Clinical outcome in patients with primary nodal diffuse large B-cell lymphomas (DLBCLs) is correlated with expression of inhibitors of the intrinsic apoptosis pathway, including X-linked inhibitor of apoptosis protein (XIAP). XIAP suppresses apoptosis through inhibiting active caspase-3, caspase-7, and caspase-9. In this study, we investigated to see if the small-molecule XIAP antagonist 1396-12 induces cell death in cultured lymphoma cells of patients with DLBCL. Treatment with this XIAP antagonist resulted in relief of caspase-3 inhibition and in induction of apoptosis in 16 of 20 tested DLBCL samples. Sensitivity to the XIAP antagonist was observed in both chemotherapy-refractory and -responsive DLBCL, but did not affect peripheral blood mononuclear cells and tonsil germinal-center B cells from healthy donors. XIAP antagonist-sensitive samples were characterized by high expression levels of XIAP, relatively low expression levels of Bcl-2, and by constitutive caspase-9 activation. These data indicate that the small-molecule XIAP antagonist can induce apoptosis in cultured DLBCL cells and therefore should be considered for possible development as a therapy for these patients. In vitro sensitivity to the XIAP antagonist can be predicted based on biological markers, suggesting the possibility of predefining patients most likely to benefit from XIAP antagonist therapy.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) account for 30% to 40% of adult non-Hodgkin lymphoma.1 At present, the standard therapy for DLBCL is a combination of intensive chemotherapy (CHOP) with rituximab.2 Although this approach results in a considerable number of patients with DLBCL in complete remission, the disease remains eventually fatal in 30% to 40% of patients.3 Fatal outcome is usually due to chemotherapy resistance manifesting in failure to achieve complete remission or the occurrence of an early relapse. Many in vitro studies have demonstrated that inhibition of the apoptosis-signaling pathways is an important factor causing chemotherapy resistance.4,,–7
Recently, using microarray expression profiling of primary nodal DLBCL, we have demonstrated that a subgroup of chemotherapy-refractory DLBCL is characterized by high expression levels of both pro- and antiapoptotic genes.8 Subsequently, we revealed that high expression levels of proapoptotic genes are associated with constitutive activation of the intrinsic, caspase-9–mediated apoptosis pathway, and that apoptosis is inhibited downstream of caspase-9 activation.9 Direct inhibitors of the downstream effector caspases of the intrinsic and extrinsic apoptosis pathways are the inhibitor of apoptosis proteins (IAPs). At present, 8 members of the IAP family have been identified in humans, including XIAP (X-linked inhibitor of apoptosis).
XIAP appears to be one of the most potent inhibitors of the apoptosis cascade and suppresses apoptosis induced by many agents, including TNF, TRAIL, Fas-L, staurosporine, etoposide, and paclitaxel.10,11 The XIAP protein inhibits caspase-3, caspase-7, and caspase-9, but not caspase-1, caspase-6, caspase-8, or caspase-10.12,13 XIAP contains 3 so-called baculoviral IAP repeat (BIR) domains.14 The second BIR domain of XIAP (BIR2) binds and inhibits caspase-3 and caspase-7, while the third BIR domain (BIR3) inhibits caspase-9.15,16 XIAP is expressed in some normal tissues and is overexpressed in many malignancies.17,–19 In DLBCL, XIAP expression is correlated with a poor clinical outcome.20 Therefore, neutralizing the effect of XIAP, resulting in selective induction of apoptosis of the tumor cells, might be a promising new therapeutic approach for chemotherapy-refractory DLBCL.
Small-molecule antagonists that specifically interfere with the inhibitory function of XIAP have been described, including the phenylurea-based compound N-[(5R)-6-[(anilinocarbonyl)amino]-5-((anilinocarbonyl)([(2R)-1-(4-cyclohexylbutyl)pyrrolidin-2-yl]-methyl)amino)hexyl]-N-methyl-N′phenylurea, also known as 1396-12.21 These phenylurea-based antagonists restore caspase-3 activity by binding the BIR2 domain of XIAP, allowing active caspase-3 to cleave substrates and to induce apoptosis.22 Small-molecule XIAP antagonists sensitize tumor cells to chemotherapy and successfully induce apoptosis of various types of tumors, including acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).21,,,–25 Moreover, phenylurea-based small-molecule XIAP antagonists produce little toxicity to normal tissues in mice.21 Currently, efforts are under way to complete preclinical development of the small-molecule XIAP antagonists for clinical use.26
In this study, we investigated to see if the small-molecule XIAP antagonist 1396-12 can induce apoptosis of isolated lymphoma cells of patients with DLBCL, including chemotherapy-refractory samples. Moreover, we examined whether the XIAP antagonist can induce apoptosis in DLBCL cell lines resistant to etoposide, and whether this antagonist can increase sensitivity to etoposide- and rituximab-induced cell death. Finally, expression levels of XIAP and other apoptosis inhibitors were determined to investigate whether they can predict sensitivity to the small-molecule XIAP antagonist.
Methods
Lymphoma samples and cell lines
A total of 20 lymphoma samples, including those from chemotherapy-refractory patients, were diagnosed and obtained between 2000 and 2005 as DLBCL at the Comprehensive Cancer Center of Amsterdam, according to the World Health Organization (WHO) criteria.27 DLBCL samples were considered responsive if patients reached complete remission (according to standard clinical evaluation, including physical examination, bone marrow biopsy, chest x-ray, and computed tomography of chest, abdomen, and pelvis) without relapse (follow-up period of 14-33 months). All other samples were considered refractory (follow-up period, 7-28 months). DLBCL samples were further subdivided into germinal-center B-cell (GCB)–like and activated B-cell (ABC)–like DLBCL using the algorithm adopted from Hans et al28 as described previously.29 Normal tonsil GC B cells and peripheral blood B cells were obtained from healthy donors and used as controls. The ethics review board of the VU University Medical Center approved collection and use of the lymphoma samples. Informed consent was obtained in accordance with the Declaration of Helsinki.
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation and frozen until further testing. Lymphoma and tonsil cell suspensions were isolated from tissue biopsies by a combination of mechanical and enzymatic dissociation and frozen until further testing.30 Primary cells were thawed 1 hour before experimental testing and cultured in Iscove modified Dulbecco medium (BioWhittaker, Cambrex, Belgium) supplemented with 10% fetal calf serum, 1% penicillin, and streptomycin at 37°C with 5% CO2 in a humidified atmosphere. Nonneoplastic T cells were removed by using super paramagnetic Dynabeads coated with anti-CD3 antibodies (Dynal, Wirral, United Kingdom). The resulting tumor cell fractions contained in all samples with less than 5% contaminating nonneoplastic cells. GC B cells were fluorescence-activated cell sorter (FACS)–sorted from tonsil B-cell suspensions using antibodies against the cell-surface markers CD38 (clone HB7; BD Biosciences, San Jose, CA) and IgD (Dako, Glostrup, Denmark).31
The DLBCL cell lines used for this study included HT, SUDHL4, and SUDHL5 (originally obtained from DSMZ, Braunschweig, Germany). As a positive control for detection of apoptosis, Nalm6 cells were used.9 Cell lines were cultured in RPMI 1640 medium (BioWhittaker) containing 10% fetal calf serum and 100 IU penicillin/100 μg/mL streptomycin.
Testing of the apoptosis-inducing effect of small-molecule XIAP antagonists
Primary cells were incubated with 10, 25, and 50 μM XIAP antagonist 1396-12 or the inactive structurally similar control 1396-28 for 4 and 24 hours.21,24 DLBCL-derived cell lines were incubated with increasing concentrations of the XIAP antagonist 1396-12 or the inactive compound 1396-28 for 4, 7, 16, and 24 hours. Cell lines were preincubated with increasing concentrations of the caspase-9 inhibitor LEHD-fmk or the pancaspase inhibitor z-VAD-fmk (Alexis Biochemicals, Lausen, Switzerland) 1 hour prior to treatment.
Etoposide (VP16; Sigma, St Louis, MO) was used to assess sensitivity to chemotherapy-induced apoptosis because it activates the intrinsic pathway9,30 and as such, is representative of the many chemotherapeutic drugs used in the treatment of DLBCL.32,,–35 Moreover, it lacks autofluorescence, making etoposide very suitable for FACS analysis. DLBCL cell lines were incubated with 500 nM etoposide for 4, 7, 16, and 24 hours at 37°C. Sensitivity to rituximab-induced cell death was determined by incubating DLBCL cells with 10 μg/mL rituximab (Roche, Basel, Switzerland) for 15 minutes at room temperature. Subsequently, normal human serum was added as source of complement and cells were incubated at 37°C for 24 hours. Dose-response curves showed that for etoposide- and rituximab-induced cell death, 500 nM etoposide and 10 μg/mL rituximab were the optimal cell death–inducing concentrations in DLBCL cell lines, respectively (data not shown).
The effect of XIAP antagonists on etoposide-induced cell death was tested with increasing concentrations of XIAP antagonist 1396-12 in combination with 500 nM for 16 hours.
Cell death of primary cells and DLBCL cell lines was determined as described previously.30 Briefly, cell death was detected using a standard number of fluorescent beads (Fluorospheres; Becton Dickinson, San Jose, CA) in combination with 7AAD (7-amino-actinomycin; ViaProbe; BD PharMingen, Erembodegem, Belgium) to determine the number of viable (7AAD−) cells per 2500 beads for each individual experiment. Fluorescence was detected using a FACSCalibur flow cytometer and analyzed using CELL-Quest software (both from Becton Dickinson). All tests were performed in triplicate.
Detection of caspase-3/7 activity
Caspase-3/7 activity was determined using a fluorogenic substrate according to the manufacturer's instructions (Roche, Mannheim, Germany). Cells were lysed and incubated with the DEVD-rhodamine 110 substrate for 1 hour at 37°C. Subsequently, the amount of free rhodamine was determined with a 492-nm excitation filter and a 535-nm emission filter using a microplate fluorescence reader (TECAN spectrafluor; TECAN Group, Männedorf, Switzerland). Levels of caspase activity were indicated as caspase activity levels of treated samples minus caspase activity levels of untreated samples. Experiments were performed in triplicate.
RT-MLPA analysis
RNA of primary cells and DLBCL cell lines were prepared using RNbee solution (Tel-test, Friendswood, TX) according to the manufacturer's recommendations. Reverse transcriptase–multiplex ligation–dependent probe amplification (RT-MLPA) was performed on total RNA as described previously.9,36 Data were analyzed with Genotype and GeneScan software (Applied Biosystems, Warrington, United Kingdom). As internal reference, the housekeeping gene β-glucuronidase (GUS-B) was used to minimize possible effects of unequal amounts of mRNA.
siRNA analysis
In SUDHL4 cells, Bcl-2 expression was down-regulated using siRNA analysis. Transfection of SUDHL4 cells was performed using Transpei (Eurogentec, San Diego, CA). Transfection efficiency of SUDHL4 was 70% to 80%. Transfection-associated cell death was negligible. Before transfection of siRNA, cells were washed with RPMI 1640 medium containing 10% fetal calf serum. Cells were transfected with 100 nM control nontargeting siRNA pool or Bcl-2–specific siRNA (both provided by Dharmacon, Lafayette, CO). Transfection efficiency was determined by FACS analysis of fluorescent siRNA siGLO Lamin A/C (Dharmacon).
Western blot analysis
Total protein extracts were prepared from SUDHL4 at 24, 48, 72, and 96 hours after transfection and after incubation with 10 μM of the XIAP antagonist for Western blot analysis, as described previously.37 Cells were washed with phosphate-buffered saline (PBS) and resuspended in lysis buffer (50 mM Tris/HCl [pH 8.0], 0.5% NP-40, and 5 mM EDTA) containing protease inhibitors. Cytosolic fractions were generated using a digitonin-based subcellular fractionation technique, as described previously.38 In brief, cells were collected, washed in ice-cold PBS (pH 7.2), and resuspended in cytosolic extraction buffer (250 mM sucrose, 70 mM KCl, 137 mM NaCl, and 4.3 mM Na2HPO4), supplemented with protease inhibitor cocktail, 250 μM PMSF, and 200 μg/mL digitonin. After incubation for 5 minutes at 4°C, the cytosolic fraction was obtained. Expression of Bcl-2, Bax and cytochrome c was detected using anti–Bcl-2 (clone 124, 0.225 μg/mL; Dako, Glostrup, Denmark), anti-Bax (1:1000; Dako) and anti–cytochrome c (1:1000; BD Pharmingen). Protein expression was visualized with the enhanced chemiluminescence technique (Amersham Pharmacia Biotech, Piscataway, NJ). Cellular β-actin (AB-1 kit, 6.25 ng/mL; Oncogene Research Products, Darmstadt, Germany) was used as loading control in each sample on the blots.
Statistical analysis
Analyses were performed using SPSS software (version 11.5; SPSS, Chicago, IL). The Mann-Whitney U test was used to compare means between groups. P values less than .05 were considered significant.
Results
XIAP antagonist 1396–12 induces apoptosis of cells from patients with DLBCL
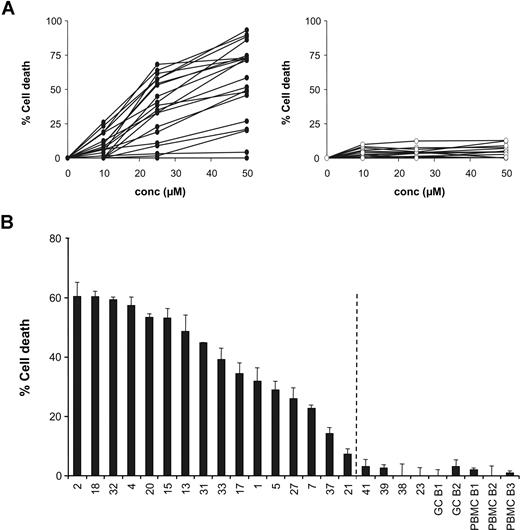
To determine the effect of small-molecule XIAP antagonists on apoptosis sensitivity, cultured lymphoma cells from 20 patients with DLBCL were treated with increasing concentrations of the XIAP antagonist 1396-12 for 4 and 24 hours. The results after 4 hours of incubation are depicted in Figure 1A. In sensitive samples, the XIAP antagonist 1396-12 (Figure 1A left) showed a significant concentration-dependent induction of apoptosis when compared with the inactive control compound 1396-28. The inactive control 1396-28 was not toxic for the cells from patients with DLBCL (Figure 1A right).
Small-molecule XIAP antagonist induces apoptosis of DLBCL patient cells. (A) Dose-response curves for each DLBCL sample after 4 hours of treatment with the XIAP antagonist (●) 1396-12 (left) or the inactive control (○) 1396-28 (right). (B) Detection of the percentage of cell death after 4 hours of treatment with 25 μM XIAP antagonist in DLBCL samples, tonsil GC B cells, and PBMC B cells. The observed percentage of XIAP antagonist-induced cell death was defined as the percentage of cell death induced by XIAP antagonist 1396-12 minus the percentage of cell death induced by the inactive compound 1396-28. When the percentage cell death greater than 2 times the standard deviation of the mean value of the nonneoplastic cells (GC B cells and PBMC B cells) was taken as the cut-off value (indicated by ¦), we found that 16 of 20 tested samples were sensitive to the XIAP antagonist.
Small-molecule XIAP antagonist induces apoptosis of DLBCL patient cells. (A) Dose-response curves for each DLBCL sample after 4 hours of treatment with the XIAP antagonist (●) 1396-12 (left) or the inactive control (○) 1396-28 (right). (B) Detection of the percentage of cell death after 4 hours of treatment with 25 μM XIAP antagonist in DLBCL samples, tonsil GC B cells, and PBMC B cells. The observed percentage of XIAP antagonist-induced cell death was defined as the percentage of cell death induced by XIAP antagonist 1396-12 minus the percentage of cell death induced by the inactive compound 1396-28. When the percentage cell death greater than 2 times the standard deviation of the mean value of the nonneoplastic cells (GC B cells and PBMC B cells) was taken as the cut-off value (indicated by ¦), we found that 16 of 20 tested samples were sensitive to the XIAP antagonist.
We found that isolated lymphoma cells from 16 of 20 tested samples were considered XIAP antagonist sensitive when the percentage cell death was more than 2 times the standard deviation of the mean value of the nonneoplastic GC B cells and PBMC B cells (Figure 1B). The lethal dose (LD50) among the lymphoma samples varied between 20 μM and more than 50 μM with a median LD50 of 39 μM after 4 hours and varied between 7 μM and more than 50 μM with a median LD50 of 13 μM after 24 hours of exposure. Peripheral blood B cells and tonsil-derived GC B cells were resistant to the XIAP antagonist (Figure 1B).
We further investigated if the XIAP antagonist also induces apoptosis in clinically chemotherapy-refractory DLBCL. Clinical data were available for 17 of the 20 DLBCL samples. The XIAP antagonist was similarly effective in samples obtained from both chemotherapy-refractory and -responsive patients with DLBCL. ABC-like DLBCL tended to be more frequently sensitive to the XIAP antagonist than GCB-like DLBCL, although this was not statistically significant (Table 1).
Characterization of DLBCL samples
| Tumor cell characteristics . | Response to the XIAP antagonist . | P . | |
|---|---|---|---|
| LD50 less than 39 μM . | LD50 39 μM or more . | ||
| Apoptosis profile* | .02 | ||
| Low | 1 | 7 | — |
| High | 9 | 3 | — |
| GCB or ABC phenotype† | NS | ||
| GCB | 4 | 7 | — |
| ABC | 5 | 3 | — |
| NI | 1 | 0 | — |
| Tumor cell characteristics . | Response to the XIAP antagonist . | P . | |
|---|---|---|---|
| LD50 less than 39 μM . | LD50 39 μM or more . | ||
| Apoptosis profile* | .02 | ||
| Low | 1 | 7 | — |
| High | 9 | 3 | — |
| GCB or ABC phenotype† | NS | ||
| GCB | 4 | 7 | — |
| ABC | 5 | 3 | — |
| NI | 1 | 0 | — |
DLBCL samples were divided into 2 groups based on the median LD50 dose of 39 μM after 4 hours of incubation with the XIAP antagonist 1396-12.
NS indicates not significant; NI, not interpretable; and —, not applicable.
Indicates expression levels of proapoptotic genes (including MCL1-short, Bax-Long, Bax-short, BCL-Rambo, BCL-G, Noxa, Bad, Bak, Bim, Bid, Harakiri, PUMA, Bik, BNIP3, BNIP3L, MAP1, BMF, AIF, Apaf-1, Apaf-1L, Apaf-1XL and Smac) and antiapoptotic genes (including: BCL-W, BCL-XL, BCL-2, BCL2-A1, Appolon, BIRC7, MCL1-long, NIAP, cIAP, cIAP2, XIAP, Survivin, c-Flip and Pi-9) as described previously.9
According to the previously described algorithm.29
Previously, we demonstrated that expression profiling of apoptosis-related genes using RT-MLPA analysis separates DLBCL in 2 groups: 1 group with relatively low levels of both pro- and antiapoptotic genes, and 1 group with high expression levels of these apoptosis-regulating genes.9 The XIAP antagonist appeared to be especially effective in DLBCL samples with high expression levels of pro- and antiapoptotic genes (Table 1; P = .02).
XIAP antagonist-induced cell death is dependent on caspase-9 activation
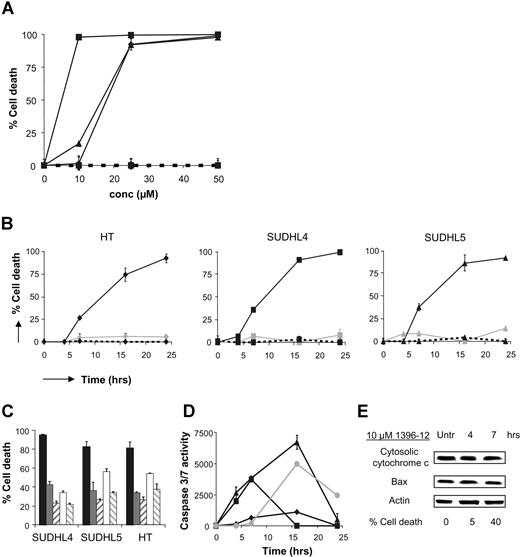
The effect of the XIAP antagonist on apoptosis sensitivity was further investigated in 3 DLBCL-derived cell lines: SUDHL4, SUDHL5, and HT. All 3 cell lines resistant to etoposide were sensitive to the XIAP antagonist (Figure 2A,B). In simultaneous experiments, addition of the pancaspase inhibitor z-VAD-fmk completely blocked killing induced by the XIAP antagonist, indicating that the induced cell death is caspase dependent. Moreover, induction with the XIAP antagonist resulted in clearly detectable caspase-3/7 activity, with a maximal increase after 7 to 16 hours of incubation. Experiments with a specific caspase-9 inhibitor, LEHD-FMK, showed that the observed cell death is caspase-9 dependent (Figure 2C,D). Previously, we demonstrated that SUDHL4, SUDHL5, and HT showed constitutive caspase-9 activation, and that incubation with the XIAP antagonist did not result in an increase in caspase-9 activation.9 As expected, no additional increase in mitochondrial cytochrome c release in the cytoplasm or Bax expression was observed after incubation with the XIAP antagonist (Figure 2E).
XIAP antagonist induces caspase-9–mediated apoptosis in etoposide-resistant DLBCL-derived cell lines. (A) Dose-response curves of the DLBCL cell lines SUDHL4 (■), SUDHL5 (▴), and HT (♦) after 24 hours of exposure to XIAP antagonist 1396-12 (solid line) or the inactive compound 1396-28 (dashed line) are shown. (B) Detection of cell death in DLBCL cell lines untreated (----) or treated with 25 μM XIAP antagonist (—) or 500 nM etoposide (solid gray line) for increasing periods of time. (C) Cell death detected in DLBCL cell lines after treatment with 25 μM XIAP antagonist in combination with increasing concentrations of the pancaspase inhibitor z-VAD-fmk or the caspase-9 inhibitor LEHD-fmk for 16 hours. (■ indicates 25 μM 1396-12; ▩, 1396-12 + 50 μM zVAD-FMK; ▨, 1396-12 + 100 μM zVAD-FMK; □, 1396-12 + 50 μM LEHD-FMK; and ▧, 1396-12 + 100 μM LEHD-FMK). (D) XIAP antagonist induced caspase-3/7 activity in DLBCL cell lines SUDHL4 (■), SUDHL5 (▴), and HT (♦) after exposure to 25 μM XIAP antagonist for 24 hours. Nalm6 cells (●) treated with etoposide were used as positive control. Error bars represent SD of the mean value of 3 experiments. (E) Expression of Bax and cytosolic cytochrome c from a time-course treatment of SUDHL4 cells with 10 μM 1396-12.
XIAP antagonist induces caspase-9–mediated apoptosis in etoposide-resistant DLBCL-derived cell lines. (A) Dose-response curves of the DLBCL cell lines SUDHL4 (■), SUDHL5 (▴), and HT (♦) after 24 hours of exposure to XIAP antagonist 1396-12 (solid line) or the inactive compound 1396-28 (dashed line) are shown. (B) Detection of cell death in DLBCL cell lines untreated (----) or treated with 25 μM XIAP antagonist (—) or 500 nM etoposide (solid gray line) for increasing periods of time. (C) Cell death detected in DLBCL cell lines after treatment with 25 μM XIAP antagonist in combination with increasing concentrations of the pancaspase inhibitor z-VAD-fmk or the caspase-9 inhibitor LEHD-fmk for 16 hours. (■ indicates 25 μM 1396-12; ▩, 1396-12 + 50 μM zVAD-FMK; ▨, 1396-12 + 100 μM zVAD-FMK; □, 1396-12 + 50 μM LEHD-FMK; and ▧, 1396-12 + 100 μM LEHD-FMK). (D) XIAP antagonist induced caspase-3/7 activity in DLBCL cell lines SUDHL4 (■), SUDHL5 (▴), and HT (♦) after exposure to 25 μM XIAP antagonist for 24 hours. Nalm6 cells (●) treated with etoposide were used as positive control. Error bars represent SD of the mean value of 3 experiments. (E) Expression of Bax and cytosolic cytochrome c from a time-course treatment of SUDHL4 cells with 10 μM 1396-12.
Sensitivity to the XIAP antagonist correlates with high expression levels of XIAP and with low levels of Bcl-2 expression
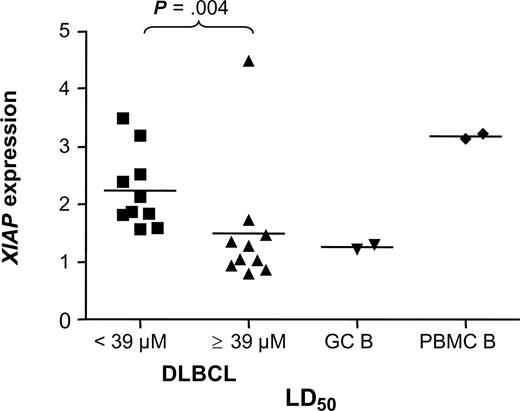
We investigated whether sensitivity to the XIAP antagonist in DLBCL samples correlates with expression levels of XIAP and with expression of other apoptosis inhibitors—Bcl-2, Bcl-XL, MCL1, and cIAP2. Expression levels were detected at the mRNA level using RT-MLPA analysis. The strongest apoptosis-inducing effect of the XIAP antagonist was observed in DLBCL samples with relatively high levels of XIAP (P = .004; Figure 3). When investigated in nonneoplastic cells, PBMCs were found to express high levels of XIAP, whereas XIAP expression levels were very low in GC B cells (Figure 3). Thus, sensitivity to 1396-12 is not determined solely by XIAP protein levels.
Levels of XIAP expression correlate with sensitivity to the XIAP antagonist.XIAP expression was detected in 20 DLBCL samples using RT-MLPA analysis. Patient DLBCL samples were divided into 2 groups based on their LD50 value for 1396-12 (LD50 < 39 μM or LD50 ≥ 39 μM). Horizontal lines represent the mean XIAP expression level for each group.
Levels of XIAP expression correlate with sensitivity to the XIAP antagonist.XIAP expression was detected in 20 DLBCL samples using RT-MLPA analysis. Patient DLBCL samples were divided into 2 groups based on their LD50 value for 1396-12 (LD50 < 39 μM or LD50 ≥ 39 μM). Horizontal lines represent the mean XIAP expression level for each group.
When comparing XIAP antagonist-sensitive DLBCL (LD50 < 39 μM) with resistant samples (LD50 values ≥ 39 μM), we found that sensitive samples were characterized by relatively high expression levels of MCL1 and cIAP2 (P = .01 and P = .005, respectively) and low expression levels of Bcl-2 (P = .04; Figure 4A). Expression levels of Bcl-XL did not significantly correlate with the response to the XIAP antagonist.
Sensitivity to the XIAP antagonist in relation to expression of Bcl-2. (A) Bcl-2 expression was detected in all the DLBCL samples tested using RT-MLPA analysis. Patient samples were divided into 2 groups: samples with an LD50 less than 39 μM or LD50 of 39 μM or greater. Horizontal lines represent the mean Bcl-2 expression level for each group. (B) Expression of Bcl-2 after transfection with siControl or siBcl-2 RNA in SUDHL4 cells. Shown are the results after 72 hours of transfection. (C) Percentage cell death after 4 hours of incubation with 10 μM XIAP antagonist in SUDHL4-transfected cells with siControl or siBcl-2 RNA. Error bars represent SD of the mean value of 3 experiments.
Sensitivity to the XIAP antagonist in relation to expression of Bcl-2. (A) Bcl-2 expression was detected in all the DLBCL samples tested using RT-MLPA analysis. Patient samples were divided into 2 groups: samples with an LD50 less than 39 μM or LD50 of 39 μM or greater. Horizontal lines represent the mean Bcl-2 expression level for each group. (B) Expression of Bcl-2 after transfection with siControl or siBcl-2 RNA in SUDHL4 cells. Shown are the results after 72 hours of transfection. (C) Percentage cell death after 4 hours of incubation with 10 μM XIAP antagonist in SUDHL4-transfected cells with siControl or siBcl-2 RNA. Error bars represent SD of the mean value of 3 experiments.
To further substantiate if Bcl-2 expression is involved in resistance to the XIAP antagonist in DLBCL cells, siRNA analysis was used to knock down Bcl-2 expression in the Bcl-2+ DLBCL cell line SUDHL4. The transfection efficiency in SUDHL4 was 70% to 80%. Time-course curves demonstrated that maximal repression of Bcl-2 expression was obtained in SUDHL4 cells 72 hours after transfection (Figure 4B). Therefore, we performed cell death assays at 72 hours. Untreated cells transfected with siBcl-2 showed an increase of 30% plus or minus 4% apoptotic cells in SUDHL4 compared with the control siRNA. Cells treated with 1396-12 showed 58% plus or minus 5% apoptosis above background levels of the inactive compound 1396-28 treatment. Addition of the XIAP antagonist to siBcl-2–transfected cells resulted in increased apoptosis, representing an additive effect compared with treatment with either 1396-12 or siBcl-2 alone. Thus, both Bcl-2 and XIAP make contributions to DLBCL survival (Figure 4C).
The XIAP antagonist does not sensitize cell lines to etoposide-induced cell death
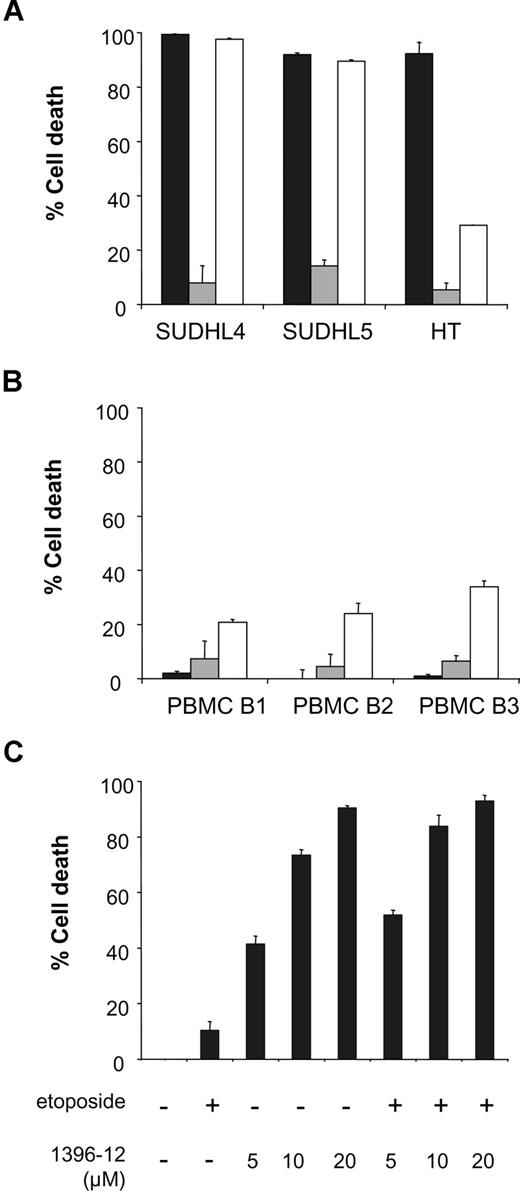
We compared sensitivity to the chemotherapeutic agents etoposide and rituximab with sensitivity to the XIAP antagonist in DLBCL-derived cell lines. SUDHL4, SUDHL5, and HT were incubated with optimized concentrations of etoposide, rituximab, and the XIAP antagonist 1396-12 for 24 hours. All 3 cell lines were highly sensitive to the XIAP antagonist and showed almost no sensitivity to etoposide-induced cell death. In addition, we found high levels of XIAP antagonist-induced cell death in HT cells that demonstrated relative low levels of rituximab-induced cell death (Figure 5A).
The XIAP antagonist does not induce apoptosis in normal PBMC B-cells and does not sensitize DLBCL cells to etoposide. (A) DLBCL cell lines SUDHL4, SUDHL5, and HT were incubated with 25 μM XIAP antagonist (■), 500 nM etoposide (▩), or 10 μg/mL rituximab (□). Cell death was detected after 24 hours of incubation. (B) PBMC B cells of 3 healthy donors were treated with 25 μM XIAP antagonist (■), 500 nM etoposide (▩), or 10 μg/mL rituximab (□). Cell death was detected after 24 hours of incubation. (C) SUDHL4 cells were incubated with increasing concentrations of the XIAP antagonist either with or without 500 nM etoposide. Cell death was measured after 16 hours of incubation. Error bars represent SD of the mean value of 3 experiments.
The XIAP antagonist does not induce apoptosis in normal PBMC B-cells and does not sensitize DLBCL cells to etoposide. (A) DLBCL cell lines SUDHL4, SUDHL5, and HT were incubated with 25 μM XIAP antagonist (■), 500 nM etoposide (▩), or 10 μg/mL rituximab (□). Cell death was detected after 24 hours of incubation. (B) PBMC B cells of 3 healthy donors were treated with 25 μM XIAP antagonist (■), 500 nM etoposide (▩), or 10 μg/mL rituximab (□). Cell death was detected after 24 hours of incubation. (C) SUDHL4 cells were incubated with increasing concentrations of the XIAP antagonist either with or without 500 nM etoposide. Cell death was measured after 16 hours of incubation. Error bars represent SD of the mean value of 3 experiments.
In normal PBMC B cells from healthy donors, almost no induction of cell death was observed by the XIAP antagonist or etoposide. However, rituximab induced 26% plus or minus 7% cell death in PBMC B cells (Figure 5B).
Furthermore, we investigated if the XIAP antagonist 1396-12 could sensitize cells to etoposide. SUDHL4 cells were treated with increasing concentrations of the XIAP antagonist with or without 500 nM etoposide. No significant additional increase in apoptosis was observed by combining the XIAP antagonist with etoposide (Figure 5C).
Discussion
Previous studies have shown that inhibition of the intrinsic apoptosis pathway is an important cause of chemotherapy resistance in DLBCL.4,,,–8 We have recently demonstrated that the majority of chemotherapy-refractory DLBCLs are characterized by constitutive caspase-9 activation with concomitant high expression levels of downstream apoptosis inhibitors, including XIAP.8,9 In these cases, resistance to chemotherapy-induced apoptosis was not due to failure to activate the intrinsic apoptosis pathway by chemotherapy, but was caused by inhibition of the downstream convergence apoptosis pathway. An important candidate that causes this downstream inhibition is XIAP, which interacts with active caspase-9 and active effector caspase-3 and caspase-7.12,13,15,16 Thus, restoring this downstream convergence pathway by blocking the function of downstream apoptosis-inhibiting proteins such as XIAP should result in “spontaneous” caspase-9–mediated apoptosis. Indeed, in the present study, we demonstrated that the small-molecule XIAP antagonist 1396-12 derepresses caspase-3/7 inhibition and induces high levels of apoptosis only in patients with DLBCL with high expression levels of XIAP and only in patients with constitutive caspase-9 activity.
The notion that the XIAP antagonist only derepresses caspase-3/7 inhibition also explains that the XIAP antagonist does not induce apoptosis in nonneoplastic GC B cells and PBMC B cells from healthy donors.24 In these cells, the intrinsic apoptosis pathway is not constitutively activated,9 and thus relieving the apoptosis blockade will not result in apoptosis. Furthermore, these findings explain why the XIAP antagonist did not sensitize DLBCL cells to etoposide-induced apoptosis, because the intrinsic pathway is already activated in XIAP-sensitive DLBCL cells, which cannot be further activated by etoposide.9
In this study, we also show that the XIAP antagonist 1396-12 is active as a single agent and can directly induce apoptosis. Compared with conventional agents used in the treatment of DLBCL, the XIAP antagonist can induce apoptosis, even in cases with relatively low levels of etoposide- or rituximab-induced cell death. In addition, almost no XIAP antagonist-induced cell death was observed in normal cells, whereas rituximab was more toxic to normal cells. Rodent toxicology studies also suggest that the XIAP antagonist is well tolerated, causing essentially no detectable hematopoietic toxicity or injury to vital organs at doses that achieve significant antitumor activity.21
To understand the variation in response to the XIAP antagonists between the DLBCL samples, we correlated the response to the XIAP antagonist with expression of XIAP and other apoptosis-inhibiting genes implicated in the intrinsic apoptosis pathway. Similar to previous observations for leukemia cells,24 the apoptosis-inducing effect of the XIAP antagonist was stronger in samples with relatively high levels of XIAP expression. However, treatment with the XIAP antagonist did not result in apoptosis in all samples with high expression of XIAP, indicating that other pathophysiologic mechanisms are also important in chemotherapy resistance.
We found that relative resistance to the XIAP antagonist in DLBCL cells correlates with high expression levels of Bcl-2. In DLBCL, Bcl-2 expression is strongly related to a poor clinical outcome, and thus probably is an important inhibitor of the intrinsic apoptosis pathway in a subset of DLBCL.20,39,40 Using siRNA analysis, we further substantiated this notion by demonstrating that knock-down of Bcl-2 expression in the DLBCL cell line SUDHL4 combined additively with the XIAP antagonist to induce apoptosis. Thus, both Bcl-2 and XIAP make contributions to survival in culture of DLBCL cells. Furthermore, DLBCLs with low Bcl-2 expression are more likely to have caspase-9 activated, and thus to respond to the XIAP antagonist.
These preclinical data indicate that the small-molecule XIAP antagonist 1396-12 can induce apoptosis of DLBCL. The in vitro sensitivity to the XIAP antagonist can be predicted based on biological markers (ie, expression levels of XIAP, Bcl-2, and levels of constitutive caspase-9 activation), suggesting the possibility of predefining patients that will benefit from XIAP antagonist therapy. Taken together, our findings indicate that further preclinical evaluations of XIAP antagonists as a possible therapy for DLBCL should be conducted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Marcel Flens from the department of Pathology of the Zaans Medical Center (Zaandam, the Netherlands) for providing us some of the fresh lymphoma biopsies used in this study.
This work was supported by grants VU 2003-2818 from the Dutch Cancer Society, SCOR LLS-7332 from the Leukemia and Lymphoma Society, U19 NCI-DDG CA -113318 from the National Institutes of Health/National Cancer Institute, and P01 CA-081534 from CLL-Chronic Lymphocytic Leukemia Research.
National Institutes of Health
Authorship
Contribution: S.A.G.M.C. performed research, analyzed data, and wrote the paper; J.C.R. contributed to interpretation of data, discussion, and paper writing; K.W. contributed to identification of the XIAP antagonists; C.P. contributed to interpretation of the results and discussion; R.H. contributed to identification of the XIAP antago-nists; E.H. contributed to interpretation of the results and discussion; J.D. performed siRNA analysis; K.C.M.C. performed research and analyzed data; P.K. performed research; C.J.H. contributed to discussion; G.J.O. contributed to discussion and paper writing; C.J.L.M.M. designed the study and contributed to discussion and paper writing; and J.J.O. designed and supervised the study and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joost J. Oudejans MD, VU University Medical Center, Department of Clinical Pathology, PO Box 7057, 1007 MB Amsterdam, the Netherlands; e-mail: jj.oudejans@vumc.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal