Abstract
TLR3 recognizes double-stranded RNA, a product associated with viral infections. Many details of TLR3-induced mechanisms have emerged from gene-targeted mice or inhibition studies in transformed cell lines. However, the pathways activated in human immune cells or cells from disease tissue are less well understood. We have investigated TLR3-induced mechanisms of human primary cells of the innate immune system, including dendritic cells (DCs), macrophages (MØs), endothelial cells (ECs), and synovial fibroblasts isolated from rheumatoid arthritis joint tissue (RA-SFs). Here, we report that while these cells all express TLR3, they differ substantially in their response to TLR3 stimulation. The key antiviral response chemokine IP-10 was produced by all cell types, while DCs and MØs failed to produce the proinflammatory cytokines TNFα and IL-6. Unexpectedly, TNFα was found secreted by TLR3-stimulated RA-SF. Furthermore, TLR3 stimulation did not activate NFκB, MAPKs, or IRF-3 in DCs and MØs, but was able to do so in ECs and RA-SF. These findings were specific for human cells, thereby revealing a complexity not previously expected. This is the first report of such cell type– and species-specific response for any TLR stimulation and helps to explain important difficulties in correlating murine models of inflammatory diseases and human inflammation.
Introduction
The discovery of Toll-like receptors (TLRs) uncovered a key mechanism used by the immune system to detect infections and tissue damage.1 There are 10 known human TLRs that recognize “molecular patterns” produced as a result of pathogenic infections. TLR3 is a member of this receptor family that is activated by double-stranded RNA (dsRNA),2 an intermediate formed by most viruses during their replication. The viral dsRNA functions as a danger signal released in the extracellular environment from dying virally infected cells, alerting inflammatory cells and contributing to systemic disease.3,4 In addition to microbial products, there is growing evidence that TLRs also recognize endogenous ligands found at sites of tissue destruction and cell death,5 and TLR3 has been shown to recognize mRNA released from dying cells.6 Although there are concerns that some of the data on endogenous ligands could be a result of contamination with microbial products, the detection of endogenous ligands would be useful in alerting the host of the presence of tissue injury induced by infection or other means. However, there is a potential drawback in that the same endogenous ligands are also found at sites of chronic inflammation and could further drive the inflammatory response in a TLR-dependent manner. There is increasing evidence that TLRs could be associated with chronic inflammatory diseases such as rheumatoid arthritis (RA).5,7 In this context, TLR3 expression is increased in the RA synovium,8,9 and the presence of TLR3 ligands might affect several aspects of the disease by acting on cells within the joint. In support of this, dsRNA can induce joint inflammation and cause arthritis in a murine disease model.10
Given the importance of TLRs in regulation of both positive and negative aspects of the immune response, there has been much interest in how their activation is controlled. The signaling pathways initiated by most TLRs are regulated by the adaptor protein MyD88 (myeloid differentiation factor 88), with some variations by the additional recruitment of MAL (MyD88 adaptor-like), TRAM (TRIF-related adaptor molecule), and TRIF (TIR-domain–containing adaptor inducing interferon β).11 However, TLR3 is unique since it does not use MyD88 but instead recruits only TRIF, which mediates the activation of NFκB and IRF-3. Activation of these pathways results in expression of various inflammatory mediators including the cytokines tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) and the chemokines IL-8 and IFN-inducible-10 (IP-10). However, as for most TLRs, little is known about TLR3-induced signaling and cellular responses in human primary cells. Previous findings are mainly the result of studies in murine cells, especially derived from knockout animals, or immortalized cell lines, which are all model systems of the human immune system. Furthermore, since many cell lines are of tumor origin, it is unclear if their responses are representative of the normal innate immune response. We therefore felt that it was important to define responses induced by TLR3 in human cell types, and in particular in cells involved in chronic inflammatory disease such as RA.
The findings presented here challenge the current beliefs that signaling pathways initiated by the viral response molecule dsRNA are mediated by NFκB, MAPK, and IRF-3 in all cell types. Our results highlight that responses induced by dsRNA/TLR3 differ between human cells of separate lineages, as well as between mouse and human. These are crucial findings for increasing the understanding of the human immune system and the inflammatory response to infection.
Materials and methods
Ethical approval for the use of human materials was granted by the Riverside Research Ethics Committee, London, United Kingdom.
Reagents
Human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) was generously provided by Beheringwerke (Marburg, Germany) and human recombinant IL-4 was from Novartis Pharma (Basel, Switzerland). Human recombinant macrophage colony-stimulating factor (M-CSF) and human IL-1α were from Genetics Institute (Madison, NJ). Escherichia coli (E coli) LPS was obtained from Sigma (St Louis, MO) and polyinosinic-polycytidylic acid (poly I:C) was from Amersham Life Science (Buckinghamshire, United Kingdom). Mouse serum was purchased from Sigma (St Louis, MO); anti–TLR3-FITC (clone 40C1285.6) (raised against a peptide corresponding to AA 54–71 in the LRR motifs of the TLR3 receptor extracellular domain) was from Imgenex (Cambridge, United Kingdom); and the corresponding IgG1-FITC–labeled isotype control was from Becton Dickinson (Oxford, United Kingdom). Protein inhibitor cocktail and chloroquine were purchased from Sigma (Poole, United Kingdom). The IRF-3 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); anti-HA, from Covance (Denver, PA); and anti-Flag, from Sigma (Poole, United Kingdom).
Cell isolation and culture conditions
Human peripheral blood mononuclear cells were isolated and differentiated for 5 to 7 days with 50 ng/mL GM-CSF, and 10 ng/mL IL-4 for dendritic cells (DCs), and for 3 to 4 days with 100 ng/mL M-CSF for macrophages (MØs), as previously described.12,13 When matured, DCs express the specific marker CD83 and MØs express varied levels of CD14. During assays, both cells were cultured at 106 cells/mL in RPMI1640 5% FCS, 1% penicillin/streptomycin. Rheumatoid arthritis synovial fibroblasts (RA-SFs) and human umbilical vein endothelial cells (HUVECs) were isolated as previously described.14,15 RA-SFs express vimentin at more than 95% and were cultured at 105 cells/mL in DMEM 10% FCS, 100 U/mL penicillin/streptomycin, and 200 μg/mL indomethacin (Sigma, Poole, United Kingdom); HUVECs were 99.19% pure16 and were cultured at 105 cells/mL on gelatin in RPMI1640, 10% FCS, 10% newborn calf serum (Gibco, Auckland, NZ), 1% penicillin/streptomycin, 15 μg/mL endothelial growth supplement (Sigma, Poole, United Kingdom), and 50 IU/mL heparin (CD Pharmaceutical, Wrexham, United Kingdom). Murine bone marrow DCs were derived as previously described17 and cultured at 105 cells/mL in DMEM 5% FCS and 1% penicillin/streptomycin.
Reverse transcriptase–polymerase chain reaction
RNA was isolated using the Qiagen RNA Blood isolation kit (Qiagen, Hilden, Germany). Total RNA was reverse transcribed with Superscript II RNase H− reverse transcriptase (Gibco, Paisley, United Kingdom) and oligo (dT) primer. The primers used were as follows: 5′-GCAAACACAAGCATTCGGAATCT-3′ and 5′-TTGAAGGCTTGGGACCAAGGCA-3′ for TLR3; 5′-TGGATACGTTTCCTTATAAG-3′ and 5′-GAAATGGAGGCACCCCTTC-3′ for TLR4; 5′-GAAGCTCAGAAGCAGTATTGGTC-3′ and 5′-GGTTGGTGTAGGATGACAAACTCC-3′ for MD-2. Amplification was performed in a Dyad polymerase chain reaction (PCR) machine (MJ Instruments, Watertown, MA) and consisted of 35 cycles with the annealing temperature 62°C for TLR3 and 40 cycles with the annealing temperature 54°C for TLR4 and MD-2.
Flow cytometry
Intracellular and extracellular TLR3 expressions were determined using flow cytometry. Cells fixed with 2% paraformaldehyde were either left untreated or permeabilized with 1% saponin. The cells were incubated with mouse antihuman TLR3-FITC or isotype control for 30 minutes on ice in the presence of 1% mouse serum. The samples were analyzed on a Becton Dickinson LSR flow cytometer.
Cytokine analysis by enzyme-linked immunosorbent assay
Supernatants were harvested after 18-hour stimulation with LPS (100 ng/mL) or poly I:C (20 μg/mL). Enzyme-linked immunosorbent assay (ELISA) reagents for measurement of human TNFα and IL-6 were from Pharmingen (San Diego, CA) and human IP-10, murine IL-6, and murine TNFα reagents were obtained from R&D Systems (Abington, United Kingdom). Absorbance of 450 nm was measured on a spectrophotometric ELISA plate reader (Labsystems Multiscan Biochromic, Cambridge, United Kingdom) using the Ascent version 2.4.2 software program (Thermo Labsystems, Cambridge, United Kingdom). Results are expressed as the mean concentration of triplicate cultures plus or minus SD.
Mixed lymphocyte reaction
Human DCs were stimulated and cultured in graded doses with 1 × 105 allogeneic elutriated T cells in quadruplicate. Proliferation was measured on day 5 by thymidine incorporation after a 16-hour pulse with [3H] thymidine (0.5 μCi/well [0.0185 MBq]; Amersham Life Science).
TaqMan real-time PCR
Total RNA was extracted using RNeasy Kit (Qiagen) and PCR was performed using an ABI PRISM 7700 Sequence Detection System and predeveloped assay reagents (PerkinElmer Applied Biosystems, Warrington, United Kingdom). The one-step real-time PCRs were run for 40 cycles, and all quantitations were normalized to GAPDH. Relative quantitation was performed using the comparative ΔΔCt method according to the manufacturer's instructions.
NFκB luciferase reporter assay
Cells were infected for 1 to 2 hours in serum-free media with a recombinant replication-deficient adenovirus containing an NFκB luciferase reporter gene, which is a modification of the pNFκB-Reporter vector (BD Clontech, Palo Alto, CA) and was kindly provided by Dr Paul McCray (University of Iowa). DCs and MØs were infected at 100:1 multiplicity of infection (moi); ECs, at 100:1 at 60% confluence; and RA-SFs, at 500:1, resulting in more than 90% infection efficiencies, as shown by us and others.12,13,18,19 The cells were cultured for 24 hours prior to 6-hour stimulation. The luciferase assay was performed as previously described.20
Western blotting
Nuclear protein fractions were extracted as previously described,21 and whole-cell lysates were prepared in 1% Triton X100, 10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1 mM EDTA, 0.1 mM Na3VO4, 5 mM NaF, 1 mM DTT, and 1 × protein inhibitor cocktail. Equal amounts of proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel and transferred to a PVDF membrane for Western blotting.
Limulus assay
The endotoxin content in all reagents was measured with the Limulus assay (BioWhittaker, Wokingham, United Kingdom) according to the manufacturer's instruction. Only batches of poly I:C with no detectable levels of endotoxin were used in this study.
Statistical analyses
Mean and standard deviation (SD) were calculated using GraphPad Prism version 3.02 (GraphPad Software, San Diego, CA).
Results
Analysis of TLR3 expression on human immune cells
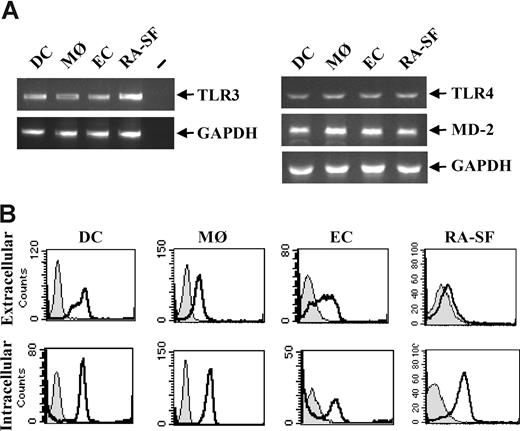
The effects of TLR3 stimulation on human immune cells were analyzed due to their key roles in infectious diseases and acute as well as chronic inflammation. Professional immune cells such as human primary dendritic cells (DCs) and macrophages (MØs), and nonprofessional immune cells such as endothelial cells (ECs) and synovial fibroblasts from RA disease tissue (RA-SFs) were examined. TLR3 expression was detected at the RNA level in all the cell types studied (Figure 1A). By fluorescence-activated cell sorting (FACS), a proportion of the cells was shown to express TLR3 extracellularly, whereas a higher level of TLR3 was found expressed intracellularly in all cell types (Figure 1B).
TLR3 is expressed on all cell types used in this study. Human DCs were generated from peripheral blood monocytes after 5- to 7-day culture in the presence of 10 ng/mL IL-4 and 50 ng/mL GM-CSF. MØs were differentiated from peripheral blood monocytes for 3 to 4 days in the presence of 100 ng/mL M-CSF. ECs were isolated from human umbilical veins and were used at passages 2 to 3. Fibroblasts were isolated from synovial tissue from RA patients undergoing joint replacement. (A) Total RNA was isolated from each cell type and 1 μg was subjected to reverse transcriptase-PCR and analyzed for TLR3, TLR4, MD-2, or GAPDH expression with PCR. − indicates PCR-negative control. (B) DCs, MØs, ECs, and RA-SFs were analyzed for both intracellular and membrane-bound TLR3 by flow cytometry. Cells stained with isotype control (filled histograms) or anti–TLR3-FITC antibody (open black histograms) are shown.
TLR3 is expressed on all cell types used in this study. Human DCs were generated from peripheral blood monocytes after 5- to 7-day culture in the presence of 10 ng/mL IL-4 and 50 ng/mL GM-CSF. MØs were differentiated from peripheral blood monocytes for 3 to 4 days in the presence of 100 ng/mL M-CSF. ECs were isolated from human umbilical veins and were used at passages 2 to 3. Fibroblasts were isolated from synovial tissue from RA patients undergoing joint replacement. (A) Total RNA was isolated from each cell type and 1 μg was subjected to reverse transcriptase-PCR and analyzed for TLR3, TLR4, MD-2, or GAPDH expression with PCR. − indicates PCR-negative control. (B) DCs, MØs, ECs, and RA-SFs were analyzed for both intracellular and membrane-bound TLR3 by flow cytometry. Cells stained with isotype control (filled histograms) or anti–TLR3-FITC antibody (open black histograms) are shown.
Differential induction of proinflammatory and interferon response cytokines by TLR3 stimulation
To evaluate the responses of TLR3 stimulation, the effects of poly I:C treatment on cytokine production by the different human immune cells were examined. The TLR4 ligand LPS was used as a control stimulus, and, as shown in Figure 1A, all cell types studied also express TLR4 and MD-2. In murine cells, LPS induces similar pathways as dsRNA, leading to production of proinflammatory cytokines such as IL-6 and TNFα, as well as expression of IFN-regulated genes (IRGs), such as the chemokine IP-10.2,22 As predicted, poly I:C induced IP-10 production from DCs to similar levels as LPS-stimulated cells (Figure 2A). However unlike LPS, poly I:C did not induce TNFα, IL-6 (Figure 2A), or IL-8 (data not shown) in human DCs. This was not due to production of the anti-inflammatory cytokine IL-10, since it was not induced by poly I:C, whereas IL-10 was produced after LPS stimulation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The induction of IP-10 but not TNFα or IL-6 was repeated in poly I:C–stimulated MØs (Figure 2A). This effect was specific for human cells, since TNFα or IL-6 was produced by murine DCs after poly I:C stimulation (Figure 2B), confirming previous studies.2,22 As poly I:C was described by another group to induce TNFα and IL-6 mRNA in human DCs,23 we investigated whether stimulation with poly I:C could increase TNFα and IL-6 mRNA levels in the absence of increased protein expression. However, studies of mRNA expression confirmed our protein studies with poly I:C inducing very little, if any, TNFα and IL-6 mRNA, whereas LPS caused high mRNA levels of both cytokines (Figure 2C). In addition, we could detect type I IFN from both poly I:C– and LPS-stimulated human DCs (data not shown). Stimulation of human DCs with poly I:C was also previously shown to induce DC activation and functional maturation.24-26 We could confirm these findings, since poly I:C stimulation of human DCs resulted in increased ability to induce an allogenic MLR (Figure 2D) and up-regulation of the costimulatory markers CD80 and CD86 and the maturation markers HLA-DR and CD83 (Figure 2D).
Poly I:C–induced expression of IFN-regulated genes but not proinflammatory cytokines is both cell-type and species specific. (A) Human DCs and MØs, (B) murine DCs, (E) ECs, and (E,F) RA-SFs were stimulated with either 100 ng/mL LPS or poly I:C (in μg/mL) as indicated. The supernatants were analyzed for IP-10, IL-6, and TNFα by ELISA. The data are mean values (± SD) from triplicates and are representative of at least 3 experiments using cells of different donors. (C) Human DCs were stimulated with either 100 ng/mL LPS or 20 μg/mL poly I:C, and total RNA was isolated and assessed for the levels of TNFα and IL-6 mRNA by Taqman PCR. The points correspond to values from 4 different donors (± SD). (D) Human DCs were stimulated with 20 μg/mL poly I:C or 100 ng/mL LPS and cocultured with 105 allogeneic T cells in quadruplicate. Proliferation was determined on day 5 using [3H] thymidine. Each point represents the mean (± SEM) from 3 independent experiments on unrelated donors. Human DCs were also examined for cell surface expression of CD80, CD86, HLA-DR, and CD83 by flow cytometry. Unstimulated DCs (black line) and DCs treated with 20 μg/mL poly I:C for 48 hours (gray line) were compared. Cells stained with isotype control (filled histograms) or anti-TLR3 antibody (open black histograms) are shown. One representative of 3 independent experiments is shown.
Poly I:C–induced expression of IFN-regulated genes but not proinflammatory cytokines is both cell-type and species specific. (A) Human DCs and MØs, (B) murine DCs, (E) ECs, and (E,F) RA-SFs were stimulated with either 100 ng/mL LPS or poly I:C (in μg/mL) as indicated. The supernatants were analyzed for IP-10, IL-6, and TNFα by ELISA. The data are mean values (± SD) from triplicates and are representative of at least 3 experiments using cells of different donors. (C) Human DCs were stimulated with either 100 ng/mL LPS or 20 μg/mL poly I:C, and total RNA was isolated and assessed for the levels of TNFα and IL-6 mRNA by Taqman PCR. The points correspond to values from 4 different donors (± SD). (D) Human DCs were stimulated with 20 μg/mL poly I:C or 100 ng/mL LPS and cocultured with 105 allogeneic T cells in quadruplicate. Proliferation was determined on day 5 using [3H] thymidine. Each point represents the mean (± SEM) from 3 independent experiments on unrelated donors. Human DCs were also examined for cell surface expression of CD80, CD86, HLA-DR, and CD83 by flow cytometry. Unstimulated DCs (black line) and DCs treated with 20 μg/mL poly I:C for 48 hours (gray line) were compared. Cells stained with isotype control (filled histograms) or anti-TLR3 antibody (open black histograms) are shown. One representative of 3 independent experiments is shown.
Given these unexpected findings in human primary DCs and MØs, we examined the responses to poly I:C in ECs, which was previously shown to produce IL-6 mRNA and adhesion molecules after poly I:C stimulation.27,28 In addition, we analyzed the responses in RA-SFs, which have also been shown to respond to poly I:C treatment.29-31 Stimulation of ECs with poly I:C induced IP-10 secretion, whereas no substantial production could be observed after LPS treatment (Figure 2E). Furthermore, poly I:C stimulation of ECs led to the production of IL-6 and IL-8, at levels comparable with LPS (Figure 2E and data not shown). Similar results were also observed for poly I:C–stimulated RA-SFs (Figure 2E). An interesting observation made in this study was that poly I:C could induce TNFα secretion by human RA-SFs (Figure 2F). This effect was specific to poly I:C since TNFα levels could not be detected after LPS stimulation (Figure 2F). It was also found specific for RA-SF, since poly I:C–stimulated human skin fibroblasts (HSFs) produced only IL-6 and not TNFα (data not shown). This is the first report describing the ability of a ligand to induce TNFα production from a human fibroblast population. Overall, these results indicated that responses induced by poly I:C in human cells differed depending on the cell lineage and can be divided into response induced by myeloid cells (DCs and MØs) and nonmyeloid cells (ECs and RA-SFs).
Poly I:C stimulation was previously shown to be blocked by inhibitors of endosomal maturation,32,33 suggesting that the TLR3 signaling is initiated intracellularly. To address this issue, we investigated if the cell type–specific difference was the result of TLR3 activation mediated from different locations in myeloid and nonmyeloid cells. However, in the presence of chloroquine, an inhibitor of endosomal acidification, IP-10 production was inhibited in both DCs and RA-SFs (Figure 3). This suggests that cells of both lineages use the intracellular TLR3 and that the distribution of the receptor is not the cause of these variations.
Poly I:C stimulation is dependent on endosomal maturation in both human dendritic cells and RA synovial fibroblasts. Human DCs and RA-SFs were incubated with chloroquine for 1 hour prior to stimulation with 20 μg/mL poly I:C for 18 hours. The supernatants were analyzed by ELISA. The data are mean values (± SD) from triplicates.
Poly I:C stimulation is dependent on endosomal maturation in both human dendritic cells and RA synovial fibroblasts. Human DCs and RA-SFs were incubated with chloroquine for 1 hour prior to stimulation with 20 μg/mL poly I:C for 18 hours. The supernatants were analyzed by ELISA. The data are mean values (± SD) from triplicates.
Cell lineage–specific activation of NFκB and MAPKs upon TLR3 stimulation
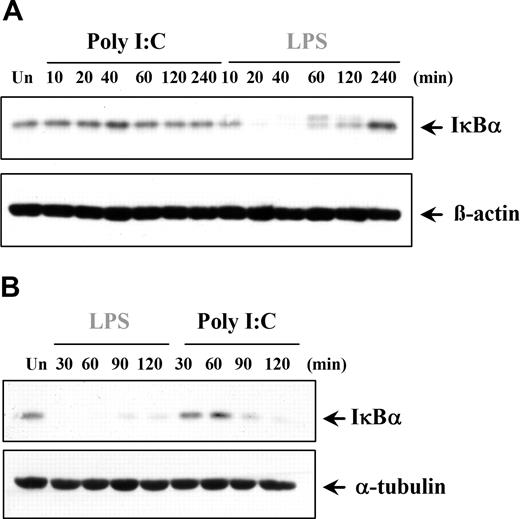
The finding that poly I:C induces only proinflammatory cytokines in certain cell types prompted the examination of downstream signaling pathways. NFκB is involved in regulating TNFα and IL-6 expression, and previous studies have claimed that dsRNA stimulation can activate NFκB.2,34-39 We examined if the cell type–specific differences observed with cytokine production were due to a variation in NFκB regulation. Interestingly, in DCs and MØs, poly I:C was unable to activate NFκB, whereas LPS was a potent activator, as expected (Figure 4A). In contrast, NFκB transcriptional activity in ECs and RA-SFs was induced by poly I:C to levels comparable with LPS stimulation (Figure 4A). Furthermore, the lack of response in the myeloid cells was again shown to be a species-specific effect of human cells since poly I:C could stimulate NFκB activity in murine DCs (Figure 4B). The NFκB results were supported by studies of poly I:C–induced IκBα degradation. In a similar manner, poly I:C did not induce IκBα degradation in either DCs (Figure 5A) or MØs (data not shown). Conversely, poly I:C stimulation of RA-SFs resulted in IκBα degradation, confirming our NFκB results (Figure 5B). The poly I:C–induced IκBα degradation in RA-SFs occurred with a delayed kinetics than LPS stimulation, which induced IκBα degradation in all cell types, as expected.
Poly I:C stimulation induces activation of NFκB in endothelial cells and RA synovial fibroblasts but not in dendritic cells and macrophages. (A) Human DCs, MØs, ECs, RA-SFs, and (B) murine DCs were infected for 1 hour with an adenovirus carrying an NFκB luciferase reporter gene. The cells were stimulated for 6 hours with 100 ng/mL LPS or poly I:C (in μg/mL) as indicated and then analyzed for luciferase activity. The data are mean values (± SD) from triplicates and are representative of at least 3 experiments using cells of different donors.
Poly I:C stimulation induces activation of NFκB in endothelial cells and RA synovial fibroblasts but not in dendritic cells and macrophages. (A) Human DCs, MØs, ECs, RA-SFs, and (B) murine DCs were infected for 1 hour with an adenovirus carrying an NFκB luciferase reporter gene. The cells were stimulated for 6 hours with 100 ng/mL LPS or poly I:C (in μg/mL) as indicated and then analyzed for luciferase activity. The data are mean values (± SD) from triplicates and are representative of at least 3 experiments using cells of different donors.
Poly I:C stimulation induces degradation of IκBα in fibroblasts but not in dendritic cells. (A) DCs and (B) RA-SFs were either left untreated (Un) or stimulated with poly I:C (20μg/mL) or LPS (100 ng/mL). The cell lysates were examined for the level of IκBα, β-actin, or α-tubulin by Western blotting. Representatives of 3 independent experiments per cell type are shown.
Poly I:C stimulation induces degradation of IκBα in fibroblasts but not in dendritic cells. (A) DCs and (B) RA-SFs were either left untreated (Un) or stimulated with poly I:C (20μg/mL) or LPS (100 ng/mL). The cell lysates were examined for the level of IκBα, β-actin, or α-tubulin by Western blotting. Representatives of 3 independent experiments per cell type are shown.
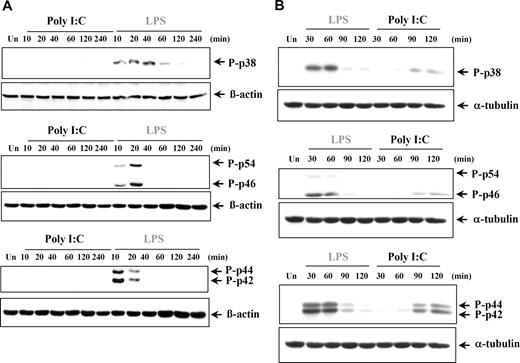
The production of proinflammatory cytokines can also be regulated by the 3 major MAPKs: p38 MAPK, JNK (p46/p54), and ERK (p42/p44) MAPKs. It was previously reported that poly I:C treatment induced phosphorylation of the MAPKs in murine cells and various cell lines.2,35,36,40-44 However in our study, poly I:C stimulation did not induce phosphorylation of p38, JNKs, or ERKs at any time point analyzed in either human DCs (Figure 6A) or MØs (data not shown), whereas LPS was found to potently induce all 3 groups of MAPKs, as expected. Conversely, stimulation of RA-SFs by both poly I:C and LPS resulted in activation of all 3 MAPKs, albeit with different kinetics (Figure 6B). To elucidate if the late activation of the MAPK in response to poly I:C was due to an indirect effect, we stimulated RA-SFs in the presence of brefeldin A, an inhibitor of the secretory pathway. Brefeldin A had no effect on poly I:C–induced ERK phosphorylation (Figure S2), indicating that the activation was not mediated by a secreted autocrine mediator.
Poly I:C treatment does not induce phosphorylation of p38, JNK, and ERK in human dendritic cells but does so in RA synovial fibroblasts. (A) Human DCs and (B) synovial fibroblasts from RA tissue were either left untreated (Un) or stimulated with LPS (100 ng/mL) or poly I:C (20μg/mL). The cell lysates were examined for phospho-p38, phospho-JNK, phospho-ERK, or β-actin by Western blotting. Representatives of 3 independent experiments are shown.
Poly I:C treatment does not induce phosphorylation of p38, JNK, and ERK in human dendritic cells but does so in RA synovial fibroblasts. (A) Human DCs and (B) synovial fibroblasts from RA tissue were either left untreated (Un) or stimulated with LPS (100 ng/mL) or poly I:C (20μg/mL). The cell lysates were examined for phospho-p38, phospho-JNK, phospho-ERK, or β-actin by Western blotting. Representatives of 3 independent experiments are shown.
IRF-3 activation in response to poly I:C stimulation is restricted to cells of nonmyeloid lineage
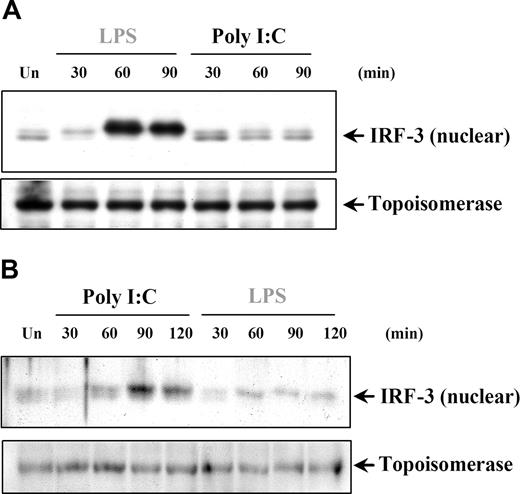
IRF-3 has been shown to have a critical role in TLR3-induced responses through the TRIF-dependent pathway leading to IFNs and IRGs, such as IP-10. We aimed to address the question of whether the induction of IRF-3 could explain the poly I:C–induced IP-10 production in DCs and MØs in the absence of NFκB and MAPK activation. IRF-3 is found in the cytoplasm as an inactive monomer and becomes activated by phosphorylation, followed by dimerization and translocation to the nucleus.45,46 The involvement of IRF-3 in poly I:C signaling was examined by measuring the change in IRF-3 nuclear levels. In this study, we could only detect IRF-3 nuclear translocation after LPS, but not poly I:C treatment of DCs (Figure 7A) and MØs (data not shown). Conversely, in both RA-SFs (Figure 7B) and ECs (data not shown), the results showed enhanced levels of nuclear IRF-3 after only poly I:C and not LPS stimulation. These results further confirmed that signaling events induced by poly I:C are tightly regulated according to the cell type involved, and differ substantially between professional and nonprofessional immune cells.
Poly I:C stimulation of RA synovial fibroblasts but not DCs activates nuclear translocation of IRF-3. (A) Human DCs and (B) RA-SFs were either left unstimulated (Un) or stimulated with poly I:C (20 μg/mL) and LPS (100 ng/mL), and the nuclear fractions were examined for nuclear IRF-3 and topoisomerase by Western blotting. Representatives of 3 independent experiments per cell type are shown.
Poly I:C stimulation of RA synovial fibroblasts but not DCs activates nuclear translocation of IRF-3. (A) Human DCs and (B) RA-SFs were either left unstimulated (Un) or stimulated with poly I:C (20 μg/mL) and LPS (100 ng/mL), and the nuclear fractions were examined for nuclear IRF-3 and topoisomerase by Western blotting. Representatives of 3 independent experiments per cell type are shown.
Discussion
This study investigated and compared the responses induced by TLR3 stimulation of human primary DCs, MØs, ECs, and RA-SFs from disease tissue. These cell types are involved in responses to infection and both acute and chronic inflammation. The results in this study have uncovered a considerable selectivity in the TLR3-induced responses of these cell types, with substantial differences observed between myeloid cells (DCs and MØs) and nonmyeloid cells (ECs and RA-SFs). Surprisingly, DCs and MØs were found to produce the IRG IP-10, but not cytokines such as TNFα, IL-6, or IL-8 after poly I:C stimulation. Studies on mRNA expression of TNFα and IL-6 in human DCs confirmed our protein data showing no poly I:C–induced cytokine production, whereas we could detect IFNβ mRNA, as shown by other groups.25,26 This contradicts a previously published study that failed to detect IFNβ but showed induction of TNFα and IL-6 mRNA, although no protein studies were performed.23 Furthermore, we showed that poly I:C stimulation of human DCs induced up-regulation of the maturation markers HLA-DR, CD80, CD86, and CD83, and T-cell stimulatory capacity, all of which have previously been demonstrated by other groups.24-26 This is the first report showing induction of DC maturation and production of IRGs from human DCs and MØs, but not proinflammatory cytokines, in response to TLR3 stimulation. Furthermore, of great interest, this phenotype was shown to be specific for human myeloid cells since both TNFα and IL-6 could be produced by murine DCs after poly I:C stimulation. Moreover, in contrast to DCs and MØs, the cells of nonmyeloid origin, ECs and RA-SFs, produced both types of cytokines after poly I:C treatment. This is a major difference in the behavior of TLR3 signaling between human and mouse and could affect how we interpret and extrapolate data between the 2 systems, especially when considering viral disease models, where TLR3 might have a role, or where cell necrosis and release of RNA may be involved.
The differences between the myeloid and nonmyeloid cell types were also reflected in the TLR3 signaling pathways. Unexpectedly, poly I:C did not induce activation of NFκB, MAPKs, or IRF3 in DCs and MØs, but was able to do so in ECs and RA-SFs. The inability of poly I:C to activate these pathways in DCs and MØs could explain the failure to produce TNFα, IL-6, and IL-8, since their expressions are regulated by NFκB. Conversely, the activation of NFκB and MAPKs in RA-SFs and ECs would also explain why poly I:C could induce IL-6 and IL-8 in these cells. Our results confirm the previously reported observation that poly I:C stimulation regulates NFκB in human ECs.27,47
We have previously shown that there are major differences in the signaling pathways of other TLRs between human cells of myeloid and nonmyeloid lineages.19,20,48 A possible explanation for our results could be cell type–specific expression of signaling molecules between cell lineages, as was shown with IKKϵ expression between murine macrophages and fibroblasts.49 Such differential expression could account for IFNβ and IP-10 production but no NFκB activation or secretion of proinflammatory cytokines in poly I:C–stimulated DCs and MØs. It is possible that these cells lack a signaling molecule similar to feckless described by Beutler et al.50 Cells from mice with a feckless mutation fail to activate NFκB or produce TNFα in response to dsRNA/TLR3, although dsRNA is still able to initiate IFNβ production. Another possibility is that additional transcription factors, such as other IRFs, are involved in TLR3 activation in human myeloid cells. As such, we have evidence that poly I:C induces a rapid and transient nuclear accumulation of IRF-1 in DCs (Figure S3). This is an interesting area that we are actively pursuing further.
An interesting finding in this study was that poly I:C stimulation of RA-SFs induced TNFα production, which was specific for poly I:C since LPS treatment failed to induce the same response. The ability of poly I:C to induce TNFα production does not appear to be a general property of all fibroblast populations. We were unable to detect TNFα production in HSFs in response to poly I:C even though IL-6 was produced by these cells. This is not the only difference we have observed between RA-SFs and HSFs, as the former48 unlike the latter are able to respond to LPS. One could therefore hypothesize that RA-SFs have an inflammatory phenotype, possibly due to their environment. It is interesting to note that poly I:C induces TNFα production in cultures of RA joint synovial membranes from which RA-SFs are derived (S.M.S. et al, manuscript in preparation). Furthermore, TNFα production by poly I:C–stimulated RA-SFs is unlikely to be caused by contamination of MØs or T cells, since we could not detect CD14- or CD3-expressing cells in our RA-SF cultures. If MØs were present, we would have observed TNFα expression after LPS and not poly I:C stimulation of these cultures. However, although unlikely, we cannot completely rule out the presence of other contaminating cell types. Historically nonmyeloid cells were not believed to be able to express TNFα, however, supporting observations were recently reported by 2 independent groups in murine embryonic fibroblasts (MEFs).51,52 They showed that LPS stimulation of MEFs induced low levels of TNFα that was a result of IRF-3 activation. We show in our human cell systems that there is a correlation between TNFα production and IRF-3 activation. As such, LPS stimulation of DCs and MØs induces TNFα production and IRF-3 activation, whereas poly I:C does not. The situation is reversed in RA-SFs and it is poly I:C stimulation and not LPS that induces both TNFα and IRF-3.
We have previously shown that NFκB is required for LPS-induced MHC class II and CD80/86 expression and antigen presentation.53,54 However, since TLR3-induced up-regulation of these molecules could be observed in the absence of a detectable NFκB activation, their expression appears to be NFκB independent. Furthermore, IRF-3 is thought to be required for TLR3-induced type I IFN and IP-10 production. However, since no IRF-3 activation could be seen in DCs and MØs, it is unclear what regulates the production of IP-10 and type I IFNs in these cell types. This is the subject of ongoing investigations.
One obvious reason for the variations between the cell types is that they have very different functions in the immune system. DCs and MØs are professional antigen-presenting cells involved in T-cell activation, whereas ECs and RA-SFs are nonprofessional immune cells. Thus, given their diverse roles in the immune response, it makes sense that the same ligand would induce individual cell type–specific responses. For example, stimulation of DCs and MØs by TLR3 appeared to selectively induce IP-10, a key chemokine targeting T lymphocytes, hence specifically enhancing recruitment of T cells to the site of inflammation. In contrast, this selectivity in cytokine production was not observed with ECs and RA-SFs. In these cells, TLR3 activation instead induced a broader range of proinflammatory mediators, suggesting participation of these cell types to chronic inflammation. We also show that TLR3 stimulation results in DC maturation, favoring increased T-cell activation. Furthermore, DCs and MØs are capable of inducing cross-presentation,55,56 and TLR3-stimulated DCs can cross-present antigens from virally infected cells to promote priming of virus-specific T cells.57-59 It is therefore possible that TLR3-stimulated DCs and MØs are involved in recruitment of T cells and induction of cross-presentation. Interestingly, TLR3 stimulation also leads to a marked increase in cross-priming toward cell-associated antigens.60 In this way, peripheral tolerance could be overcome by dsRNA and thus trigger an autoimmune organ-specific disease.60
The results presented in this study differ from the literature using other cell systems, and they indicate that the signaling pathways induced by poly I:C in human primary cell types and cells from disease tissue are complex. While these observations have raised the question of why differences exist between cell types stimulated with the same ligand, they also highlight the importance of studying signaling pathways in relevant cell types and the danger in directly transferring the results observed in cell lines or murine systems to human systems. By characterizing the pattern of activation induced by each cell type in response to the TLR3 ligand, this study increases the understanding of the possible contributions of each cell to the innate host responses to viral infections or tissue damage. The differences in TLR3 stimulation between the cell types are subject to further investigation, in order to fully understand the specific role of TLR3 in the human immune response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Arthritis Research Campaign of the United Kingdom and the Kennedy Institute of Rheumatology Trustees.
Authorship
Contribution: A.M.L. and S.K.D. performed research; C.M., L.M.W., and S.M.S. contributed new reagents/analytic tools; A.M.L, M.F., and B.M.F. designed research, analyzed data, and wrote the paper. B.M.F. and M.F. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian M. Foxwell, Kennedy Institute of Rheumatology Division, Faculty of Medicine, Imperial College of Science, Technology and Medicine, 1 Aspenlea Road, Hammersmith, London, W6 8LH, United Kingdom; e-mail: b.foxwell@imperial.ac.uk.

![Figure 2. Poly I:C–induced expression of IFN-regulated genes but not proinflammatory cytokines is both cell-type and species specific. (A) Human DCs and MØs, (B) murine DCs, (E) ECs, and (E,F) RA-SFs were stimulated with either 100 ng/mL LPS or poly I:C (in μg/mL) as indicated. The supernatants were analyzed for IP-10, IL-6, and TNFα by ELISA. The data are mean values (± SD) from triplicates and are representative of at least 3 experiments using cells of different donors. (C) Human DCs were stimulated with either 100 ng/mL LPS or 20 μg/mL poly I:C, and total RNA was isolated and assessed for the levels of TNFα and IL-6 mRNA by Taqman PCR. The points correspond to values from 4 different donors (± SD). (D) Human DCs were stimulated with 20 μg/mL poly I:C or 100 ng/mL LPS and cocultured with 105 allogeneic T cells in quadruplicate. Proliferation was determined on day 5 using [3H] thymidine. Each point represents the mean (± SEM) from 3 independent experiments on unrelated donors. Human DCs were also examined for cell surface expression of CD80, CD86, HLA-DR, and CD83 by flow cytometry. Unstimulated DCs (black line) and DCs treated with 20 μg/mL poly I:C for 48 hours (gray line) were compared. Cells stained with isotype control (filled histograms) or anti-TLR3 antibody (open black histograms) are shown. One representative of 3 independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-02-072934/7/m_zh80220709130002.jpeg?Expires=1767696654&Signature=DCElPTYv-PhxALR7605UIch2iijt4FaSC41Z31M~Ya69hpT0tcbKlmg8WSQ1MVbURDm8jkqgVO~W2qEQnl6BQNxifbGc1uPtfz4rb5DJZQ3lbiwPGNQF5EocAscNhla-kymha26Q9jQP~-rwW-l3vGeWTw6dU6IS4cGMdA8EoryWG3M~yKyNm6f9BwDLvMTtMOBRI3yoR-11dxFwf8O3vcC~Ar5PQtkvnDTHCcdVBbpTvDoclmcZwGbAzUq~-M6Fn5oplXHtsVdgaEdsocYc0cCHaXseYWgQRsqHlx3uRcW3guOW9pAN9P7tgNvT38UV3tbDaBEAvVGNdnVPsLGcUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal