Herbs have successfully been used in traditional Chinese medicine for centuries. However, their curative mechanisms remain largely unknown. In this study, we show that Wogonin, derived from the traditional Chinese medicine Huang-Qin (Scutellaria baicalensis Georgi), induces apoptosis in malignant T cells in vitro and suppresses growth of human T-cell leukemia xenografts in vivo. Importantly, Wogonin shows almost no toxicity on T lymphocytes from healthy donors. Wogonin induces prolonged activation of PLCγ1 via H2O2 signaling in malignant T cells, which leads to sustained elevation of cytosolic Ca2+ in malignant but not normal T cells. Subsequently, a Ca2+ overload leads to disruption of the mitochondrial membrane. The selective effect of Wogonin is due to its differential regulation of the redox status of malignant versus normal T cells. In addition, we show that the L-type voltage-dependent Ca2+ channels are involved in the intracellular Ca2+ mobilization in T cells. Furthermore, we show that malignant T cells possess elevated amounts of voltage-dependent Ca2+ channels compared with normal T cells, which further enhance the cytotoxicity of Wogonin for malignant T cells. Taken together, our data show a therapeutic potential of Wogonin for the treatment of hematologic malignancies.
Introduction
Induction of apoptosis in cancer cells is one of the strategies of anticancer therapy. Apoptotic cell death can be induced through the extrinsic or the intrinsic signaling pathways that are ultimately coupled to the activation of effector caspases.1,2 The extrinsic pathway involves ligation of death receptors followed by the formation of the death-inducing-signaling-complex (DISC) and activation of, for example, pro-caspase-8. Caspase-8 activates caspase-3, which cleaves target proteins leading to apoptosis. Intrinsic death stimuli directly or indirectly activate the mitochondrial pathway by inducing release of cytochrome c and formation of a cytosolic multiprotein complex, the apoptosome, composed of Apaf-1 and pro-caspase-9. Caspase-9 is activated at the apoptosome and, in turn, activates pro-caspase-3. This death pathway is largely controlled by the proapoptotic (eg, Bax, Bad, Bid, and Bak) and antiapoptotic (eg Bcl-2 and Bcl-xL) Bcl-2 family proteins.1,2 Caspase-8 may also induce cleavage of Bid, which induces the translocation of the proapoptotic Bcl-2 family proteins Bax and/or Bak to the mitochondrial membrane.3
Many stimuli, such as growth factor deprivation, ionizing radiation, and reactive oxygen species (ROS), may trigger the intrinsic death pathway. Recently, Ca2+ signals have been implicated to play an important role in regulation of cell death and survival.1 Certain apoptotic stimuli induce release of Ca2+ from stores in the endoplasmic reticulum (ER), which causes Ca2+ overload of the mitochondria leading to the release of cytochrome c as part of a stress response. ROS, such as ·O2− and its reduced product H2O2, have been considered as cytotoxic byproducts of cellular metabolism. Recent evidence indicates that H2O2 can also serve as a signaling molecule to modulate various physiologic functions, including mobilization of intracellular Ca2+ through activation of phospholipase Cγ1 (PLCγ1), a key enzyme involved in Ca2+ signaling.4,–6
Over the past decades, much effort has been invested into the search for agents that can differentially induce apoptotic death in cancer cells. In recent years, traditional Chinese herbal remedies have gradually gained considerable attention as a new source of anticancer drugs. Although their curative mechanisms are still largely unknown, some of the drugs have been used to treat cancer.7 Extracts of the radix of the traditional Chinese herb Huang-Qin (Scutellaria baicalensis Georgi) are among the most popular herbal remedies used in China and several oriental countries for clinical treatment of hyperlipemia, atherosclerosis, hypertension, dysentery, common cold, and inflammatory diseases, such as atopic dermatitis. Huang-Qin extracts show low toxicity in different animals (The grand dictionary of Chinese herbs, 1977). The active components of Huang-Qin are confirmed to be flavonoids. Wogonin (5,7-dihydroxy-8-methoxyflavone) is one of the major bioactive flavonoids of Scutellaria baicalensis Georgi, which has been shown to have antioxidant,8 antiviral,9 antithrombotic,10 and anti-inflammatory activities.11 Wogonin has also been shown to exert cytostatic and proapoptotic effects on several tumor cell lines.12,–14 However, the molecular mechanisms by which Wogonin exerts its proapoptotic effect on tumor cells are largely unknown. We have recently shown that Wogonin can sensitize resistant leukemia cells to tumor necrosis factorα- and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by differential regulation of the redox status in malignant versus normal lymphocytes.15 This prompted us to further investigate the therapeutic potential of Wogonin for anticancer treatment. In this study, we show that Wogonin selectively induces apoptosis in malignant but not in normal peripheral blood T cells. The selective effect of Wogonin is due to its selective activation of PLCγ1 via H2O2 signaling and consequently triggering intracellular Ca2+ release in malignant but not in nonmalignant T cells. Furthermore, we show that Wogonin significantly inhibits growth of xenografted human tumor cells in vivo in mice.
Methods
Cell lines and culture
The malignant T-cell lines CEM, Molt-4, DND-41, Jurkat J16, J16-neo, J16 bcl-2, Jurkat A3, Jurkat A3 deficient in FADD, Jurkat cells deficient in LAT (J-IATdef/J-CaM2), SLP76 (J-SLP76def/J14), PLCγ1 (PLCγ1def/J-γ1), and retransfected in LAT (J-LATretran/J-CaM2/LAT)), SLP76 (J-SLP76retran/J14–76-11), and PLCγ1 (J-PLCγ1retran/J-γ1/PLCγ1)16,–18 (all deficient cell lines purchased from American Type Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum, 50 μg/mL gentamicin (Invitrogen), 6 mM HEPES (Invitrogen), and 2 mM l-glutamine (Invitrogen) at 37°C and 5% CO2. The human T-cell clones K234 (kindly provided by Dr C. Falk, National Center of Tumor Diseases, Heidelberg, Germany), D894/25, and D596/6 (kindly provided by Dr O. Janssen, University Hospital of Kiel, Germany) were cultured as described previously.19
Preparation of human T cells from peripheral blood
Human peripheral T cells were prepared as described previously20 and were more than 90% CD3+. For activation, resting T cells (day 0) were cultured at 2 × 106 cells/mL with 1 μg/mL phytohemagglutinin (PHA) for 16 hours (day 1). Day 1 T cells were then washed 3 times and cultured for an additional 5 days in the presence of 25 U/mL interleukin-2 (IL-2; day 6).
Determination of mitochondrial membrane potential
To measure Δψm, Wogonin treated or untreated cells (5 × 105/mL) were incubated with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1, 5 μg/mL; FL-1; Invitrogen) for 20 minutes at room temperature in the dark followed by analysis on a flow cytometer (FACScan).
Determination of apoptosis
Cells were plated in triplicates and treated for the indicated periods of time at 37°C with different reagents: Wogonin (> 98% pure) (Wako Pure Chemical Industries, Osaka, Japan), zVAD-fmk (Bachem, Weil am Rhein, Germany), Nifedipin (Calbiochem, Schwalbach, Germany), Ru-360, and FK-506 (Calbiochem). Apoptotic cell death was examined by 2 parameters: forward scatter/side scatter (FSC/SSC) index of apoptotic-like change in cell size and granularity by FACScan and by analysis of DNA fragmentation according to the method of Nicoletti et al.21 Specific apoptosis was calculated as (percentage of experi-mental apoptosis − percentage of spontaneous apoptosis)/(100 − percentage of spontaneous apoptosis) × 100.
In vitro caspase activity assay
For a source of caspases, Jurkat J16 cells were lysed in 1% Triton X-100. The lysate was incubated for 30 minutes with 50 μM fluorigenic caspase substrate Ac-IETD-AFC, selective for caspase-8 and -10 (Alexis, Lausen, Switzerland), in a buffer containing 100 mM NaCl, 50 mM HEPES, pH 7.4, 0.1% CHAPS, 10 mM DTT, and 10% sucrose in the presence or absence of Wogonin. Samples were analyzed in duplicates on a fluorimetric plate reader (Wallac Victor, PerkinElmer Life and Analytical Sciences, Waltham, MA), and enzymatic activity was determined by calibrating against a slandered curve of free AFC (7-amino-4-trifluoromethylcoumarin). As positive control, a lysate from Jurkat cells, treated with anti-CD95 agonist antibody, was used.
H2O2 measurement
Cells were stained for 30 minutes with 5 μM of the H2O2-sensitive fluorescent dye dichlorofluorescein diacetate (DCFDA, FL-1) (Invitrogen) at 37°C in the dark, and then treated with Wogonin (50 μM) or αCD3 antibodies (plate-coated at 30 μg/mL) for indicated times and then washed 3 times with phosphate-buffered saline (PBS) and subsequently assayed by FACScan.
Analysis of PLCγ1 phosphorylation by flow cytometry
Jurkat cells unstimulated or stimulated with αCD3 (coated at 30 μg/mL), Wogonin (50 μM), or H2O2 (20 μM) were fixed by BD cytofix buffer (BD Biosciences, Heidelberg, Germany) for 10 minutes, then permeabilized in BD Phosflow PermBuffer III on ice for 30 minutes, stained with Alexa Fluor 488 antiphospho-PLCγ1 (pY783) antibody (BD Biosciences PharMingen, Heidelberg, Germany) and then analyzed by FACScan. The phosphorylated PLCγ1 was quantified as the increase in mean fluorescence intensity (MFI), calculated by the following formula: increase in MFI (%) = [(MFIstimulated − MFIunstimulated)/MFIunstimulated] × 100 as described.18
Determination of intracellular calcium
Cells were loaded with either 1 μM Fluo-4 (Invitrogen) for 30 minutes or 1 μM Indo-l (Invitrogen) for 60 minutes at 37°C in the dark, washed 3 times with PBS and resuspended in PBS. The loaded cells were measured by flow cytometry in a FACScan (BD Biosciences) after adding reagents: 1 μM ionomycine (Sigma-Aldrich, Taufkirchen, Germany) 100 μM (±)-Bay K 8644 (Calbiochem) or 5 to 50 μM Wogonin (Wako Pure Chemical Industries). Calcium influx was assayed for 300 to 512 seconds. In the inhibition experiments, nifedipine (Calbiochem) was dissolved in Me2SO as a stock solution of 200 mM and stored in the dark at −20°C. Cells were preincubated with 100 to 300 μM nifedipine or Me2SO without extracellular Ca2+ (in PBS) for 10 minutes. Cells were then stimulated with 0.5 μM of ionomycin to induce Ca2+ release.
Western blot analysis
A total of 106 cells were sedimented and lysed for 15 minutes in ice-cold lyses buffer (29 mM Tris-HCl, pH 7.4, 137 mM NaCl, 10% [w/v] glycerin, 1% [v/v] Triton X-100, 2 mM EDTA, 1 mM PMSF, 0.4 mM NaVa4, 10 mM NaF, complete protease inhibitor cocktail; Roche Diagnostics, Mannheim, Germany). After removing the cell debris by centrifugation at 16 200g for 15 minutes, equal amounts of proteins were separated on a 12% SDS-PAGE, blotted onto a nitrocellulose membrane (GE Healthcare, Little Chalfont, United Kingdom) and blocked with 5% nonfat drymilk in PBS/Tween (0.05% Tween-20 in PBS). The following antibodies were used: caspase-9 mAb (Santa Cruz Biotechnology, Santa Cruz, CA), caspase-3 polyclonal antibody (Cell Signaling Technology, Danvers, MA), phospho-Bad (7E11 mAb) and Bcl-xL (Transduction Laboratories, Heidelberg, Germany), Bax (SC-B9), and Bcl-2 (N-19) (Santa Cruz Biotechnology), and Tubulin (Sigma-Aldrich). The caspase-9 specific inhibitor z-LEHD-fmk was purchased from R&D system (Minneapolis, MN). For stripping, blots were incubated for 30 minutes in a buffer containing 62.5 mM Tris/HCl, pH 6.8, 2% SDS, and 100 mM β-mercaptoethanol at 56°C. The blots were washed 6 times for 10 minutes in PBS/Tween and blocked again in 5% nonfat drymilk.
In vivo mouse studies
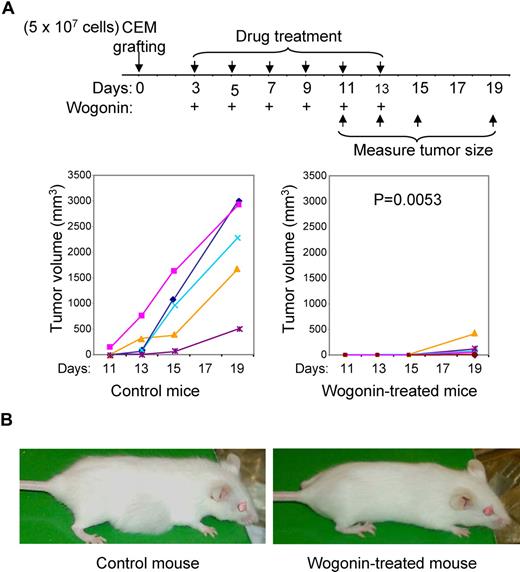
Immunodeficient mice (H-2qRag−/−γc−/−) were implanted subcutaneously in the right dorsal flank region with CEM (5 × 107 cells). Three days after xenografting, Wogonin (200 mg/kg body weight, dissolved in DMSO and diluted in olive oil) was administered by intraperitoneal injection as indicated in Figure 6A. The control group was treated in an analogous manner with the vehicle. The tumor size was measured with a micrometer caliper at the indicated times and the tumor volume (V) was calculated by the formula V = (a2 × b)/2, where “a” is the width and “b” is the length in millimeters.22 All protocols using and maintaining animals were approved by the German Animal Protection Authority (Office Regierungspräsidium Karlsruhe, Karlsruhe, Germany). Treated and control animals were compared for differences in tumor growth after end of treatment using the nonparametric method of Koziol et al in a one-sided statistical test at the significance level of 0.05.23
Results
Wogonin selectively induces apoptosis in malignant versus normal T cells
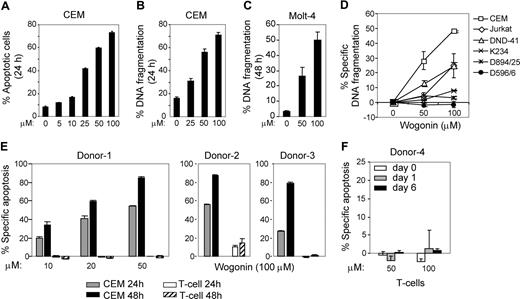
To investigate the effect of Wogonin on malignant cells, we used different human leukemia T-cell lines as a model system. At concentrations of 25 to 100 μM, Wogonin induces 40% to 70% of the human leukemia CEM T cells to undergo apoptotic-like changes in cell size and granularity within 24 hours (Figure 1A) accompanied by DNA fragmentation (Figure 1B). In addition, Wogonin induces apoptosis in leukemic T-cell lines Molt-4, Jurkat, and a cell line DND-41, which contains a p53 mutation (Figure 1C,D). In contrast to malignant T-cell lines, Wogonin showed almost no toxicity on normal T-cell clones (Figure 1D). Because most drugs used in clinical practice as anticancer agents so far have a broad, nonspecific spectrum of activity. Therefore, we further tested whether Wogonin exerts differential effects on the viability of tumor versus normal T lymphocytes. CEM cells and primary T cells isolated from peripheral blood of healthy donors were treated with Wogonin in parallel. At concentrations of approximately 50 to 100 μM, Wogonin showed no toxicity to normal peripheral blood T cells (Figure 1E). Freshly isolated (resting) peripheral blood T cells are known to be resistant toward activation-induced cell death, whereas T cells activated for several days in culture become sensitive toward activation-induced cell death.20 To investigate whether T cells at different activation stages show different susceptibilities to Wogonin, peripheral blood T cells freshly isolated (day 0), 16 hours PHA-activated (day 1), or PHA-activated and further cultured for 5 days (day 6) in IL-2–containing medium were subjected to Wogonin treatment. In all cases, no (50 μM) or a very low (100 μM) toxicity was seen when the cells were treated with Wogonin (Figure 1F). Thus, Wogonin can selectively induce apoptosis in malignant T cells.
Wogonin exclusively induces apoptosis in malignant and not in normal T cells. (A,B) Wogonin induces a dose-dependent increase in apoptotic cell death in CEM leukemia T cells. CEM cells were incubated with different doses of Wogonin for 24 hours. Apoptotic changes in cell size and granularity were quantified by a decrease in FSC/SSC (A), and the apoptotic cell death was analyzed by FACS for DNA fragmentation (B). Results are representative of 4 separate experiments, and each experiment was carried out in triplicate. (C) The leukemic T-cell line Molt-4 was treated by different doses of Wogonin, and apoptotic cell death was determined by fluorescence-activated cell sorting (FACS) for DNA fragmentation. Results are representative of 2 separate experiments done in triplicate. (D) Leukemic T-cell lines CEM, Jurkat, and DND-41 and normal T-cell clones K234, D894/25, and D596/6 were treated with different doses of Wogonin for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Results are representative of 2 separate experiments done in triplicate. (E) CEM and normal T cells freshly isolated from peripheral blood of 3 representative healthy donors were treated with 10 to 50 μM (left panel) or 100 μM (right panel) of Wogonin for 24 and 48 hours. (F) Resistance of nonmalignant T cells to Wogonin-induced apoptosis is not dependent on the activation stages of T cells. Freshly isolated peripheral blood T cells (day 0), 16 hours PHA-activated (day 1), or PHA-activated and further cultured in the presence of IL-2 for 5 days (day 6) were treated with Wogonin. Results are representative of 3 donors analyzed in duplicate. Error bars represent SD.
Wogonin exclusively induces apoptosis in malignant and not in normal T cells. (A,B) Wogonin induces a dose-dependent increase in apoptotic cell death in CEM leukemia T cells. CEM cells were incubated with different doses of Wogonin for 24 hours. Apoptotic changes in cell size and granularity were quantified by a decrease in FSC/SSC (A), and the apoptotic cell death was analyzed by FACS for DNA fragmentation (B). Results are representative of 4 separate experiments, and each experiment was carried out in triplicate. (C) The leukemic T-cell line Molt-4 was treated by different doses of Wogonin, and apoptotic cell death was determined by fluorescence-activated cell sorting (FACS) for DNA fragmentation. Results are representative of 2 separate experiments done in triplicate. (D) Leukemic T-cell lines CEM, Jurkat, and DND-41 and normal T-cell clones K234, D894/25, and D596/6 were treated with different doses of Wogonin for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Results are representative of 2 separate experiments done in triplicate. (E) CEM and normal T cells freshly isolated from peripheral blood of 3 representative healthy donors were treated with 10 to 50 μM (left panel) or 100 μM (right panel) of Wogonin for 24 and 48 hours. (F) Resistance of nonmalignant T cells to Wogonin-induced apoptosis is not dependent on the activation stages of T cells. Freshly isolated peripheral blood T cells (day 0), 16 hours PHA-activated (day 1), or PHA-activated and further cultured in the presence of IL-2 for 5 days (day 6) were treated with Wogonin. Results are representative of 3 donors analyzed in duplicate. Error bars represent SD.
Wogonin induces apoptosis through the intrinsic death pathway
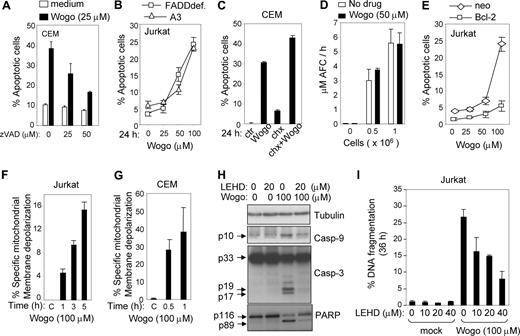
Wogonin-induced cell death can be inhibited by the pan-caspase inhibitor zVAD-fmk indicating involvement of a caspase-dependent apoptotic process (Figure 2A). To investigate whether a receptor-mediated death pathway24,25 is involved in Wogonin-induced apoptosis, Jurkat T cells deficient of the adapter molecule FADD (FADDdef) (Fas/Apo-1–associated death domain protein) were treated with different doses of Wogonin. The experiment showed that Jurkat FADDdef cells were as sensitive to Wogonin as the parental cells (Figure 2B). Treatment with cycloheximide had no inhibitory effect on Wogonin-induced cell death, indicating that Wogonin-mediated apoptosis does not require newly synthesized proteins (Figure 2C). Wogonin itself does not influence caspase-8 activity directly, as shown in an in vitro caspase-8 activity assay (Figure 2D). These results indicate that Wogonin-induced apoptotic cell death is unlikely to be regulated through the extrinsic (receptor-mediated) pathway. In contrast, Jurkat cells stably expressing the antiapoptotic protein Bcl-2 were resistant to Wogonin-induced apoptosis (Figure 2E). On Wogonin treatment, depolarization of mitochondrial membrane potential was observed in both Jurkat (Figure 2F) and CEM cells (Figure 2G). Caspase-9 and caspase-3 were activated on Wogonin treatment, and their activation was blocked by the caspase-9 specific inhibitor z-LEHD-fmk (Figure 2H). In addition, z-LEHD-fmk largely inhibited the cleavage of the caspase-3 target protein poly (ADP-ribose) polymerase (Figure 2H) and prevented apoptosis induced by Wogonin (Figure 2I). These data strongly suggest that Wogonin-induced apoptotic cell death is mainly mediated through the intrinsic (mitochondrial) pathway.
Wogonin-induced apoptosis is caspase-dependent but death receptor–independent. (A) Wogonin-induced apoptosis involves caspases. CEM cells were treated with 25 μM Wogonin in the presence or absence of different doses of the pan-caspase inhibitor zVAD-fmk for 24 hours. Apoptotic cells were analyzed by FSC/SSC. (B) The death receptor system is not required for Wogonin-induced apoptosis. FADD-deficient (FADDdef) and parental (A3) Jurkat cells were treated with different doses of Wogonin for 24 hours. (C) Wogonin-induced apoptosis does not require newly synthesized proteins. CEM cells were treated with 25 μM Wogonin in the presence or absence of cycloheximide (chx) (1 μg/mL). (D) Wogonin does not activate caspase-8 in vitro. Cell lysates from Jurkat cells were subjected to an in vitro caspase activity assay with or without 50 μM Wogonin. Results are the average of 2 assays in triplicate measurements. (E) Overexpression of Bcl-2 prevents Wogonin-induced apoptosis. Jurkat cells stably expressing Bcl-2 and control Jurkat cells (neo) were treated with different doses of Wogonin for 24 hours. Apoptotic cells were analyzed by FSC/SSC in triplicates. (F,G) Wogonin induces depolarization of the mitochondrial membrane potential. Jurkat and CEM cells were treated with 100 μM Wogonin for different times as indicated, and the mitochondrial membrane potential was determined by FACS. (H) Wogonin induces activation of caspase-9 and -3. Jurkat T cells were treated with 100 μM Wogonin for 8 hours in the absence or presence of 20 μg/mL caspase-9 inhibitor z-LEHD-fmk. Cell lysates were subjected to Western blot analysis with specific antibodies as indicated. (I) Jurkat T cells were treated with 100 μM Wogonin for 36 hours in the absence or presence of different amounts of z-LEHD-fmk. Apoptotic cell death was determined by FACS for DNA fragmentation. Error bars represent SD.
Wogonin-induced apoptosis is caspase-dependent but death receptor–independent. (A) Wogonin-induced apoptosis involves caspases. CEM cells were treated with 25 μM Wogonin in the presence or absence of different doses of the pan-caspase inhibitor zVAD-fmk for 24 hours. Apoptotic cells were analyzed by FSC/SSC. (B) The death receptor system is not required for Wogonin-induced apoptosis. FADD-deficient (FADDdef) and parental (A3) Jurkat cells were treated with different doses of Wogonin for 24 hours. (C) Wogonin-induced apoptosis does not require newly synthesized proteins. CEM cells were treated with 25 μM Wogonin in the presence or absence of cycloheximide (chx) (1 μg/mL). (D) Wogonin does not activate caspase-8 in vitro. Cell lysates from Jurkat cells were subjected to an in vitro caspase activity assay with or without 50 μM Wogonin. Results are the average of 2 assays in triplicate measurements. (E) Overexpression of Bcl-2 prevents Wogonin-induced apoptosis. Jurkat cells stably expressing Bcl-2 and control Jurkat cells (neo) were treated with different doses of Wogonin for 24 hours. Apoptotic cells were analyzed by FSC/SSC in triplicates. (F,G) Wogonin induces depolarization of the mitochondrial membrane potential. Jurkat and CEM cells were treated with 100 μM Wogonin for different times as indicated, and the mitochondrial membrane potential was determined by FACS. (H) Wogonin induces activation of caspase-9 and -3. Jurkat T cells were treated with 100 μM Wogonin for 8 hours in the absence or presence of 20 μg/mL caspase-9 inhibitor z-LEHD-fmk. Cell lysates were subjected to Western blot analysis with specific antibodies as indicated. (I) Jurkat T cells were treated with 100 μM Wogonin for 36 hours in the absence or presence of different amounts of z-LEHD-fmk. Apoptotic cell death was determined by FACS for DNA fragmentation. Error bars represent SD.
Wogonin causes a sustained cytosolic Ca2+ overload
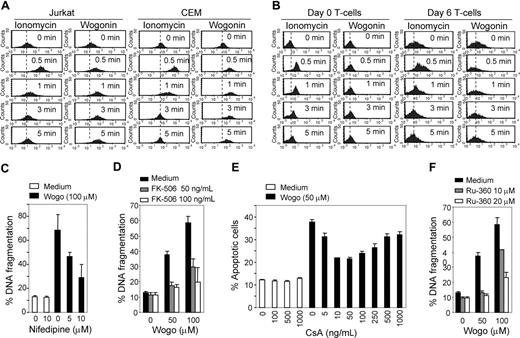
Mitochondria are the major site of ROS production. Accumulation of ROS may lead to initiation of apoptosis.26 However, Wogonin is a ROS scavenger, and we have previously shown that ROS is not involved in Wogonin-induced apoptosis.15 Recently, growing evidence demonstrates that Ca2+ may play a central role in regulation of cell death.1,27 Triggering release of intracellular Ca2+ stores and activation of the Ca2+ pathway are sufficient to induce apoptosis.28,29 Bcl-2 can increase the capacity of mitochondria to store Ca2+ and to buffer the Ca2+ influx into the cytosol.29,30 To investigate whether Wogonin has a direct effect on intracellular Ca2+ homeostasis, CEM, Jurkat, and normal peripheral blood T cells were loaded with a Ca2+ indicator dye and then treated with Wogonin in the absence of extracelluar Ca2+. Interestingly, a rise in cytosolic Ca2+ concentration was seen in malignant but not in (neither resting nor 6 day-activated) T cells isolated from healthy donors on Wogonin treatment (Figure 3A,B). As a control, ionomycin, a Ca2+ stimulus commonly used in combination with PMA to mimic antigen signals for T-cell activation, was used throughout the experiment. In contrast to Wogonin, ionomycin stimulation was shown to increase cytosolic Ca2+ in both malignant and normal T cells. Besides, ionomycin only triggered a transient elevation of Ca2+, which stopped after approximately 2 minutes in all cell types tested (Figure 3A,B). Cells treated with ionomycin alone did not undergo apoptosis (data not shown). In comparison, a prolonged increase in cytosolic Ca2+ occurred in malignant Jurkat and CEM T cells on Wogonin treatment (Figure 3A). These results indicate that Wogonin selectively induces apoptosis by exclusive induction of Ca2+ release from intracellular stores into the cytosol in malignant T cells. To confirm that the Wogonin-induced loss of intracellular Ca2+ homeostasis plays a crucial role in cell death, we carried out experiments with a Ca2+ channel antagonist, nifedipine.31 In the presence of nifedipine, Wogonin-induced apoptotic cell death was reduced by approximately 50% (Figure 3C). In addition, inhibition of apoptotic cell death induced by Wogonin was observed in the presence of the calcineurin inhibitor FK506 (Figure 3D). Reduced apoptosis was also observed in cells treated with Wogonin in the presence of cyclosporine A (CsA)32 (Figure 3E). These data demonstrate that Wogonin-induced apoptotic killing involves a Ca2+-sensitive mechanism.
Ca2+ is involved in Wogonin-induced apoptosis. (A,B) Wogonin mobilizes intracellular Ca2+ in malignant but not in normal T cells. Jurkat, CEM, naive (day 0), and effector (day 6) normal T cells were treated in PBS with either 0.5 μM ionomycin or 50 μM Wogonin as indicated. The intracellular Ca2+ release was monitored by FACS. Results are representative of 3 independent experiments. (C) The Ca2+ influx antagonist Nifedipine prevents Wogonin-induced apoptosis. CEM cells were treated with 100 μM Wogonin in the presence or absence of different amounts of nifedipine (added 2 hours before Wogonin treatment) for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Results are representative of 3 independent experiments in triplicate measurements. (D) The calcineurin inhibitor FK506 inhibits Wogonin-induced apoptosis. CEM cells were treated with 50 to 100 μM Wogonin in the presence or absence of different amounts of FK506 (added 2 hours before Wogonin treatment) for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Results are representative of 2 independent experiments in triplicate measurements. (E) CEM cells were treated with 50 μM Wogonin in the presence or absence of different concentrations of CsA (preincubated for 2 hours before Wogonin treatment) for 24 hours. Apoptotic cell death was analyzed by a decrease in FSC/SSC. Results are representative of 3 independent experiments. (F) Effects of the mitochondrial uniporter antagonist RU-360 on Wogonin-induced apoptosis. CEM cells were treated with Wogonin in the presence or absence of RU-306 for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Error bars represent SD.
Ca2+ is involved in Wogonin-induced apoptosis. (A,B) Wogonin mobilizes intracellular Ca2+ in malignant but not in normal T cells. Jurkat, CEM, naive (day 0), and effector (day 6) normal T cells were treated in PBS with either 0.5 μM ionomycin or 50 μM Wogonin as indicated. The intracellular Ca2+ release was monitored by FACS. Results are representative of 3 independent experiments. (C) The Ca2+ influx antagonist Nifedipine prevents Wogonin-induced apoptosis. CEM cells were treated with 100 μM Wogonin in the presence or absence of different amounts of nifedipine (added 2 hours before Wogonin treatment) for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Results are representative of 3 independent experiments in triplicate measurements. (D) The calcineurin inhibitor FK506 inhibits Wogonin-induced apoptosis. CEM cells were treated with 50 to 100 μM Wogonin in the presence or absence of different amounts of FK506 (added 2 hours before Wogonin treatment) for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Results are representative of 2 independent experiments in triplicate measurements. (E) CEM cells were treated with 50 μM Wogonin in the presence or absence of different concentrations of CsA (preincubated for 2 hours before Wogonin treatment) for 24 hours. Apoptotic cell death was analyzed by a decrease in FSC/SSC. Results are representative of 3 independent experiments. (F) Effects of the mitochondrial uniporter antagonist RU-360 on Wogonin-induced apoptosis. CEM cells were treated with Wogonin in the presence or absence of RU-306 for 24 hours. Apoptotic cell death was analyzed by FACS for DNA fragmentation. Error bars represent SD.
Several studies have also shown that elevation of cytosolic Ca2+ can lead to rapid Ca2+ accumulation within mitochondria. Ca2+ overload can rupture the mitochondrial membrane, leading to release of cytochrome c and to DNA fragmentation.33,34 Inhibition of mitochondrial Ca2+ uptake by an inhibitor (RU-360) of the mitochondrial uniporter can attenuate apoptotic cell death.35 Therefore, we carried out experiments with RU-360. In the presence of RU-360, Wogonin-induced apoptosis was down-regulated in a dose-dependent manner, suggesting that disruption of the mitochondrial membrane via Ca2+ overload may be also involved in Wogonin-induced apoptosis (Figure 3F).
PLCγ1 is essential for Wogonin-induced cell death
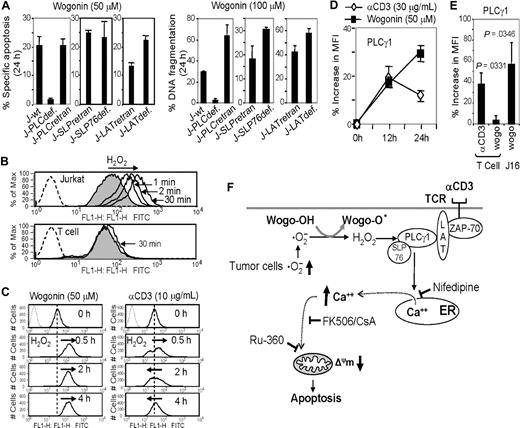
PLCγ1 is a key enzyme that contributes to T-cell antigen receptor (TCR)–mediated signaling via triggering Ca2+ release from ER stores.16 Therefore, we speculated that PLCγ1 might be involved in Wogonin-mediated apoptosis. To investigate this assumption, the wild-type (J-wt), PLCγ1-deficient (J-PLCγ1def), and PLCγ1-reconstituted (J-PLCγ1recon) Jurkat cells were subjected to Wogonin treatment. As expected, Jurkat cells deficient in PLCγ1 were resistant to Wogonin-induced apoptosis (Figure 4A). The sensitivity of J-PLCγ1def cells to Wogonin-mediated apoptosis was restored after PLCγ1 was reconstituted in these cells (Figure 4A). On normal T cell activation via TCR ligation, linker for activation of T cell (LAT) is phosphorylated and SRC homology-2(SH2)-domain-containing leukocyte protein of 76 kDa (SLP76) is recruited to LAT. LAT and SLP76 serve as a scaffold for PLCγ1.36 In contrast to PLCγ1, Jurkat cells deficient in the T-cell adapter proteins LAT (J-LATdef) or SLP56 (J-SLP76def) were still sensitive to Wogonin treatment (Figure 4A). These data demonstrates that PLCγ1 is essential for death signaling induced by Wogonin.
PLCγ1 is required for Wogonin-induced apoptosis in malignant T cells. (A) PLCγ1def Jurkat cells are resistant to Wogonin-induced apoptosis. Jurkat deficient either in PLCγ1 (J- PLCγ1def), SLP76 (J-SLP76def), or LAT (J-LATdef) together with the retransfected J-PLCγ1retran, J-SLP76retran, and J-LATretran cells were subjected to Wogonin treatment. Apoptotic cells were determined by either a decrease in fetal calf serum/SSC (left panel) or DNA fragmentation (right panel). Results are representative of 3 or 4 independent experiments. Error bars represent SD. (B) Wogonin generates rapid and strong H2O2 signals in malignant T cells. Jurkat and normal (day 6) T cells were treated with Wogonin (50 μM) for the indicated times. The H2O2 products were monitored by oxidation-sensitive fluorescent dye by FACS. (C) Wogonin generates long-lasting H2O2 signals compared with αCD3 stimulation. Jurkat cells were stimulated with either Wogonin (50 μM) or αCD3 (coated at 30 μg/mL) for the different times as indicated. The H2O2 products were monitored by FACS. (D) Wogonin induces phosphorylation of PLCγ1. Jurkat cells were stimulated with either αCD3 (coated at 30μg/mL) or Wogonin (50 μM) for indicated times. The expression levels of activated PLCγ1 were analyzed by FACS with antiphospho-PLCγ1 antibodies. (E) Wogonin induces stronger activation of PLCγ1 in malignant than in normal T cells (P = .035, n = 4). Jurkat and normal T cells isolated from peripheral blood of healthy donors were treated with Wogonin (50 μM) for 24 hours. In parallel, T cells activated by αCD3 for 24 hours were used as a positive control to demonstrate that the T cells used were capable to express activated PLCγ1. The expression levels of phosphor-PLCγ1 were analyzed as in panel D). Error bars represent SD. (F) Schematic summary of the discovered mechanism by which Wogonin induces malignant T cells to undergo apoptosis. Tumor cells produce ROS at elevated rates and Wogonin shows stronger and sustained generation of H2O2 in the malignant but not in normal T cells. H2O2 in turn serves as a signaling molecule to activate PLCγ1 and consequently induces Ca2+ release from intracellular stores. Cytosolic Ca2+ overload leads to disrupt the mitochondrial membrane. Nifedipine, FK-506, CsA, and Ru-360 were shown to block the Wogonin-induced apoptosis pathway at different levels.
PLCγ1 is required for Wogonin-induced apoptosis in malignant T cells. (A) PLCγ1def Jurkat cells are resistant to Wogonin-induced apoptosis. Jurkat deficient either in PLCγ1 (J- PLCγ1def), SLP76 (J-SLP76def), or LAT (J-LATdef) together with the retransfected J-PLCγ1retran, J-SLP76retran, and J-LATretran cells were subjected to Wogonin treatment. Apoptotic cells were determined by either a decrease in fetal calf serum/SSC (left panel) or DNA fragmentation (right panel). Results are representative of 3 or 4 independent experiments. Error bars represent SD. (B) Wogonin generates rapid and strong H2O2 signals in malignant T cells. Jurkat and normal (day 6) T cells were treated with Wogonin (50 μM) for the indicated times. The H2O2 products were monitored by oxidation-sensitive fluorescent dye by FACS. (C) Wogonin generates long-lasting H2O2 signals compared with αCD3 stimulation. Jurkat cells were stimulated with either Wogonin (50 μM) or αCD3 (coated at 30 μg/mL) for the different times as indicated. The H2O2 products were monitored by FACS. (D) Wogonin induces phosphorylation of PLCγ1. Jurkat cells were stimulated with either αCD3 (coated at 30μg/mL) or Wogonin (50 μM) for indicated times. The expression levels of activated PLCγ1 were analyzed by FACS with antiphospho-PLCγ1 antibodies. (E) Wogonin induces stronger activation of PLCγ1 in malignant than in normal T cells (P = .035, n = 4). Jurkat and normal T cells isolated from peripheral blood of healthy donors were treated with Wogonin (50 μM) for 24 hours. In parallel, T cells activated by αCD3 for 24 hours were used as a positive control to demonstrate that the T cells used were capable to express activated PLCγ1. The expression levels of phosphor-PLCγ1 were analyzed as in panel D). Error bars represent SD. (F) Schematic summary of the discovered mechanism by which Wogonin induces malignant T cells to undergo apoptosis. Tumor cells produce ROS at elevated rates and Wogonin shows stronger and sustained generation of H2O2 in the malignant but not in normal T cells. H2O2 in turn serves as a signaling molecule to activate PLCγ1 and consequently induces Ca2+ release from intracellular stores. Cytosolic Ca2+ overload leads to disrupt the mitochondrial membrane. Nifedipine, FK-506, CsA, and Ru-360 were shown to block the Wogonin-induced apoptosis pathway at different levels.
Wogonin selectively activates PLCγ1 in malignant versus normal T cells via H2O2 signaling
Tumor cells are known to have altered redox regulation and produce ROS at elevated rates in vitro and in vivo.37 We have recently shown that malignant T cells produce significantly higher levels of the free radical ·O2− than normal T cells and that Wogonin is capable to reduce the ·O2− level by converting it into a more reduced state, H2O2.15 Wogonin-mediated production of H2O2 was rapid and could be detected as soon as 1 minute after treatment (Figure 4B top panel). Because of higher levels of ROS in malignant than in normal T cells,15,37 higher amounts of H2O2 were generated in such cells than in normal T cells after Wogonin treatment (Figure 4B). H2O2 is an important second messenger in TCR signaling.17,18 In contrast to TCR signaling, a stronger and sustained production of H2O2 was observed in Wogonin than in TCR-triggered (by αCD3) T cells (Figure 4C). Recent studies demonstrate that H2O2 may serve as a signaling molecule to induce intracellular Ca2+ mobilization via activation of PLCγ1.4,–6 Therefore, Wogonin might be able to activate PLCγ1 via H2O2 signaling. Indeed, Wogonin treatment induced PLCγ1 phosphorylation in Jurkat T cells. Corresponding to the prolonged H2O2 production by Wogonin, a sustained PLCγ1 activation was observed in cells treated by Wogonin than in those treated by αCD3 stimulation (Figure 4D). In normal T cells, Wogonin treatment led to a very low production of H2O2 (Figure 4B); therefore, no or very little PLCγ1 activation was observed (Figure 4E). The experiments demonstrate that Wogonin activates PLCγ1 in malignant T cells, leading to triggering of intracellular Ca2+ release and induction of apoptosis in these cells (summarized in Figure 4F).
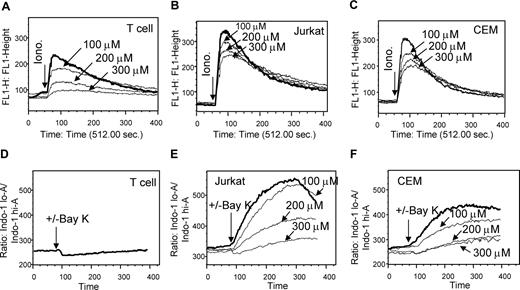
Malignant T cells express abnormal levels of VDCC Ca2+ channels
Malignant T cells have been shown to express at least 10 times higher levels of Ca2+ release-activated calcium (CRAC) channels than normal peripheral blood T cells.38,39 Therefore, another explanation for the selective effect of Wogonin could be an increased number of Ca2+ channels expressed in tumor cells versus normal cells. The CRAC channel is the major channel involved in the Ca2+ entry pathway from the extracellular matrix. Therefore, Wogonin-induced prolonged intracellular Ca2+ release in malignant T cells cannot be explained by an overexpression of CRAC. Recently, the L-type voltage-dependent Ca2+ channel (VDCC), known to mediate Ca2+ mobilization in excitable cells, has been shown to play a critical role in Ca2+ mobilization in nonexcitable cells, including T lymphocytes,31,40 Because nifedipine, the Ca2+ channel antagonist of L-type VDCC, significantly blocked Wogonin-induced apoptosis (Figure 3C), VDCC might play an important role in regulation of intracellular Ca2+ mobilization. To investigate this possibility, the efficiency of nifedipine on blocking intracellular Ca2+ release in Jurkat and CEM cells was compared with that in normal T cells. Cells were stimulated with ionomycin in the absence of extracellular Ca2+ (in PBS) to trigger Ca2+ release from the intracellular stores. The experiments showed that nifedipine could block ionomycin-induced release of Ca2+ from intracellular stores demonstrating that the VDCC channel is involved in regulation of the intracellular Ca2+ mobilization (Figure 5). The experiments also showed that ionomycin stimulation induced a much higher level of Ca2+ release in Jurkat and CEM cells than in normal T cells; 300 μM nifedipine, which almost completely blocked the Ca2+ release from the intracellular stores in normal T cells, only partially inhibited Ca2+ release in Jurkat and CEM cells (Figure 5A-C). This observation indicates that malignant lymphocytes might express higher levels of VDCC channels than normal T cells. To further examine this assumption, cells were stimulated with the VDCC 1,4-dihydropyridine L-type channel agonist, (±)-Bay K 864431 in the absence or presence of nifedipine. Up to 100 μM, (±)-Bay K 8644 did not show any induction of intracellular Ca2+ release in normal T cells (Figure 5D). In contrast, the same amount of (±)-Bay K 8644 led to a strong induction of Ca2+ release in Jurkat and CEM cells (Figure 5E,F). The (±)-Bay K 8644-induced Ca2+ release in Jurkat and CEM cells was specifically inhibited by nifedipine (Figure 5E,F). Thus, malignant T cells may express higher levels of VDCC channels than nonmalignant T cells. The data indicate that the abnormal levels of Ca2+ channels in tumor cells may also account for the different responses to Wogonin treatment of malignant versus normal T cells.
Malignant T cells express different numbers of Ca2+ channels than normal T cells. (A-C) Effects of nifedipine on blocking intracellular Ca2+ release in malignant and normal T cells. Human peripheral blood T cells (A), Jurkat (B), and CEM (C) T cells were loaded with 1 μM Fluo-4 in PBS (without extracellular Ca2+) and preincubated with 100 to 300 μM nifedipine for 10 minutes. Cells were then stimulated with 0.5 μM ionomycin to induce the intracellular Ca2+ release. (D-F) Effects of Bay K 8644 on induction of intracellular Ca2+ release in malignant and normal T cells. Human peripheral blood T cells (D), Jurkat (E), and CEM (F) T cells were loaded with 1 μM Indol-1 and pretreated with or without nifedipine for 10 minutes and then stimulated with 100 μM Bay K 8644. The intracellular Ca2+ release was monitored by FACS. Results are representative of 3 independent experiments.
Malignant T cells express different numbers of Ca2+ channels than normal T cells. (A-C) Effects of nifedipine on blocking intracellular Ca2+ release in malignant and normal T cells. Human peripheral blood T cells (A), Jurkat (B), and CEM (C) T cells were loaded with 1 μM Fluo-4 in PBS (without extracellular Ca2+) and preincubated with 100 to 300 μM nifedipine for 10 minutes. Cells were then stimulated with 0.5 μM ionomycin to induce the intracellular Ca2+ release. (D-F) Effects of Bay K 8644 on induction of intracellular Ca2+ release in malignant and normal T cells. Human peripheral blood T cells (D), Jurkat (E), and CEM (F) T cells were loaded with 1 μM Indol-1 and pretreated with or without nifedipine for 10 minutes and then stimulated with 100 μM Bay K 8644. The intracellular Ca2+ release was monitored by FACS. Results are representative of 3 independent experiments.
Wogonin suppresses growth of xenografted human tumor cells in vivo
A major goal of anticancer research is to find drugs that should have minimal side effects and maximal efficacy. The selectivity of Wogonin shown above prompted us to further investigate the effect of Wogonin in vivo. We xenografted the human CEM tumor cells into the H-2qRag−/−γc−/− immunodeficient mice. Three days after xenografting, one group of mice was treated (intraperitoneally) with 200 mg/kg body weight of Wogonin each second day (Figure 4A). The acute lethal toxicity of Wogonin is LD50 of 3.9 g/kg.41 The tumor size was measured after xenografing. The experiment showed that all control mice developed tumors in 2 weeks after xenografting. In contrast, no visible tumors were seen in Wogonin-treated mice (Figure 6A,B). The absence of tumor growth in the Wogonin-treated mice was not due to nontake of the xenografts. After stopping Wogonin-treatment, 4 of 6 treated mice developed small tumors (Figure 6A). Similar results were obtained from 2 further independent experiments (data not shown). All experiments showed that Wogonin significantly inhibited growth of the xenografted human tumor cells in vivo.
Effect of Wogonin on tumor growth in vivo. (A) Eleven H-2qRag−/−γc−/− mice were xenografted with 5 × 107 CEM and 3 days after grafting 6 mice were randomly chosen for Wogonin treatment (200 mg/kg) at each second day until day 13 as indicated. Tumor size was measured 11 days after grafting. Results are representative of 3 independent experiments. (B) Examples of an untreated and a Wogonin-treated mouse at day 15.
Effect of Wogonin on tumor growth in vivo. (A) Eleven H-2qRag−/−γc−/− mice were xenografted with 5 × 107 CEM and 3 days after grafting 6 mice were randomly chosen for Wogonin treatment (200 mg/kg) at each second day until day 13 as indicated. Tumor size was measured 11 days after grafting. Results are representative of 3 independent experiments. (B) Examples of an untreated and a Wogonin-treated mouse at day 15.
Discussion
So far, much effort has been devoted to search for agents that can specifically induce apoptotic cell death in cancer cells. Minimizing side effects and maximizing efficacy became a major goal in the development of apoptosis inducers. The radix of Haung-Qin has been used as a remedy in traditional Chinese medicine because of its low toxicity and strong anti-inflammatory activity. The principal active component of Huang-Qin, Wogonin, has attracted our attention because it shows almost no toxicity on normal peripheral blood T cells. Using normal and malignant T cells as a model system, we show that Wogonin induces malignant T cells to undergo apoptosis through the intrinsic death pathway by differentially activating PLCγ1 and mobilizing the intracellular Ca2+ stores in malignant but not in normal T cells. Loss of intracellular Ca2+ homeostasis is known to play a crucial role in induction of cell death.1,27
One known Ca2+-regulated Bcl-2-associated proapoptotic protein is Bad. In nonapoptotic cells, Bad is phosphorylated and sequestered by the cytosolic protein 14-3-3 avoiding its hetero-dimerization with Bcl-2 and Bcl-xL at the mitochondrial membrane.42 In the presence of an apoptotic stimulus (eg, Ca2+), the Ca2+/calmodulin-dependent phosphatase calcineurin dephosphorylates Bad proteins.43 The calcineurin inhibitor FK506 could inhibit Wogonin-induced apoptosis indicating that Bad may play a role in Wogonin-induced cell death. It has been shown that CsA, in addition to its inhibition of calcineurin, can block opening of the mitochondrial permeability transition pore (MPTP) by binding to cyclophylin D (CyP-D), a soluble protein that facilitates pore opening.32 Thus, CsA may protect against cell death by both inhibition of calcineurin-mediated dephosphorylation of Bad through an interaction with CyP-A and inhibition of MPTP opening though an interaction with CyP-D. In the experiments shown here, CsA treatment, however, did not exhibit a more pronounced inhibition of apoptosis than that by FK506 application. It has been shown that the MPTP can open at high Ca2+ concentrations in the presence of CsA44 and in CyP-D–deficient cells.45,,–48 Therefore, it has been suggested that CyP-D is not required for the MPTP to open at very high matrix Ca2+ levels.32 We show that Wogonin induces a high and sustained elevation of Ca2+ concentrations. Therefore, the high Ca2+ level triggered by Wogonin might induce a CyP-D–independent (CsA-insensitive) MPTP opening. In addition, the overall role of the MPT in apoptosis has been controversially discussed because there are several studies showing that apoptosis is not inhibited by CsA in various cells isolated from CyP-D–deficient mice. Thus, in such mice, thymocytes and hepatocytes undergo apoptosis in response to various stimuli.45,47,49,50 Although both CsA and FK506 inhibit calcineurin activity, FK506 exerts a better prevention of apoptosis than CsA in the experiment shown here. This may be because CsA is more toxic to the cells than FK506. The concentrations of CsA used may inhibit other targets and have a secondary effect on apoptosis.
Previous studies have also demonstrated that certain anticancer drugs (eg, staurosporine or adriamycin) induce Ca2+ release from the ER. Depletion of the ER Ca2+ pool can trigger secondary changes in mitochondrial Ca2+ levels that contribute to cytochrome c release and cell death.35,51 In those cases, blocking mitochondrial Ca2+ uptake by an inhibitor of the mitochondrial uniporter (RU-360) could attenuate staurosporine-induced apoptosis. In our experiments, a strong reduction in Wogonin-induced apoptosis was also observed in the presence of RU-306 (Figure 4F). Therefore, direct disruption of the mitochondrial membrane via Ca2+ overloading may also be one of the mechanisms for Wogonin-induced apoptosis.
Physiologically, T cell activation via TCR engagement initiates sequential activation of protein tyrosine kinase of the Src family LCK and the Syk family protein tyrosine kinase, ZAP-70. ZAP-70 phosphorylates several downstream targets, including the transmembrane adaptors LAT and SLP76. Together, LAT and SLP76 activate PLCγ1, resulting in cleavage of the inositol phospholipids, PI(4,5)P2, and in generation of the second messengers IP3 and DAG. IP3 binds to the IP3 receptor on ER and induces Ca2+ release from the ER.36 Depletion of Ca2+ stores is sensed by an as yet not fully characterized signaling mechanism, which triggers entry of Ca2+ through CRAC channels in the plasma membrane and leads to activation of a large number of genes.52 In this study, we showed that Wogonin induced an elevated activation of PLCγ1 compared with TCR stimulation (by αCD3), which correlates with a prolonged release of Ca2+ from intracellular stores in malignant T cells. However, Wogonin did not trigger Ca2+ release in normal T cells. One explanation for the difference in PLCγ1 activation in malignant and normal T cells is that tumor cells produce more ROS15,37 and Wogonin shows stronger and long-lasting shifts of ·O2− to H2O2 in the malignant but not in normal T cells (Figure 4B,C).15 H2O2 in turn serves as a signaling molecule to activate PLCγ1 (Figure 4D,E).4,–6 Thus, we showed that J-PLCγdef cells, in contrast to J-SLP76def and J-LATdef cells, are almost completely resistant to Wogonin-induced apoptosis (Figure 4A). These data strongly suggest that Wogonin induces apoptosis through activation of PLCγ and a PLCγ-mediated Ca2+ pathway.
It has been shown that Jurkat T cells express 100 to 400 CRAC channels, whereas resting peripheral blood T cells express only 15 such channels.38,39 Therefore, another possible explanation for the differential effect of Wonognin on malignant and normal T cell is that tumor cells may harbor disrupted or aberrant Ca2+ channels. Recently, the L-type VDCC channel has been implicated in regulation of Ca2+ influx induced by TCR stimulation.31,40 However, the role of VDCC in intracellular Ca2+ mobilization is unknown. Using the L-type VDCC Ca2+ channel antagonist, nifedipine, and agonist, (±)-Bay K 8644, in the absence of extracellular Ca2+, we demonstrate that VDCC is involved in intracellular Ca2+ mobilization (Figure 5). Furthermore, we show that Jurkat and CEM cells are less (at least 50%) sensitive to nifedipine than normal T cells. In addition, Bay K 8644, an agonist that induces L-type Ca2+ channel opening, was shown to induce an intracellular Ca2+ release in Jurkat and CEM T cells but not in human peripheral blood T cells (Figure 5). These observations indicate that, apart from the CRAC channels,38,39 malignant T cells may also express more VDCC L-type Ca2+ channels than normal T cells. This assumption is supported by the observation that the mRNA expression levels of 2 pore-forming subunits α1S and α1C of the L-type VDCC channel are elevated in colon cancer cell lines and in colon patient tissues.53,54 Taken together, malignant T cells may express different numbers and types of Ca2+ channels than normal T cells. This may be another factor that accounts for the different responses to Wogonin treatment of malignant versus normal T cells.
Wogonin has also been reported to have cytostatic effects (in vitro) on other types of cancer cell lines, such as hepatocellular carcinoma, bladder cancer, and myelogenous leukemia cell lines.12,–14 A previous animal experiment showed that oral administration in mice with the roots of Huang-Qin at a dose of 10 mg per mouse per day resulted in inhibition of bladder tumor growth by approximately 30%.12 However, in these experiments, the precise compounds which inhibited tumor growth were not defined. Using purified compounds, we show that the control mice developed large tumors in comparison to Wogonin-treated mice, which only showed small or no tumors at all. We noticed some interindividual variability of the rate of tumor growth, although all mice had the same genetic background. Therefore, some mice may need higher doses of Wogonin to prevent tumor growth. Thus, further work is needed to better understand the pharmacokinetics of Wogonin.
Taken together, we show that Wogonin selectively induces apoptosis in malignant T cells through the mitochondria pathway via 2 important mechanisms (Figure 4F). First, Wogonin induces the generation of a much stronger H2O2 signal in malignant than in normal T cells. This leads to stronger activation of PLCγ1 in malignant T cells that triggers intracellular Ca2+ release. Second, we found a higher expression (and/or function) of VDCC in malignant versus normal T cells. This may further enhance the cytotoxicity of Wogonin for malignant T cells. Furthermore, we have previously shown that Wogonin is capable to shift the free radical ·O2− to a more reduced nonradical product, H2O2, and thereby attenuate NF-κB activity and enhance sensitivity of leukemia cells to tumor necrosis factorα- and TRAIL-induced apoptosis.15 Thus, our results demonstrate that the Chinese herbal compound Wogonin may be an attractive compound for the development of new drugs against hematologic malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. Köhler, K. Kappes, N. Stephan, K. Palfi, C. Stumpf, and M. K. Treiber for technical assistance and Dr C. Falk and Dr O. Janssen for normal T-cell clones.
This work was supported by the Deutsche Krebshilfe in Germany.
Authorship
Contribution: S.B., S.C.F., M.G., W.W.M., and A.M. performed experiments; K.G. and P.H.K. analyzed data; L.E. performed statistical analysis; M.L.-W designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Min Li-Weber, Tumor Immunology Program D030, German Cancer Research Center, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; e-mail: m.li-weber@dkfz-heidelberg.de.
References
Author notes
S.B. and S.C.F. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal