGAATGAGGG-3′. Cell lines were grown in DMEM supplemented with 10% (293T, HCT116) fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) or in RPMI 1640 supplemented with 15% (Kasumi-1) or 10% (MV4-11, K562, ML-1) FBS (Invitrogen). Human bone marrow cells, which were obtained through the Ohio State Leukemia Tissue Bank from patients who gave informed consent in accordance with the Declaration of Helsinki on an IRB-approved protocol, were grown in RPMI 1640 supplemented with 15% human serum and granulocyte-macrophage colony-stimulating factor (GM-CSF) plus Cytokine Cocktail (R&D Systems, Minneapolis, MN).
Chemicals and antibodies
Bortezomib is commercially available from Millennium Pharmaceuticals (Cambridge, MA) and decitabine was purchased from Sigma-Aldrich (St Louis, MO). These compounds were dissolved in PBS sterilized by filtration through a 0.22-μm syringe filter and stored at −80°C. The antibodies used were as follows: anti-HDAC1 (Upstate Biotechnology, Billerica, MA); anti-Sp1, β-actin, and rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA); anti-DNMT1 (New England Biolabs, Beverly, MA); and NF-κB (p65) (Cell Signaling Technology, Danvers, MA).
Proteasome inhibition assay
MV4-11 cells were incubated with 60 nM bortezomib for the indicated time and stored at −80°C. A spectrofluorometric assay was used to assess the proteasome activity based on a previously described method.45
In vivo xenograft models
Athymic nu/nu mice were purchased from Charles River (Wilmington, MA). Four- to 6-week-old animals (n = 3 to 5) were injected with 107 MV4-11 or K562 cells subcutaneously. When tumor size approached approximately 50 mm3, the animals received a single dose (2 mg/kg) of bortezomib or vehicle alone (intravenous bolus). After 48 hours, the tumors were excised for protein, RNA expression, and DNA methylation analysis.
Cell transfection, immunoprecipitation, and Western blot
Sp1 shRNA constructs were introduced into leukemia cell line by Nucleofector kits (Amaxa, Gaithersburg, MD) according to the manufacturer's instruction. Whole cellular lysates were prepared by harvesting the cells in 1× cell lysis buffer (20 mM HEPES [pH 7.6], 150 mM NaCl, and 0.1% NP40 supplemented with 1 mM b-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, 1 mM NaF, 1 mM benzimedin, and protease inhibitors [protease inhibitor cocktail set III; Calbiochem-Novabiochem, San Diego, CA]). Approximately 200 μg nuclear extract or 1 mg total protein lysates was precleared with 70 μL of 50% slurry of protein A or G agarose beads (Upstate Biotechnology) for 2 hours at 4°C. Agarose beads (70 μL) were coated with 2 to 5 μg antibodies at 4°C overnight. Precleared protein extracts were incubated with antibody-coated agarose beads for an additional 2 hours at 4°C. Bound proteins were resolved by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Amersham, Piscataway, NJ). The protein blots were incubated with indicated antibodies, and signals were developed using a Chemiluminescent Detection kit (Amersham).
Quantification of global DNA methylation
Electrophoretic mobility-shift assays and antibody-supershift assays
Three pairs of oligonucleotides derived from the human DNMT1 promoter regions that contain putative Sp1-binding sites were chemically synthesized, and complementary oligos were annealed and labeled with 32P-dCTP and Klenow. All reactions were processed on ice except for those indicated. The oligo sequences used were as follows: DNMT1/Sp1–1F: 5′-GGGCTCCGCGTGGGGGGGGTGTGTGCCCGCCTTGCGC-3′; DNMT1/Sp1–1R: 5′-GCGCAAGGCGGGCACACACCCCCCCCAC-GCGGAG-3′; DNMT1/Sp1–2F: 5′-GGGCATGGCCGGCTCCGTTCCATCCTTC-3′; DNMT1/Sp1–2R: 5′-GAAGGATG GAACGGAGCCGGCCATG-5′; DNMT1/Sp1–3F: 5′-CCCGGACTGGGGTGGTAGA CGCCG-3′; DNMT1/Sp1–3R: 5′-GGGCGGCGTCTACCACCCCAGTCCGGG-3′. Total protein extracts were isolated from MV4-11 cells using M-PER (Mammalian Protein Extraction Reagent; Pierce, Rockford, IL), and nuclear extracts were prepared using NE-PER (Nuclear and Cytoplasmic Extraction Reagent; Pierce). Electrophoretic mobility-shift assays (EMSA) with total extracts or nuclear extracts and 32P-labeled DNMT1 promoter oligos were performed as previously described.48 For antibody-supershift assays, 2 μg antibodies (Sp1, NF-κB [p65]) or control rabbit IgG was added after the binding reactions had proceeded for 10 minutes, and further incubated for another 20 minutes before gel loading. Alternatively, nuclear extracts were preincubated with individual antibody at 4°C for 16 hours before adding to the binding reactions.
Quantitative real-time reverse-transcription–polymerase chain reaction
Quantitative real-time reverse-transcription–polymerase chain reaction (RT-PCR) for expression of DNMT1, Sp1, and RIL (primer and probes available upon request) was performed using 2 μg total RNA prepared with Trizol reagent and reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen). The comparative cycle threshold (CT) method was used to determine the expression levels of DNMT1, Sp1, and RIL relative to an internal control 18S, as previously reported.49
RIL gene-promoter methylation analysis
ML-1 cells were treated with bortezomib or decitabine for 24 or 48 hours and genomic DNA was extracted. DNA (1 μg) from these cells was digested by HpaII or BstuI for 2 hours. The digested DNA was cleaned by PCR purification kit (QIAGEN Science) and applied to PCR using primers specific for RIL gene promoter (primers available upon request). The cycle number and the amount of template were optimized to ensure assessment of amplicon levels within the linear range phase of the PCR. The PCR products were resolved on 1.0% agarose gel containing ethidium bromide and analyzed under UV light.
Results
Bortezomib inhibits the 26S ubiquitin-proteasome system and causes accumulation of polyubiquitinated proteins in AML cells
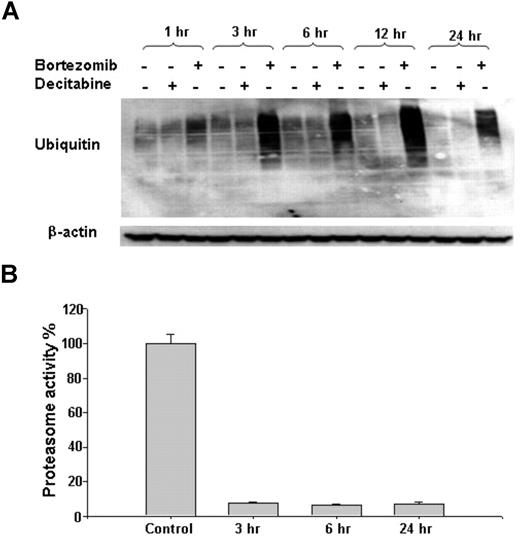
Bortezomib inhibits the 26S proteasome by binding to the chymotrypsin-like site in the 20S core complex. To test the proteasome inhibitory activity of this compound in human AML cells, we first assessed the levels of ubiquitinated proteins in MV4-11 cell line after incubation with bortezomib (60 nM) or decitabine (2.5 μM), as a negative control. We observed that bortezomib, not decitabine, blocked ubiquitination-mediated protein degradation, leading in turn to the accumulation of polyubiquitinated proteins (Figure 1A). Similar results were observed in other AML cell lines (ie, Kasumi-1 and K562) (data not shown). Consistent with increased levels of ubiquitinated proteins, exposure to 60 nM bortezomib for 3 or more hours resulted in approximately 90% reduction in 26S proteasome activity (Figure 1B). Of note, although cell viability decreased significantly at 72 hours, it was not affected before 12 hours and only partially decreased after 24-hour treatment with 20 nM or 60 nM bortezomib (data not shown), indicating that inhibition of proteasome activity precedes and, most likely, accounts for bortezomib-induced cell death.
Inhibitory effect of bortezomib on 26S proteasome system in AML cells. (A) Accumulation of polyubiquitinated proteins after bortezomib treatment. MV4-11 cells were treated with 60 nM bortezomib or 2.5 μM decitabine for the indicated time points. (B) Bortezomib (60 nM) exposure resulted in reduction of proteasome activity compared with untreated control. Error bars represent SD.
Inhibitory effect of bortezomib on 26S proteasome system in AML cells. (A) Accumulation of polyubiquitinated proteins after bortezomib treatment. MV4-11 cells were treated with 60 nM bortezomib or 2.5 μM decitabine for the indicated time points. (B) Bortezomib (60 nM) exposure resulted in reduction of proteasome activity compared with untreated control. Error bars represent SD.
Sp1 protein binds to the DNMT1 gene promoter in human cancer cells
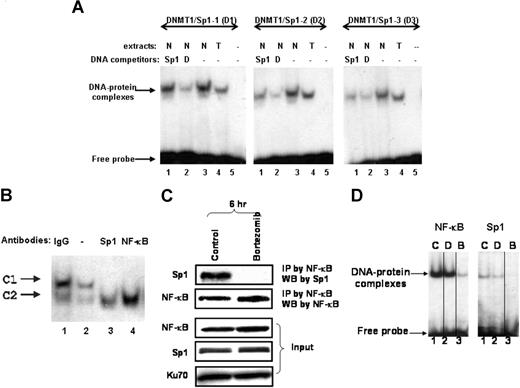
It has been reported that DNMT1 promoter activity is regulated by the Sp1 zinc finger protein in murine cells.15 Analysis of the human DNMT1 promoter identified 4 putative binding sites for the transcription factor Sp1 (data not shown). Three double-stranded DNMT1 promoter probes, which spanned different regions of the DNMT1 promoter containing Sp1-binding sites, were generated for DNA-protein interaction studies. In electrophoretic mobility shift assays (EMSAs) with nuclear (N) or total (T) lysates from MV4-11 cells, the use of all 3 probes yielded slower migrating DNA-protein complexes (Figure 2A lanes 3-4). The specificity of DNA binding was demonstrated by competition with 20-fold excess unlabeled DNMT1 promoter probes (Figure 2A lane 2). In addition, unlabeled DNA oligos containing the consensus Sp1-binding site efficiently competed away protein binding to 2 of 3 probes (ie, DNMT1-Sp1-1 and -2 probes), supporting the specific interaction of DNMT1 promoter with Sp1 protein (Figure 2A lane 1). These results indicated that Sp1 protein was enriched on DNMT1 gene promoter.
Sp1 binds to the DNMT1 promoter. (A) Sp1 bound to putative Sp1-binding sites on DNMT1 promoter. EMSA assay of MV4-11 total extracts (T) or nuclear extracts (N) was performed using 3 different 32P-labeled DNMT1 promoter probes: DNMT1-Sp1-1 (abbreviated as D1); DNMT1-Sp1-2 (D2); and DNMT1-Sp1-3 (D3). Unlabeled oligos containing the consensus binding sites for Sp1 (Sp1) or unlabeled DNMT1 promoter probes (D) were added for competition with the 32P-labeled probes, thereby testing for DNA-binding specificity. (B) Antibody competition assays. Nuclear lysates were preincubated with Sp1 and NF-κB (p65) antibodies before formation of protein-DNA complex, thereby testing for protein-binding specificity. (C) Sp1 was physically associated with NF-κB and this association was disrupted by bortezomib. MV4-11 cells were treated with 60 nM bortezomib for 6 hours, and nuclear extracts were then applied to immunoprecipitation with NF-κB (p65) antibody. The immunocomplex was blotted with Sp1 antibody (top panel). Input controls (bottom panels). (D) Bortezomib inhibited NF-κB and Sp1 protein–binding activity. EMSA was performed with nuclear extracts prepared from MV4-11 cells untreated or treated with decitabine or bortezomib for 24 hours. DNA-consensus sequences containing Sp1- or NF-κB–binding sites were used as probes, shown on the top of each panel. C indicates untreated; D, decitabine (2.5 μM); and B, bortezomib (60 nM). Vertical lines have been inserted to indicate repositioned gel lanes.
Sp1 binds to the DNMT1 promoter. (A) Sp1 bound to putative Sp1-binding sites on DNMT1 promoter. EMSA assay of MV4-11 total extracts (T) or nuclear extracts (N) was performed using 3 different 32P-labeled DNMT1 promoter probes: DNMT1-Sp1-1 (abbreviated as D1); DNMT1-Sp1-2 (D2); and DNMT1-Sp1-3 (D3). Unlabeled oligos containing the consensus binding sites for Sp1 (Sp1) or unlabeled DNMT1 promoter probes (D) were added for competition with the 32P-labeled probes, thereby testing for DNA-binding specificity. (B) Antibody competition assays. Nuclear lysates were preincubated with Sp1 and NF-κB (p65) antibodies before formation of protein-DNA complex, thereby testing for protein-binding specificity. (C) Sp1 was physically associated with NF-κB and this association was disrupted by bortezomib. MV4-11 cells were treated with 60 nM bortezomib for 6 hours, and nuclear extracts were then applied to immunoprecipitation with NF-κB (p65) antibody. The immunocomplex was blotted with Sp1 antibody (top panel). Input controls (bottom panels). (D) Bortezomib inhibited NF-κB and Sp1 protein–binding activity. EMSA was performed with nuclear extracts prepared from MV4-11 cells untreated or treated with decitabine or bortezomib for 24 hours. DNA-consensus sequences containing Sp1- or NF-κB–binding sites were used as probes, shown on the top of each panel. C indicates untreated; D, decitabine (2.5 μM); and B, bortezomib (60 nM). Vertical lines have been inserted to indicate repositioned gel lanes.
To demonstrate that Sp1 protein is present in the DNA-protein complexes formed between the MV4-11 lysates and the DNMT1 promoter probes, antibody-supershift assays were performed. Initial EMSA experiments with short (30 minutes) incubation of the binding reaction in the presence of anti-Sp1 antibody led to only a low amount of the complex being supershifted (not shown). Therefore, we next performed EMSA experiments with nuclear extracts preincubated with the anti-Sp1 antibody for 16 hours before adding the 32P-labeled DNMT1/Sp1 probe. Pretreatment with anti-Sp1 antibody (Figure 2B lane 3) completely abolished the formation of only one of the 2 detectable DNA-protein complexes (ie, named C1), supporting that the antibody interfered with the ability of Sp1 to bind the DNMT1 promoter. Our data, therefore, indicated that complex C1, but not complex C2, contained Sp1 protein. Furthermore, pretreatment with anti–NF-κB (p65) antibody (Figure 2B lane 4) also completely abrogated the formation of complex C1, supporting that NF-κB was part of the protein-binding complex in MV4-11 cell lysates and suggesting that NF-κB interplays with Sp1 for DNMT1 transactivation.
The physical interaction between Sp1 and NF-κB was further supported by the observation that Sp1 was coimmunoprecipitated with NF-κB (p65) in untreated MV4-11 cells (Figure 2C). In contrast, Sp1 and NF-κB did not coimmunoprecipitate in bortezomib-treated cells (Figure 2C), indicating that bortezomib disrupted this protein-protein interaction. Furthermore, bortezomib exposure significantly reduced the DNA-binding activity of both Sp1 and NF-κB, as shown in the EMSA experiments using consensus DNA-binding probes for these 2 proteins (Figure 2D lane B). In contrast, the DNA-protein complex remained intact in cells untreated (Figure 2D lane C) or treated (Figure 2D lane D) with decitabine.
Changes in Sp1 protein levels correlate with changes in DNMT1 transcription
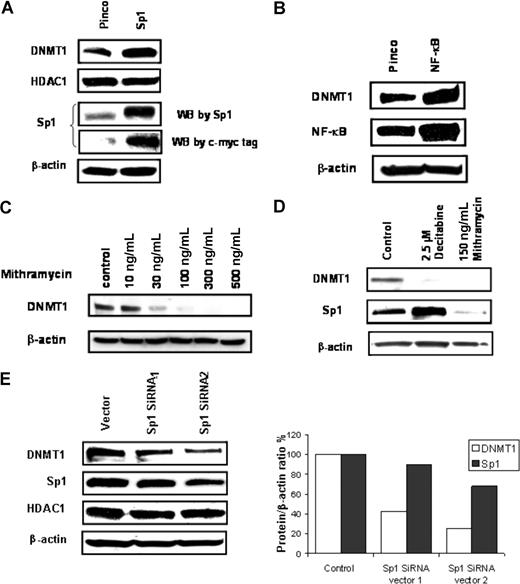
To further support the hypothesis that the Sp1/NF-κB complex coparticipates in DNMT1 transcriptional activation, the Sp1 gene was cloned into a viral vector (Pinco) and stable Sp1 expression in 293T cells was established. Increased levels of DNMT1 protein, but not histone deacetylase 1 (HDAC1), were observed in cells overexpressing Sp1 compared with cells transfected with the empty vector (Figure 3A). Notably, overexpression of NF-κB (p65) in 293T cells also augmented the expression of DNMT1 protein (Figure 3B). Treatment of MV4-11 cells with mithramycin A, an inhibitor of Sp1-binding activity,50 led to dose-dependent reduction of DNMT1 levels (Figure 3C). Moreover, both DNMT1 and Sp1 protein levels were decreased in mithramycin A–treated AML primary cells (Figure 3D). Finally, when Sp1 expression was knocked down using Sp1 shRNA, a concurrent decrease in DNMT1, but not HDAC1 (Figure 3E), was observed, thereby supporting a role of Sp1 protein in regulating DNMT1 expression.
Coregulation of DNMT1, Sp1, and NF-κB. (A) Western blot analysis of DNMT1 in 293T cells stably transfected with empty vector (Pinco) or Sp1-myc-tagged-Pinco. (B) Western blot analysis of DNMT1 expression in 293T cells transiently transfected with empty vector (Pinco) or NF-κB (p65)-Pinco. (C) Mithramycin A depleted Sp1 expression and down-regulated DNMT1 expression in MV4-11 cells. (D) Mithramycin A down-regulates both Sp1 and DNMT1 expression in AML primary cells. (E) Sp1 shRNA concurrently decreased Sp1 and DNMT1 proteins (left panel: immunoblot gel; right panel: quantification graph). MV4-11 cells were transfected with pSuper.retro.neo + GFP vector with DNA stuffer sequences (vector) or Sp1 shRNAs (1 or 2) and cultured for 2 weeks. Total cell lysates were subjected to Western blot for Sp1 and DNMT1.
Coregulation of DNMT1, Sp1, and NF-κB. (A) Western blot analysis of DNMT1 in 293T cells stably transfected with empty vector (Pinco) or Sp1-myc-tagged-Pinco. (B) Western blot analysis of DNMT1 expression in 293T cells transiently transfected with empty vector (Pinco) or NF-κB (p65)-Pinco. (C) Mithramycin A depleted Sp1 expression and down-regulated DNMT1 expression in MV4-11 cells. (D) Mithramycin A down-regulates both Sp1 and DNMT1 expression in AML primary cells. (E) Sp1 shRNA concurrently decreased Sp1 and DNMT1 proteins (left panel: immunoblot gel; right panel: quantification graph). MV4-11 cells were transfected with pSuper.retro.neo + GFP vector with DNA stuffer sequences (vector) or Sp1 shRNAs (1 or 2) and cultured for 2 weeks. Total cell lysates were subjected to Western blot for Sp1 and DNMT1.
Bortezomib-induced inhibition of the 26S proteasome represses Sp1 expression and abrogates its DNA-binding activity
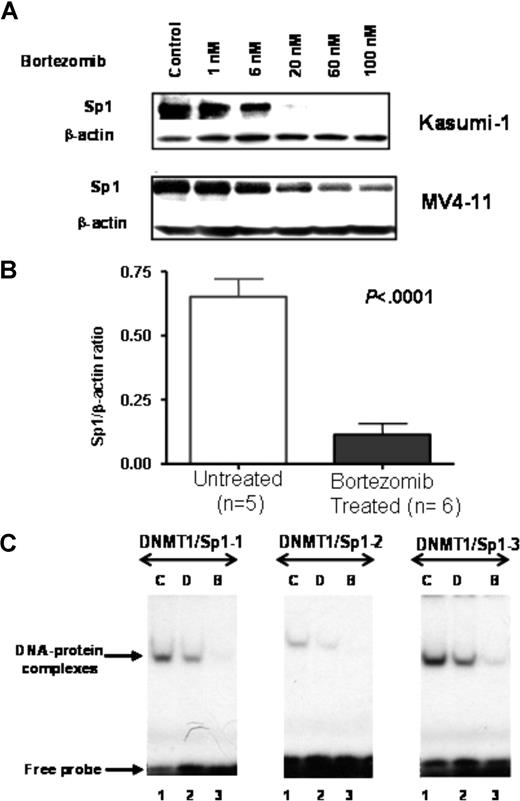
We next studied whether bortezomib-induced proteasome inhibition alters Sp1 protein expression and activity. MV4-11 and Kasumi-1 cells were exposed to bortezomib (0, 1, 6, 20, 60, and 100 nM) for 24 hours and Sp1 expression was examined. Bortezomib treatment induced a significant decrease in Sp1 protein level in a dose-dependent manner (Figure 4A). Importantly, when the activity of bortezomib was tested in vivo, we observed more than 80% down-regulation of Sp1 levels in tumors from MV4-11 xenografts following 48-hour treatment with a single dose (2 mg/kg) of bortezomib, compared with tumors from untreated controls (P < .001, pairwise t test; Figure 4B). No toxicity was observed in the treated animals.
Bortezomib inhibits Sp1 protein expression and function. (A) Reduced Sp1 protein expression in Kasumi-1 and MV4-11 cells treated with indicated dosage of bortezomib for 24 hours. (B) Reduced Sp1 protein level in MV4-11 xenografts following 48-hour exposure to a single dose (2 mg/kg) of bortezomib (intravenous bolus). Error bars represent SD. (C) Bortezomib abolished Sp1 and NF-κB binding to DNMT1 promoter. EMSA was performed with nuclear extracts prepared from MV4-11 cells untreated or treated with bortezomib or decitabine. The DNMT1/Sp1 probes used are shown on the top of each panel. C indicates untreated; D: decitabine (2.5 μM); and B: bortezomib (60 nM).
Bortezomib inhibits Sp1 protein expression and function. (A) Reduced Sp1 protein expression in Kasumi-1 and MV4-11 cells treated with indicated dosage of bortezomib for 24 hours. (B) Reduced Sp1 protein level in MV4-11 xenografts following 48-hour exposure to a single dose (2 mg/kg) of bortezomib (intravenous bolus). Error bars represent SD. (C) Bortezomib abolished Sp1 and NF-κB binding to DNMT1 promoter. EMSA was performed with nuclear extracts prepared from MV4-11 cells untreated or treated with bortezomib or decitabine. The DNMT1/Sp1 probes used are shown on the top of each panel. C indicates untreated; D: decitabine (2.5 μM); and B: bortezomib (60 nM).
To determine the effect of bortezomib on the Sp1 binding to the DNMT1 promoter, EMSAs were performed with nuclear extracts from MV4-11 cells treated with bortezomib (60 nM for 24 hours). As shown in Figure 4C, bortezomib decreased the Sp1 protein binding to the DNMT1 promoter, while no obvious alteration of the Sp1 DNA binding was observed in decitabine-treated cells. In addition, Western blot analysis on the same extracts showed that bortezomib decreased Sp1 protein expression (data not shown).
Bortezomib causes down-regulation of DNMT1 gene expression
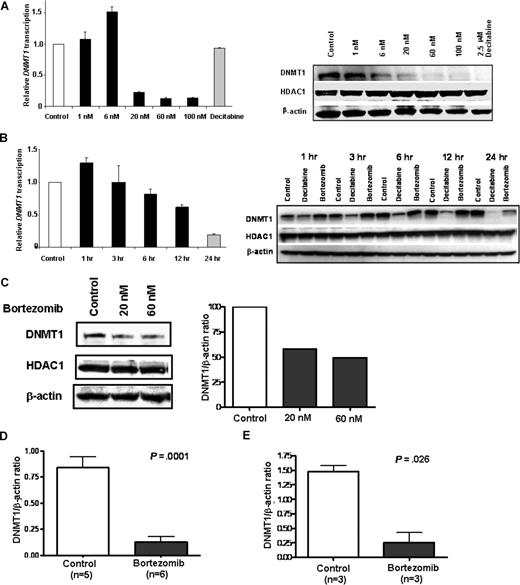
As Sp1 interacts with NF-κB to transactivate DNMT1, and bortezomib negatively affects Sp1 expression, Sp1/NF-κB physical interaction, and Sp1 binding to the DNMT1 gene promoter, we next postulated whether bortezomib down-regulates DNMT1 expression and induces DNA hypomethylation. MV4-11 cells were incubated with different concentrations of bortezomib (0, 1, 6, 20, 60, or 100 nM) for 24 hours, or with a fixed concentration (60 nM) for variable periods of time, and DNMT1 mRNA levels were measured by real-time RT-PCR. We observed that bortezomib treatment significantly reduced DNMT1 mRNA expression in a dose- and time-dependent fashion (Figure 5A,B left panels). To demonstrate whether a decrease in the DNMT1 protein corresponded to the lower levels of DNMT1 transcription induced by bortezomib, we examined protein lysate from bortezomib-treated MV4-11 cells by Western blot and observed that DNMT1 protein levels were significantly decreased in a dose- and time-dependent fashion (Figure 5A,B right panels). In contrast, we did not observe significant changes in the levels of control proteins (ie, HDAC1), supporting a specific activity of bortezomib on DNMT1 expression. DNMT1 mRNA and protein levels were also decreased in other bortezomib-treated hematopoietic and nonhematopoietic cell lines (ie, Kasumi-1, K562, THP-1, Jurkat, Eol-1, NB4, ML-1, and HCT116; data not shown).
Bortezomib treatment down-regulates DNMT1 expression in vitro and in vivo. (A) Bortezomib decreased DNMT1 mRNA transcription (left) and protein expression (right) in a dose-dependent manner. (B) Bortezomib decreased DNMT1 mRNA (left) and protein (right) in a time-dependent fashion. MV4-11 cells were incubated with different concentration of bortezomib (0, 1, 6, 20, 60, and 100 nM) for 24 hours or 60 nM bortezomib for indicated time periods. DNMT1 mRNA levels were examined by quantitative real-time RT-PCR (A,B left panel), and DNMT1 protein levels were detected by Western blot (A,B right panel). (C) Down-regulation of DNMT1 protein expression was observed in AML patient primary blasts treated ex vivo with bortezomib. Primary cells were exposed to 20 nM or 60 nM bortezomib for 48 hours, and the cell lysates were applied to Western blot for DNMT1 protein (left panel). Quantification of DNMT1 protein level normalized by β-actin is shown (right panel). (D,E) DNMT1 protein expression was repressed in tumors from MV4-11 (D) and K562 (E) xenograft mice following treatment with a single dose (2 mg/kg) of bortezomib (intravenous bolus). Error bars represent SD.
Bortezomib treatment down-regulates DNMT1 expression in vitro and in vivo. (A) Bortezomib decreased DNMT1 mRNA transcription (left) and protein expression (right) in a dose-dependent manner. (B) Bortezomib decreased DNMT1 mRNA (left) and protein (right) in a time-dependent fashion. MV4-11 cells were incubated with different concentration of bortezomib (0, 1, 6, 20, 60, and 100 nM) for 24 hours or 60 nM bortezomib for indicated time periods. DNMT1 mRNA levels were examined by quantitative real-time RT-PCR (A,B left panel), and DNMT1 protein levels were detected by Western blot (A,B right panel). (C) Down-regulation of DNMT1 protein expression was observed in AML patient primary blasts treated ex vivo with bortezomib. Primary cells were exposed to 20 nM or 60 nM bortezomib for 48 hours, and the cell lysates were applied to Western blot for DNMT1 protein (left panel). Quantification of DNMT1 protein level normalized by β-actin is shown (right panel). (D,E) DNMT1 protein expression was repressed in tumors from MV4-11 (D) and K562 (E) xenograft mice following treatment with a single dose (2 mg/kg) of bortezomib (intravenous bolus). Error bars represent SD.
To further explore the potential clinical relevance of our findings, primary bone marrow mononuclear cells from AML patients were cultured and treated in vitro with 20 nM or 60 nM bortezomib for 48 hours. Following treatment, whole cell extracts were analyzed for DNMT1 expression by Western blot. Similar to the effects observed in cell lines, bortezomib induced a reduction of the DNMT1 protein in AML primary cells compared with untreated controls, while no significant changes in HDAC1 were observed (Figure 5C).
The ability of bortezomib to down-regulate DNMT1 expression was also evaluated in vivo in nude mice engrafted with MV4-11 or K562 cells. The tumor-bearing mice were treated with a single injection of 2.0 mg/kg bortezomib or vehicle alone (intravenous bolus). The tumors were excised after 48 hours and analyzed by Western blot. DNMT1 protein levels were found significantly decreased in tumors of mice engrafted with MV4-11 (P = .001; pairwise t test) or K562 (P = .026; pairwise t test) cells and treated with bortezomib compared with untreated controls (Figure 5D,E). No significant alterations occurred in the levels of other proteins (ie, HDAC1), suggesting a specific activity of bortezomib on DNMT1 both in vitro and in vivo.
A decrease in DNMT1 transcription correlates with a decrease in global genomic DNA methylation in vitro and in vivo
Given the inhibitory effects of bortezomib on DNMT1 transcription, we next investigated whether this compound also induced DNA hypomethylation. MV4-11 cells were treated with 20 nM bortezomib for 24 hours. Genomic DNA was isolated and hydrolyzed and assessed for levels of global DNA methylation by LC-MS/MS.47 Approximately 50% reduction in global DNA methylation was observed in bortezomib-treated cells compared with untreated controls (Figure 6A). To test whether this effect could be reproduced in vivo, DNA samples from MV4-11 and K562 xenograft tumors were analyzed by LC-MS/MS.47 We observed DNA hypomethylation in the tumor cells from bortezomib-treated animals compared with untreated controls (Figure 6B,C). This effect correlated with the aforementioned changes in DNMT1 protein levels, suggesting that bortezomib acted as a hypomethylator both in vitro and in vivo through decreasing DNMT1 expression. Very similar results were observed in other leukemia cell lines (ie, Kasumi-1 and THP-1; data not shown). Other anticancer drugs with different mechanism of action, such as depsipeptide, trichostatin A (TSA), and 17-allylaminodemethoxygeldanamycin (17AAG), failed to induce global DNA hypomethylation, supporting a specific effect of bortezomib on cellular epigenetic profiles.
Bortezomib induces global DNA hypomethylation in vitro and in vivo. (A) Global DNA methylation was found to be decreased in MV4-11 cells treated in vitro with 20 nM bortezomib for 24 hours. (B,C) Global DNA hypomethylation was promoted in tumors from MV4-11(B) or K562 (C) xenograft mice following treatment with a single dose (2 mg/kg) of bortezomib (intravenous bolus). DNA (500 ng) was hydrolyzed and subjected to LC-MS/MS assay. Error bars represent SD.
Bortezomib induces global DNA hypomethylation in vitro and in vivo. (A) Global DNA methylation was found to be decreased in MV4-11 cells treated in vitro with 20 nM bortezomib for 24 hours. (B,C) Global DNA hypomethylation was promoted in tumors from MV4-11(B) or K562 (C) xenograft mice following treatment with a single dose (2 mg/kg) of bortezomib (intravenous bolus). DNA (500 ng) was hydrolyzed and subjected to LC-MS/MS assay. Error bars represent SD.
Bortezomib treatment increases expression of epigenetically silenced genes
A recent report showed that RIL, a LIM domain gene mapping to 5q31.1, is frequently methylated and silenced in several cancer and leukemia cell lines, and decitabine treatment restores RIL expression.51 Therefore, to assess whether bortezomib could reactivate methylation-silenced genes, we used HCT116 and ML-1 cells, in which the RIL gene is highly methylated.51 In both cell lines, RIL expression was significantly increased by bortezomib exposure (ie, ∼ 2-fold at 3 hours and > 5-fold at 12 hours in HCT116 cells [Figure 7A left panel] and ∼ 4-fold at 24 hours and > 15-fold at 48 hours in ML-1 cells [Figure 7A right panel]) compared with untreated controls. DNMT1 protein levels were concomitantly down-regulated by bortezomib in both cell lines (Figure 7B). To investigate the methylation status of RIL gene promoter after bortezomib treatment, genomic DNA from bortezomib-treated ML-1 cells was digested with the restriction enzyme HpaII cutting only nonmethylated sites or BstuI cutting only methylated sites. Following digestion, DNA was analyzed by RT-PCR using primers specific to the RIL gene promoter. Efficient digestion by HpaII or BstuI leads to weaker amplification bands in hypomethylated or hypermethylated RIL promoters, respectively, compared with undigested controls. Consistent with the re-expression data and similar to decitabine-treated controls, these data provide the evidence that the RIL gene promoter is hypomethylated in ML-1 cells following bortezomib treatment (Figure 7C).
Bortezomib induces re-expression of methylation-silenced genes via promoter hypomethylation. (A) Bortezomib increased expression of RIL gene reportedly to be methylated and down-regulated in HCT116 and ML-1 cells. HCT116 and ML-1 cells were treated with bortezomib for indicated time points and dosage; RIL gene expression was measured by real-time RT-PCR. Error bars represent SD. (B) Bortezomib induced DNMT1 down-regulation in HCT116 and ML-1 cells. HCT116 and ML-1 cells were treated with bortezomib, and Western blot was performed using antibodies against DNMT1 or HDAC1 (control). (C) Hypomethylation of RIL promoter by bortezomib treatment in ML-1 cells. DNA (1 μg) from bortezomib-treated or untreated ML-1 cells were digested by HpaII or BstuI, and PCR was performed using primers specific for RIL gene promoter. HpaII indicates no digestion, then hypermethylated; BstuI, no digestion, then hypomethylated.
Bortezomib induces re-expression of methylation-silenced genes via promoter hypomethylation. (A) Bortezomib increased expression of RIL gene reportedly to be methylated and down-regulated in HCT116 and ML-1 cells. HCT116 and ML-1 cells were treated with bortezomib for indicated time points and dosage; RIL gene expression was measured by real-time RT-PCR. Error bars represent SD. (B) Bortezomib induced DNMT1 down-regulation in HCT116 and ML-1 cells. HCT116 and ML-1 cells were treated with bortezomib, and Western blot was performed using antibodies against DNMT1 or HDAC1 (control). (C) Hypomethylation of RIL promoter by bortezomib treatment in ML-1 cells. DNA (1 μg) from bortezomib-treated or untreated ML-1 cells were digested by HpaII or BstuI, and PCR was performed using primers specific for RIL gene promoter. HpaII indicates no digestion, then hypermethylated; BstuI, no digestion, then hypomethylated.
Discussion
The ubiquitin-proteasome system regulates several fundamental cellular processes, such as cell-cycle progression, differentiation, and apoptosis.22 The proteasome inhibitor bortezomib inhibits the 26S proteasome machinery via the formation of a pseudotetrahedral complex and is postulated to influence gene expression by affecting the proteosome-mediated degradation of transcription factors such as NF-κB.22 Recent studies indicated that in the mouse DNMT1 is a target for the zinc finger protein Sp1,15 which also serves as a broad transcriptional coregulator with NF-κB.18,19 Since NF-κB is constitutively activated20,21 and DNMT1 is frequently overexpressed in AML cells,3,4 we hypothesized that epigenetic regulatory pathways dependent on DNMT1 in cancer cells could be modulated by bortezomib-induced inhibition of the 26S proteasome, possibly by interfering with Sp-1/NF-κB–mediated transactivation.22
To test our hypothesis, we first studied the effect of bortezomib treatment on Sp1. We found that inhibition of 26S proteasome by bortezomib leads to depletion of Sp1 in vitro and in vivo, disruption of the Sp1/NF-κB (p65) physical interaction, and inhibition of Sp1 DNA–binding activity. Notably, levels of NF-κB (p65) are not obviously altered by bortezomib treatment, although drug exposure affects the activity of this protein as supported by a decrease in DNA binding and disruption of the Sp1/NF-κB coimmunoprecipitation complex. It has previously been reported that the expression and functional activity of Sp1 is regulated by posttranscriptional modifications and at least in part by proteasomal degradation.16,17 To our knowledge, however, this is the first report that bortezomib treatment induces Sp1 depletion. Whether this is a direct result of bortezomib-mediated inhibition of the 26S proteasome or relates to indirect interference of this drug on specific phases of the cell cycle that are preferentially associated with Sp1 expression remains to be fully elucidated. Nevertheless, our data add another target to a long list of proteins whose changes in expression or activity mediate bortezomib antitumor effect.52
We next demonstrated that bortezomib induces a marked decrease in DNMT1 transcription, and this in turn leads to global DNA hypomethylation and re-expression of epigenetically silenced genes. Transactivation of DNMT1 by Sp1 has previously been reported. Kishikawa et al showed that Sp1 binds to the DNMT1 gene promoter and forced Sp1 expression in Drosophila SL2 cells, which lack the Sp family of transcription factors, enhances transcription of DNMT1 gene promoter.15 We reported here that in human cancer cells, DNMT1 is coregulated through both Sp1 and NF-κB (p65). Sp1 down-regulation by mithramycin A or shRNA leads to DNMT1 depletion via transcriptional inhibition, while forced expression of Sp1 and NF-κB (p65) in 293T cells results in DNMT1 up-regulation. As bortezomib reduces Sp1 expression levels, Sp1 and NF-κB physical interaction, and Sp1/NF-κB promoter–binding activity, we concluded that bortezomib acts as a DNA hypomethylator through interference with Sp1-mediated DNMT1 transactivation. However, inhibition of DNMT1 transcription by bortezomib could also occur through alternative mechanisms. It has been shown that the 26S complex has a broad impact on gene transcription by regulating levels of RNA polymerase II53 and its recruitment on transcriptionally activated genes.26,54 Moreover, inhibition of the 19S moiety and 20S complex decreases RNA elongation and termination, respectively.53 This suggests that interference with 26S complex activity by bortezomib could result in generalized transcriptional down-regulation. In our experiments, however, the effect of bortezomib on DNMT1 expression appeared specific, as levels of other proteins (ie, HDAC1) remain unchanged following drug exposure. Interestingly, a paradoxical increase in DNMT1 expression was observed in cells exposed to bortezomib at very low doses (ie, 1 and 6 nM) or for very short time (ie, 1 hour) (Figure 5A,B). Although this observation remains to be fully elucidated from a mechanistic standpoint, it is possible that at very low doses or short duration of bortezomib exposure, mechanisms that interfere with the hypomethylating effects of this drug might be activated (eg, transient increase in ubiquitinated, but active Sp1). Furthermore, these data underscore the importance for dose optimization and selection of optimal time points for correlative studies that intend to demonstrate the hypomethylation activity of bortezomib in upcoming clinical trials.
Bortezomib treatment ultimately caused global DNA hypomethylation, both in vitro and in vivo, and restored the expression of the RIL gene in HCT116 and ML-1 cells, where this gene is silenced via hypermethylation.51 Consistent with these results, we also showed that bortezomib induces hypomethylation of the RIL gene promoter. Our study suggests that bortezomib induces hypomethylation through pathways of DNMT1 depletion that are different from those of azanucleosides. Ghoshal et al showed that one of the possible hypomethylating mechanisms of the azanucleosides is DNMT1 depletion via covalent trapping of the protein on DNA followed by selective proteasomal degradation.55 In contrast, we showed that bortezomib as a single agent induces DNMT1 depletion by interfering with DNMT1 gene transactivation as supported by a decrease of DNMT1 mRNA in cells treated with bortezomib, and not decitabine (Figure 5A). Altogether, these data support bortezomib as an effective nonazanucleoside hypomethylator and a novel strategy for broadening epigenetic-targeting approaches to azanucleoside-resistant malignancies. Of note, the development of bortezomib as an epigenetic-targeting agent in human cancer will be facilitated by the fact that this is an already FDA-approved agent with a known and relatively benign toxicity profile.
Finally, we showed here that bortezomib has hypomethylating activity in AML, a disease where hypermethylation-mediated gene silencing is a well-characterized event and found to be a suitable target for therapeutic intervention. Cortes et al recently reported limited clinical responses in a phase 1 study of bortezomib as a single agent in patients with refractory or relapsed acute leukemia.56 Data from this study, however, should not discourage additional trials testing the hypomethylating activity and clinical effectiveness of bortezomib in AML. In fact, like decitabine, which is shown to be most effective as a hypomethylating agent when used at doses at least one log lower than those maximally tolerated, it is possible that to effectively elicit hypomethylating and anticancer activities of bortezomib in AML, treatment doses and schedules different from those currently recommended by the “package insert” of this commercially available drug may be required.
In summary, we have demonstrated that bortezomib causes down-regulation of DNMT1 via the Sp1/NF-κB pathway and induces global DNA hypomethylation in human leukemia cells, both in vitro and in vivo. Importantly, bortezomib appears to induce DNA hypomethylation with mechanisms different from those provided by currently available hypomethylating azanucleosides. Therefore, based on our results, we propose testing response to bortezomib in clinical trials rationally designed to target DNA hypomethylation and reverse expression of epigenetically silenced genes in cancer. Because of the difference in mechanisms of activity, it would not be unreasonable to test treatment with bortezomib in combination with azanucleosides for synergistic antileukemic activity in hematologic malignancies where myelotoxicity does not constitute dose-limiting toxicity (ie, acute leukemia and high-risk MDS).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Christopher J. Hickey for the technical support.
This work was supported in part by CA90469, CA102031, and CA095512 grants from the National Cancer Institute (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: S.L. designed all the experiments, performed bench work, and wrote the paper; Z.L. designed and performed the assays testing changes in the DNA methylation and wrote the paper; Z.X., J.P., L.H., T.V., and M.T. performed bench work and critically reviewed the paper; J.Y., R.B.K., R.A.B., P.P., R.G., and D.P. designed experiments and critically reviewed the paper; E.L. performed EMSA experiments and critically reviewed the paper; W.B., J.C.B., and M.A.C. provided patients' samples and critically reviewed the paper; K.K.C. designed the global methylation assay and critically reviewed paper; L.-C.W. designed EMSA experiments and critically reviewed the paper; G.M. designed all the experiments, wrote the paper, and provided administrative and financial support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guido Marcucci or Shujun Liu, The Ohio State University, 458A Starling-Loving Hall, 320 West 10th Ave, Columbus, Ohio 43210; e-mail: guido.marcucci@osumc.eduorshujun.liu@osumc.edu.
References
Author notes
S.L. and Z.L. contributed equally to this work.