Abstract
All-trans retinoic acid (ATRA) plus anthracycline chemotherapy is the reference treatment of newly diagnosed acute promyelocytic leukemia (APL), whereas the role of cytosine arabinoside (AraC) remains disputed. We performed a joint analysis of patients younger than 65 years included in Programa para el Estudio de la Terapéutica en Hemopatía Maligna (PETHEMA) LPA 99 trial, where patients received no AraC in addition to ATRA, high cumulative dose idarubicin, and mitoxantrone, and APL 2000 trial, where patients received AraC in addition to ATRA and lower cumulative dose daunorubicin. In patients with white blood cell (WBC) count less than 10 × 109/L, complete remission (CR) rates were similar, but 3-year cumulative incidence of relapse (CIR) was significantly lower in LPA 99 trial: 4.2% versus 14.3% (P = .03), although 3-year survival was similar in both trials. This suggested that AraC is not required in APL with WBC count less than 10 × 109/L, at least in trials with high-dose anthracycline and maintenance treatment. In patients with WBC of 10 × 109/L or more, however, the CR rate (95.1% vs 83.6% P = .018) and 3-year survival (91.5% vs 80.8%, P = .026) were significantly higher in APL 2000 trial, and there was a trend for lower 3-year CIR (9.9% vs 18.5%, P = .12), suggesting a beneficial role for AraC in those patients.
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia characterized by its morphology, t(15;17) translocation leading to PML-RARa fusion gene, and by a life-threatening coagulopathy.1-5 All-trans retinoic acid (ATRA) combined with anthracycline-based chemotherapy yield complete remission (CR) rate in approximately 90% of newly diagnosed APLs and 25% to 30% subsequently relapse.6 Maintenance treatment (especially combining continuous 6-mercaptopurine [6MP] and methotrexate [MTX] to ATRA) appears to further reduce the relapse risk to 10% to 15%.6-9 Pretreatment white blood cell (WBC) count is the main factor associated with relapse in APL and a predictive model for relapse risk (Sanz score) based on pretreatment WBC and platelet counts allowing for the distinction among low-risk patients (with WBC count < 10 × 109/L and platelet count > 40 × 109/L), intermediate-risk patients (with WBC count < 10 × 109/L and platelet count < 40 × 109/L), and high-risk patients (with WBC count ≥ 10 × 109/L).10
Another sizable subset of patients die in CR from complications of consolidation treatment, mainly from infection due to chemotherapy-induced myelosuppression.7,11-13 To decrease mortality in CR, the Programa para el Estudio de la Terapéutica en Hemopatía Maligna (PETHEMA) group reduced the intensity of consolidation chemotherapy by avoiding cytosine arabinoside (AraC) in the chemotherapy regimen.8,9 They observed CR rates comparable with regimens using a combination of AraC with anthracycline, a lower rate of death in CR (2%) and a low cumulative incidence of relapse (10%). On the other hand, the French-Belgian-Swiss APL group, in a randomized trial in patients with WBC count less than 10 × 109/L that tested the role of AraC in addition to ATRA and anthracyclines, found a significantly higher cumulative incidence of relapse and lower survival in patients treated without AraC.14
To assess those discrepancies between PETHEMA and French-Belgian-Swiss results, particularly regarding the role of AraC, we performed a joint analysis of results of LPA 99 (PETHEMA group) and APL 2000 (French-Belgian-Swiss APL group) studies. We restricted, in APL 2000 trial, the joint analysis to patients who received AraC, excluding low-risk patients who were randomized to receive induction treatment without AraC.
Methods
Design of LPA 99 and APL 2000 trials
Eligibility criteria for both studies were a diagnosis of de novo APL with demonstration of the t(15;17) or PML/RAR rearrangement, no cardiac contraindication to anthracycline chemotherapy, and a signed informed consent. Informed consent was obtained in accordance with the Declaration of Helsinki, and both protocols were approved by the Research Ethics Board of each participating hospital.
LPA 99 trial.
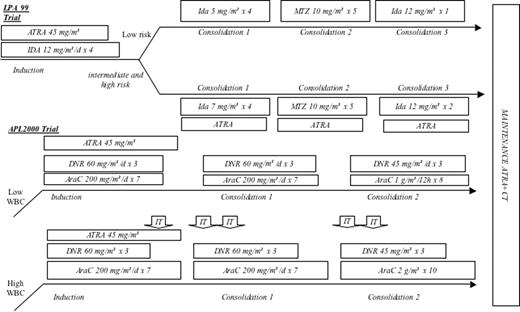
The induction regimen consisted of oral ATRA (45 mg/m2 per day), divided into 2 daily doses and maintained until complete hematologic remission, and idarubicin (12 mg/m2 per day) given on days 2, 4, 6, and 8. After CR achievement, low-risk patients according to Sanz score received 3 monthly consolidation courses consisting of idarubicin (5 mg/m2 per day) on days 1 through 4, mitoxantrone (10 mg/m2 per day) on days 1 through 5, and idarubicin (12 mg/m2 per day) on day. Intermediate- and high-risk patients received ATRA (45 mg/m2 per day given during 15 days) combined with reinforced consolidation chemotherapy (idarubicin 7 mg/m2 per day during the first course and idarubicin for 2 consecutive days instead of one in the third course). Maintenance therapy consisted of 6-mercaptopurine (50 mg/m2 per day), methotrexate (15 mg/m2 per week), and intermittent ATRA (45 mg/m2 per day) for 15 days every 3 months. Maintenance therapy was continued for 2 years (Figure 1).8
APL 2000 trial.
Patients 60 years or younger with WBC count less than 10 × 109/L were randomized to receive the reference ATRA plus CT treatment of the previous APL 93 trial (ie, ATRA 45 mg/m2 per day until hematologic CR and chemotherapy with daunorubicin (DNR) 60 mg/m2 per day during 3 days and AraC 200 mg/m2 per day during 7 days) or the same treatment but without AraC (AraC-negative group). After CR achievement, consolidation treatment consisted of 2 intensive chemotherapy courses with DNR 60 mg/m2 per day for days 1 to 3 and AraC 200 mg/m2 per day for days 1 to 7 and daunorubicin 45 mg/m2 per day for days 1 to 3 and AraC 1 g/m2 per 12 hours for days 1 to 4 in the AraC-positive arm, which were similar but without AraC in the AraC-negative arm.14
Patients 60 years or younger with a WBC count of 10 × 109/L or more received the same treatment as those 60 years or younger with WBC count less than 10 × 109/L included in AraC-positive group, but with increased dose of AraC (2 g/m2 per 12 hours during 5 days in patients younger than 50 years, and 1.5 g/m2 per 12 hours during 5 days in patients aged 50 to 60 years) with central nervous system (CNS) prophylaxis (consisting of 5 intrathecal injections of MTX 15 mg, AraC 50 mg, and depomedrol).
Patients older than 60 years with WBC count less than 10 × 109/L received the same treatment as patients 60 years or younger with WBC count less than 10 × 109/L included in the AraC-negative group (ie, ATRA 45 mg/m2 per day until hematologic CR and chemotherapy with daunorubicin 60 mg/m2 per day during 3 days) followed by consolidation treatment consisting of 2 intensive chemotherapy courses with DNR 60 mg/m2 per day for days 1 to 3 and daunorubicin 45 mg/m2 per day for days 1 to 3. Patients older than 60 years with initial WBC count of 10 × 109/L or more received the same treatment as patients 60 years or younger with WBC count less than 10 × 109/L included in the AraC-positive group (ie, ATRA 45 mg/m2 per day until hematologic CR and chemotherapy with daunorubicin 60 mg/m2 per day during 3 days and AraC 200 mg/m2 per day during 7 days) followed by consolidation treatment with 2 intensive chemotherapy courses with DNR 60 mg/m2 per day for days 1 to 3 and AraC 200 mg/m2 per day for days 1 to 7 and daunorubicin 45 mg/m2 per day for days 1 to 3 and AraC 1 g/m2 per 12 hours for days 1 to 4.14
For all patients, maintenance was the same as in LPA 99 trial.
Eligibility criteria for the joint study
A joint analysis of the 2 studies was made. It included all patients from LPA 99 younger than 65 years, and patients from APL 2000 trial younger than 65 years who were randomized or assigned to treatment arms with AraC.
Study end points
Event-free survival (EFS), cumulative incidence of relapse (CIR), and overall survival (OS) were defined as the study end points. Complete remission (CR) and relapse were defined according to International Working Group criteria.15 Early death was defined as death occurring during induction therapy or during the period of aplasia that followed chemotherapy. Total deaths included deaths irrespective of their cause (early deaths, deaths after relapse, deaths in CR).
Statistical analysis
Statistical analysis was performed at the reference date of January 1, 2007 (in 410 patients included in LPA 99 before August 2004, and 178 patients included in APL 2000 before February 2004) dealing with the main end point and overall survival.
In APL 2000 trial, treatment was stratified on initial WBC count (more or less than 10 × 109/L) and age, whereas in PETHEMA LPA 99 trial, it was stratified according to Sanz score, where initial WBC count (10 × 109/L) discriminated high-risk patients and low- and intermediate-risk patients. For this reason, joint analysis of the 2 trials was performed according to initial WBC count, separating low- and intermediate-risk patients (with WBC count < 10 × 109/L), on the one hand, from high-risk patients (with WBC count ≥ 10 × 109/L) on the other hand.
Baseline characteristics and CR rates in the 2 groups were compared by nonparametric tests (exact Fisher test for qualitative variables, Kruskal-Wallis test for quantitative variables). Censored (EFS, DFS, OS)16,17 end points were estimated by the nonparametric Kaplan-Meier method, then compared between randomized groups by the log-rank test, after checking for proportional hazards over time. Cox models allowed estimating hazard ratio (HR) of event with 95% confidence intervals (95% CIs).18-20
In estimating relapses, we took into account for competing risks deaths in first CR using the cumulative incidence curves, then compared by the Gray test, while the Fine and Gray model was used to estimate subdistribution HR (SHR).21,22
Crude estimates were computed. Estimations were finally adjusted for potential predictors including age, sex, platelet count, and WBC count. Such adjustments were performed on the basis of multivariate regression models, which differed according to the end point: Logistic model for CR rates, Cox model for censored end points, and Fine and Gray model for the cumulative incidence of relapse.
Type I error was fixed at the 5% level. All tests were 2 tailed. Statistical analysis was performed using SAS version 9.1 software (SAS, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria) software packages.
Results
Initial patient characteristics and overall treatment results
In LPA 99 trial, 410 consecutive patients younger than 65 with newly diagnosed APL from 74 institutions from Spain, Argentina, the Netherlands, and Czech Republic were registered between September 1999 and August 2004. Median follow-up was 67 months.
In APL 2000 trial, between January 2000 and February 2004, 178 APL patients younger than 65 years from 63 centers from France, Switzerland, and Belgium, randomized or assigned to receive AraC, were selected for the present joint study. Median follow-up was 62 months.
Main clinical and biologic presenting features of patients included in each trial are summarized in Table 1. Significantly higher WBC count (median: 7.1 × 109/L vs 2.5 × 109/L, P < .001), higher platelet count (median: 32 × 109/L vs 22 × 109/L, P < .001), and older age (median: 43 vs 37 years, P = .016) were observed in APL 2000 trial compared with LPA 99 trial, while no significant difference was seen for other pretreatment factors.
Pretreatment characteristics and outcome of APL patients included in the joint analysis
| Characteristics . | LPA 99 trial, N = 410 . | APL 2000 trial, N = 178 . | P . | Adjusted P* . |
|---|---|---|---|---|
| Age, y, median (Q1-Q3) | 37 (24-49) | 43 (29-51) | .016 | — |
| Age younger than 60 y, no. (%) | 378 (92.2) | 167 (93.8) | .60 | — |
| Male sex, no. (%) | 200 (48.8) | 80 (44.9) | .42 | — |
| WBC count, 109/L, median (Q1-Q3) | 2.5 (1.2-10.5) | 7.1 (1.5-27.5) | < .001 | — |
| Platelet count, 109/L, median (Q1-Q3) | 22 (13-38) | 32 (19-58) | <.001 | — |
| Fibrinogen level, g/L, median (Q1-Q3) | 1.5 (1.0-2.3) | 1.5 (1.0-2.4) | .97 | — |
| Sanz score, no. (%) | ||||
| Low risk | 73 (17.8) | 43 (24.2) | <.001 | — |
| Intermediate risk | 233 (56.8) | 53 (29.8) | — | — |
| High risk | 104 (25.4) | 82 (46.0) | — | — |
| Complete remission, no. (%) | 381 (92.9) | 173 (97.2) | .053 | .018 |
| Deaths in CR, no. (%) | 5/381 (1.3) | 5/173 (2.9) | .049 | .18 |
| Relapses, no. (3-y cumulative incidence, %) | 33/381 (7.4) | 16/173 (12.0) | .32 | .33 |
| Total events, no. (3-y EFS, %) | 66 (85.3) | 26 (86) | .97 | .81 |
| Total deaths, no. (3-y OS, %) | 43 (90.5) | 11 (93.7) | .14 | .086 |
| Characteristics . | LPA 99 trial, N = 410 . | APL 2000 trial, N = 178 . | P . | Adjusted P* . |
|---|---|---|---|---|
| Age, y, median (Q1-Q3) | 37 (24-49) | 43 (29-51) | .016 | — |
| Age younger than 60 y, no. (%) | 378 (92.2) | 167 (93.8) | .60 | — |
| Male sex, no. (%) | 200 (48.8) | 80 (44.9) | .42 | — |
| WBC count, 109/L, median (Q1-Q3) | 2.5 (1.2-10.5) | 7.1 (1.5-27.5) | < .001 | — |
| Platelet count, 109/L, median (Q1-Q3) | 22 (13-38) | 32 (19-58) | <.001 | — |
| Fibrinogen level, g/L, median (Q1-Q3) | 1.5 (1.0-2.3) | 1.5 (1.0-2.4) | .97 | — |
| Sanz score, no. (%) | ||||
| Low risk | 73 (17.8) | 43 (24.2) | <.001 | — |
| Intermediate risk | 233 (56.8) | 53 (29.8) | — | — |
| High risk | 104 (25.4) | 82 (46.0) | — | — |
| Complete remission, no. (%) | 381 (92.9) | 173 (97.2) | .053 | .018 |
| Deaths in CR, no. (%) | 5/381 (1.3) | 5/173 (2.9) | .049 | .18 |
| Relapses, no. (3-y cumulative incidence, %) | 33/381 (7.4) | 16/173 (12.0) | .32 | .33 |
| Total events, no. (3-y EFS, %) | 66 (85.3) | 26 (86) | .97 | .81 |
| Total deaths, no. (3-y OS, %) | 43 (90.5) | 11 (93.7) | .14 | .086 |
— indicates not applicable.
Adjusted for age, sex, WBC count, and platelet count.
The distribution of patients in the 3 risk groups (according to Sanz score) was as follows: low-risk group, 24.2% versus 17.8%; intermediate risk group, 29.8% versus 56.8%; and high-risk group, 46% versus 25.4% (P < .001) in APL 2000 and LPA 99 trials, respectively. The imbalance between the 2 trials resulted from the fact that we restricted, in APL 2000 trial, the joint analysis to patients who received AraC, excluding low-risk patients who were randomized years with WBC or assigned to receive induction treatment without AraC. For those reasons, comparisons between the 2 studies were adjusted for age, sex, and WBC and platelet counts.
Overall, in LPA 99 trial, CR was obtained in 381 (92.9%) of 410 patients, while 28 (6.8%) patients had early death and 1 (0.2%) had resistant leukemia. The cumulative incidence of relapse (CIR) at 2 and 3 years was 5.5% and 7.4%, respectively, and 5 patients (1.3%) died in CR.
In APL 2000 trial, CR was obtained in 173 (97.2%) of 178 patients, while 5 (2.8%) patients had early death and no patient had resistant leukemia. The cumulative incidence of relapse at 2 and 3 years was 4.5% and 12.0%, respectively, and 5 (2.9%) patients died in CR.
Comparisons in the different risk groups
Low- and intermediate-risk groups.
Main presenting features of the 402 low- and intermediate-risk patients (306 treated in LPA 99 trial, 96 treated in APL 2000 trial) are summarized in Table 2.
Pretreatment characteristics and outcome of the patients (low- and intermediate-risk groups) included in the joint analysis
| Characteristics . | LPA 99 trial, N = 306 . | APL 2000 trial, N = 96 . | P . | Adjusted P* . |
|---|---|---|---|---|
| Age, y, median (Q1-Q3) | 39 (27-50) | 43 (32-51.5) | .15 | — |
| Age younger than 60 y, no. (%) | 281 (91.8) | 94 (97.9) | .036 | — |
| Male sex, no. (%) | 148 (48.4) | 41 (42.7) | .35 | — |
| WBC count, 109/L, median (Q1-Q3) | 1.6 (1.0-3.2) | 1.8 (1.0-3.8) | .73 | — |
| Platelet count, 109/L, median (Q1-Q3) | 23 (13-40) | 36 (20-63) | <.001 | — |
| Fibrinogen level, g/L, median (Q1-Q3) | 1.6 (1.1-2.5) | 1.8 (1.1-2.7) | .58 | — |
| Sanz score, no. (%) | ||||
| Low risk | 73 (23.9) | 43 (44.8) | <.001 | — |
| Intermediate risk | 233 (76.1) | 53 (55.2) | — | — |
| Complete remission, no. (%) | 294 (96.1) | 95 (99.0) | .32 | .28 |
| Deaths in CR, no. (%) | 4/294 (1.4) | 2/95 (2.1) | .38 | .57 |
| Relapses, no. (3-y cumulative incidence, %) | 16/294 (4.2) | 9/95 (14.3) | .03 | .005 |
| Total events, no. (3-y EFS, %) | 31 (91.4) | 12 (89.4) | .16 | .12 |
| Total deaths, no. (3-y OS, %) | 20 (93.8) | 4 (95.6) | .53 | .59 |
| Characteristics . | LPA 99 trial, N = 306 . | APL 2000 trial, N = 96 . | P . | Adjusted P* . |
|---|---|---|---|---|
| Age, y, median (Q1-Q3) | 39 (27-50) | 43 (32-51.5) | .15 | — |
| Age younger than 60 y, no. (%) | 281 (91.8) | 94 (97.9) | .036 | — |
| Male sex, no. (%) | 148 (48.4) | 41 (42.7) | .35 | — |
| WBC count, 109/L, median (Q1-Q3) | 1.6 (1.0-3.2) | 1.8 (1.0-3.8) | .73 | — |
| Platelet count, 109/L, median (Q1-Q3) | 23 (13-40) | 36 (20-63) | <.001 | — |
| Fibrinogen level, g/L, median (Q1-Q3) | 1.6 (1.1-2.5) | 1.8 (1.1-2.7) | .58 | — |
| Sanz score, no. (%) | ||||
| Low risk | 73 (23.9) | 43 (44.8) | <.001 | — |
| Intermediate risk | 233 (76.1) | 53 (55.2) | — | — |
| Complete remission, no. (%) | 294 (96.1) | 95 (99.0) | .32 | .28 |
| Deaths in CR, no. (%) | 4/294 (1.4) | 2/95 (2.1) | .38 | .57 |
| Relapses, no. (3-y cumulative incidence, %) | 16/294 (4.2) | 9/95 (14.3) | .03 | .005 |
| Total events, no. (3-y EFS, %) | 31 (91.4) | 12 (89.4) | .16 | .12 |
| Total deaths, no. (3-y OS, %) | 20 (93.8) | 4 (95.6) | .53 | .59 |
— indicates not applicable.
Adjusted for age, sex, WBC, and platelet count.
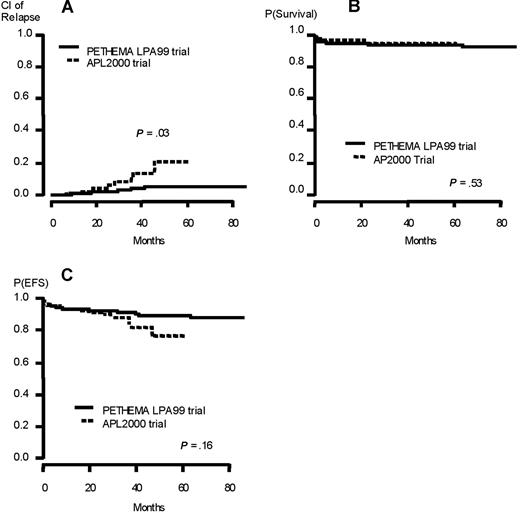
The CR rates were 99% in APL 2000 trial versus 96.1% in LPA 99 trial (P = .32). Nine patients relapsed in APL 2000 trial compared with 16 in LPA 99 trial. No extramedullary relapse was seen in APL 2000 trial but 2 in LPA 99 trial (both involving the CNS), both in the intermediate-risk group (after 24 and 86 months). The 3-year CIR and rate of death in CR were 14.3% versus 4.2% (P = .03) and 2.1% versus 1.4% (P = .38) in APL 2000 and LPA 99 trials, respectively. The 3-year EFS and overall survival were 89.4% versus 91.4% (P = .16) and 95.6% versus 93.8% (P = .53) in APL 2000 and LPA 99 trials, respectively (Figure 2).
Outcome of the patients (low- and intermediate-risk group) included in the joint analysis. (A) Cumulative incidence of relapse. (B) Overall survival. (C) Event-free survival.
Outcome of the patients (low- and intermediate-risk group) included in the joint analysis. (A) Cumulative incidence of relapse. (B) Overall survival. (C) Event-free survival.
Median duration of hospital stay was 72 days compared with 50 days in APL 2000 and LPA 99 trials, respectively (P < .001).
High-risk group.
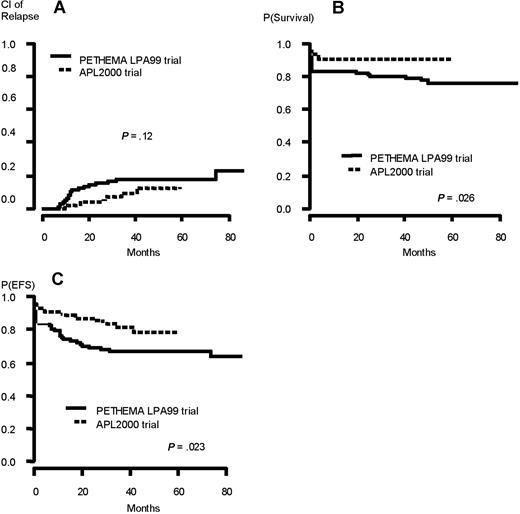
Their presenting characteristics (82 treated in APL 2000 trial, 104 treated in LPA 99 trial) are summarized in Table 3. The CR rate was 95.1% in APL 2000 trial and 83.6% in LPA 99 trial (P = .018). There were 4 induction deaths (4.9%) in APL 2000 trial (3 from sepsis and 1 from bleeding) and 17 (16.4%) in LPA 99 trial (12 from bleeding, 3 from differentiation syndrome, and 2 from infection). Seven patients relapsed in APL 2000 trial compared with 17 in LPA 99 trial. Two relapses involved the CNS in LPA 99 (at 13 and 30 months from CR) and none in APL 2000. The 3-year CIR and rate of deaths in CR were 9.9% versus 18.5% (P = .12) and 3.8% versus 1.1% (P = .24) in APL 2000 and LPA 99 trials, respectively. The 3-year EFS and overall survival were 82.2% versus 67.3% (P = .023) and 91.5% versus 80.8% (P = .026) in APL 2000 and LPA 99 trials, respectively (Figure 3). Median duration of hospital stay was 71 days in APL 2000 compared with 50 days in LPA 99 trial (P < .001).
Pretreatment characteristics and outcome of the patients (high-risk group) included in the joint analysis
| Characteristics . | LPA 99 trial, N = 104 . | APL 2000 trial, N = 82 . | P . | Adjusted P* . |
|---|---|---|---|---|
| Age, y, median (Q1-Q3) | 34 (21.5-42.5) | 41 (27-51) | .007 | — |
| Age younger than 60 y, no. (%) | 97 (93.3) | 73 (89.0) | .43 | — |
| Male sex, no. (%) | 52 (50.0) | 39 (47.6) | .77 | — |
| WBC, 109/L, median (Q1-Q3) | 27.3 (15.4-54.1) | 30.1 (14.3-58.5) | .89 | — |
| Platelets, 109/L, median (Q1-Q3) | 19 (12-34.5) | 27 (16-54) | .001 | — |
| Fibrinogen level, g/L, median (Q1-Q3) | 1.2 (0.7-1.8) | 1.25 (0.9-1.8) | .22 | — |
| CR, no. (%) | 87 (83.6) | 78 (95.1) | .018 | .001 |
| Deaths in CR, no. (%) | 1/87 (1.1) | 3/78 (3.8) | .24 | .22 |
| Relapses, no. (3-y cumulative incidence, %) | 17/87 (18.5) | 7/78 (9.9) | .12 | .15 |
| Total events, no. (3-y EFS, %) | 35 (67.3) | 14 (82.2) | .023 | .024 |
| Total deaths, no. (3-y OS, %) | 23 (80.8) | 7 (91.5) | .026 | .022 |
| Characteristics . | LPA 99 trial, N = 104 . | APL 2000 trial, N = 82 . | P . | Adjusted P* . |
|---|---|---|---|---|
| Age, y, median (Q1-Q3) | 34 (21.5-42.5) | 41 (27-51) | .007 | — |
| Age younger than 60 y, no. (%) | 97 (93.3) | 73 (89.0) | .43 | — |
| Male sex, no. (%) | 52 (50.0) | 39 (47.6) | .77 | — |
| WBC, 109/L, median (Q1-Q3) | 27.3 (15.4-54.1) | 30.1 (14.3-58.5) | .89 | — |
| Platelets, 109/L, median (Q1-Q3) | 19 (12-34.5) | 27 (16-54) | .001 | — |
| Fibrinogen level, g/L, median (Q1-Q3) | 1.2 (0.7-1.8) | 1.25 (0.9-1.8) | .22 | — |
| CR, no. (%) | 87 (83.6) | 78 (95.1) | .018 | .001 |
| Deaths in CR, no. (%) | 1/87 (1.1) | 3/78 (3.8) | .24 | .22 |
| Relapses, no. (3-y cumulative incidence, %) | 17/87 (18.5) | 7/78 (9.9) | .12 | .15 |
| Total events, no. (3-y EFS, %) | 35 (67.3) | 14 (82.2) | .023 | .024 |
| Total deaths, no. (3-y OS, %) | 23 (80.8) | 7 (91.5) | .026 | .022 |
— indicates not applicable.
Adjusted for age, sex, white blood cell count, and platelet count.
Outcome of the patients (high-risk group) included in the joint analysis. (A) Cumulative incidence of relapse. (B) Overall survival. (C) Event-free survival.
Outcome of the patients (high-risk group) included in the joint analysis. (A) Cumulative incidence of relapse. (B) Overall survival. (C) Event-free survival.
Discussion
The 2 trials (LPA 99 and APL 2000) analyzed together here shared many similarities including frontline treatment with ATRA and anthracycline-based chemotherapy followed by anthracycline-based consolidation treatment and a maintenance treatment combining intermittent ATRA and continuous 6-mercaptopurine and methotrexate, which are now widely considered as references in the treatment of APL. Differences between the trials were mainly the type of anthracycline used and the addition (or not) of AraC to anthracycline (Figure 1).8,14
The joint analysis we performed suggests that, in patients with WBC count less than 10 × 109/L, the PETHEMA approach yields even fewer relapses than a classical ATRA plus DNR plus AraC regimen, while being less myelosuppressive, and therefore leading to significantly shorter hospital stays and less mortality in CR. The lower rate of relapse seen in PETHEMA regimen may result from several factors. One reason may be the anthracycline used (idarubicin and mitoxantrone instead of DNR). The superiority of idarubicin over daunorubicin has been suggested in several randomized AML trials, although the cumulative dose of daunorubicin may have been lower than that of idarubicin in some of those studies.23-25 In addition, those studies did not specifically address APL.23-25 A higher anthracycline cumulative dose may also have contributed to the superiority of the PETHEMA approach in patients with low WBC count. Indeed, the PETHEMA group used cumulative doses of idarubicin and mitoxantrone of 80 (100 for intermediate-risk patients) and 50 mg/m2, respectively. Using a 1- to 5-dose equivalence between idarubicin (or mitoxantrone) and daunorubicin (although there is no clear consensus on this point)26 the cumulative anthracycline dose used in the LPA 99 trial would correspond to 130% to 150% of that used in APL 2000 (495 mg/m2 of DNR). Interestingly, before the ATRA era, the Italian GIMEMA group randomized idarubicin alone versus idarubicin plus AraC in the treatment of newly diagnosed APL.27 The cumulative dose of anthracycline was higher in patients treated with idarubicin alone (60 mg/m2) than in those treated with idarubicin-AraC (48 mg/m2). The 8-year event-free survival rate was significantly better for the patients treated with idarubicin alone (35% vs 23%) suggesting not only that cytarabine could be avoided in the treatment of APL when high cumulative doses of an anthracycline are used, but also a dose-effect relationship with anthracycline in APL. Other retrospective analyses published before the ATRA era also suggested a benefit for high-dose anthracycline in APL.28-30 Of note, no cardiac toxicity was observed in APL 2000 and LPA 99 trials. Long-term toxicity of high cumulative anthracycline doses is difficult to estimate after a median follow-up of only 5 years, but longer experience of the Spanish PETHEMA group over the past 12 years with high cumulative doses of idarubicin in APL has so far revealed no long-term cardiac toxicity (M.A.S, oral communication, 2006).
Finally, the difference between APL 2000 and LPA 99 trials could have been due to the addition of ATRA during consolidation courses in the LPA 99 trial in intermediate-risk patients, which may have contributed to a lower relapse risk in this group, although such benefit would, of course, have to be confirmed in a randomized trial.
The better results obtained in LPA 99 trial in low- and intermediate-risk patients suggest that, at least with high doses of idarubicin during induction and consolidation, and possibly with addition of ATRA during consolidation courses, AraC is not required for the treatment of low- and intermediate-risk APL. Furthermore, avoiding AraC reduced myelosuppression in LPA 99 trial, leading to a mortality in CR of 1% compared with 3% in APL 2000.
In patients with high WBC counts, by contrast, APL 2000 results yielded better 2-year EFS (87.7% vs 69.2%) and survival (91.5% vs 81.7%) and a trend for fewer relapses, suggesting a beneficial role for AraC in this subset of patients, possibly at high dose. Indeed, in APL 2000 trial, higher doses of AraC were used in the last consolidation course (2 g/m2 per 12 hours during 5 days). A significantly higher CR rate (95.1% vs 83.6%) was also obtained in APL 2000 trial, suggesting that AraC during induction might play a role in APL patients with high initial WBC count.
The Italian GIMEMA group compared results of 2 successive treatment regimens in patients who had achieved CR with ATRA and idarubicin, one with high-dose AraC (and also etoposide and 6-thioguanine) during consolidation courses (AIDA-2000) and one with idarubicin alone (AIDA 04-93 trial).31 Although the incidence of relapse was similar between the 2 trials for low and intermediate risk, a significantly higher incidence of relapse was observed in the AIDA-0493 versus AIDA-2000 study in high-risk patients (29% vs 2%), also suggesting a role for AraC in this group of patients. A prospective German study evaluating the role of an intensified double induction (6-thioguanine, Ara C, DNR/high-dose AraC, mitoxantrone [TAD/HAM]) regimen with AraC at conventional dose followed by high-dose (3 g/m2 per 12 hours during 3 days) and maintenance therapy found a CR rate of 92% and a 2-year overall survival and relapse-free survival of 88% and 96%, respectively.32 A higher initial leukocyte count was not identified as a risk factor for relapse, contrary to previous studies of that group with conventional dose AraC, suggesting a high antileukemic effectiveness of high-dose AraC in high-risk patients. In our study, we found only a favorable trend for a lower CIR in the APL 2000 study (P = .15).
In conclusion, this study suggests that ATRA combined with a high cumulative dose of idarubicin without AraC but with maintenance treatment, like the PETHEMA approach, may give excellent results with limited toxicity in low- or intermediate-risk APL (ie, with WBC count < 10 × 109/L). Those results will have to be confirmed in randomized trials. We are currently randomizing, in our ongoing APL 2006 trial (conducted in France, Belgium, and Switzerland) in low- and intermediate-risk APL, consolidation treatment between idarubicin plus AraC and idarubicin plus ATRA (and also idarubicin + arsenic trioxide).
On the other hand, present data suggest that the addition of AraC to ATRA and anthracyclines in high-risk patients may result in a trend toward lower incidence of relapse, and to better survival. Preliminary results suggest that arsenic derivatives may also be useful in the consolidation treatment of APL with high WBC counts, in combination or perhaps instead of AraC.33 We are also testing, in APL 2006 trial, the role of arsenic derivatives in patients with high WBC counts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the program Hospitalier de Recherche Clinique and the CHU of Lille-France, the Association pour la Recherche sur le Cancer and the Ligue Contre le Cancer (Comité du Nord), grant 2006/0137 from the Fundación para la Investigación Hospital Universitario La Fe-Ayudas Bancaja, and grant RD06/0020/0031from the Red Temática de Investigación Cooperativa en Cáncer.
Additional study participants are listed in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Authorship
Contribution: L.A., M.A.S., P.M., H.D., P.F., and L.D. conceived the study, and collected, analyzed, and interpreted the data; L.A., M.A.S. P.M., and P.F. wrote the paper; S.C did the statistical analyses; L.A., M.A.S., P.M., P.C., E.R., E.V., A.G., A.P., F.H., C.R., A.M.S., J.S., J.-Y.C., S.M.-M., T.P., X.T., S.B., R.P., J.B., T.L., A.V., S.N., N.I., H.D., A.F., D.B., and P.F. recruited the patients and collected the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Fenaux, Assistance Publique-Hôpitaux de Paris (AP-HP), Hôpital Avicenne, Service d'Hématologie Clinique, Paris 13 University, 125 rue de Stalingrad, 93009 Bobigny, France; e-mail: pierre.fenaux@avc.aphp.fr.
References
Author notes
L.A. and M.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal