Abstract
Initial analysis of the Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial of relapsed multiple myeloma patients showed significantly longer time to progression, higher response rate, and improved survival with single-agent bortezomib versus high-dose dexamethasone. In this updated analysis (median follow-up: 22 months), survival was assessed in both arms, and efficacy updated for the bortezomib arm. Median survival was 29.8 months for bortezomib versus 23.7 months for dexamethasone, a 6-month benefit, despite substantial crossover from dexamethasone to bortezomib. Overall and complete response rates with bortezomib were 43% and 9%, respectively; among responding patients, 56% improved response with longer therapy beyond initial response, leading to continued improvement in overall quality of response. Higher response quality (100% M-protein reduction) was associated with longer response duration; response duration was not associated with time to response. These data confirm the activity of bortezomib and support extended treatment in relapsed multiple myeloma patients tolerating therapy. This study is registered at http://clinicaltrials.gov (Study ID NCT00048230).
Introduction
The international, randomized, phase 3 Assessment of Proteasome Inhibition for Extending Remissions (APEX) trial led to full approval of bortezomib in 2005 for treatment of multiple myeloma (MM) patients who have received at least one prior therapy. In the initial APEX report, single-agent bortezomib demonstrated superior efficacy to high-dose dexamethasone in terms of significantly longer median time to progression (TTP, 6.2 vs 3.5 months, P < .001), higher response rates (38% vs 18%, P < .001), and improved survival (1-year survival rate: 80% vs 66%, P = .003; hazard ratio for overall survival [OS]: 0.57, P = .001).1
As a result, the high-dose dexamethasone arm was halted at interim analysis following the recommendations of an independent data-monitoring committee. A companion crossover study, which offered single-agent bortezomib initially only to patients with progressive disease (PD) on high-dose dexamethasone, was opened to all patients randomized to high-dose dexamethasone.
In this updated analysis, we report whether bortezomib continues to provide improved survival compared with high-dose dexamethasone. Other updated efficacy parameters for the bortezomib arm are reported. The relationship between duration of response (DOR) and quality of response as measured by M-protein reduction was analyzed, as was the relationship between DOR and time to first response (TTR). In addition, we report an exploratory analysis comparing patients who initially received bortezomib on APEX with those who first received high-dose dexamethasone and crossed over to receive bortezomib on the companion study.
Methods
Approval was obtained from the institutional review boards of all participating institutions (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Informed consent was obtained in accordance with the Declaration of Helsinki. APEX study details have been reported.1 Patients with MM and measurable disease following 1 to 3 prior therapies were randomized to receive bortezomib (Millennium Pharmaceuticals [Cambridge, MA] and Johnson & Johnson Pharmaceuticals Research & Development [Raritan, NJ]), 1.3 mg/m2 on days 1, 4, 8, and 11 for eight 3-week cycles, then on days 1, 8, 15, and 22 for three 5-week maintenance cycles, or dexamethasone 40 mg on days 1 to 4, 9 to 12, and 17 to 20 for four 5-week cycles, then days 1 to 4 for five 4-week cycles. Patients were assessed for disease status and survival every 3 weeks for 39 weeks. Follow-up occurred every 6 weeks until PD, then every 3 months for survival. Patients on the companion crossover study received single-agent bortezomib as administered according to the dose and schedule of the bortezomib treatment arm of the APEX study.
Updated analyses of survival of patients originally randomized to bortezomib and dexamethasone were performed. Updated efficacy analyses were performed for all 333 patients in the bortezomib arm, following treatment completion in 85 patients still receiving bortezomib at initial analysis (the dexamethasone arm was halted; additional analysis was not possible). These analyses included response rate (European Blood and Bone Marrow Transplant Group [EBMT] criteria),2 TTP, TTR, DOR, and best M-protein reduction (a key component of EBMT criteria) as measures of response quality. Response and survival with single-agent bortezomib were compared between patients who crossed over to bortezomib after PD on dexamethasone and patients initially randomized to bortezomib. Patients in this analysis were matched for age (< 65 years or ≥ 65 years), prior thalidomide, baseline albumin (< 35 g/L [3.5 g/dL] or ≥ 35 g/L [3.5 g/dL]), and number of therapies prior to APEX enrollment.
Results and discussion
Patients' baseline characteristics have been described.1 Median length of bortezomib therapy in this updated analysis was 6 cycles, as in initial analysis; 39% of patients completed the planned 8 cycles compared with 29% in the initial analysis.
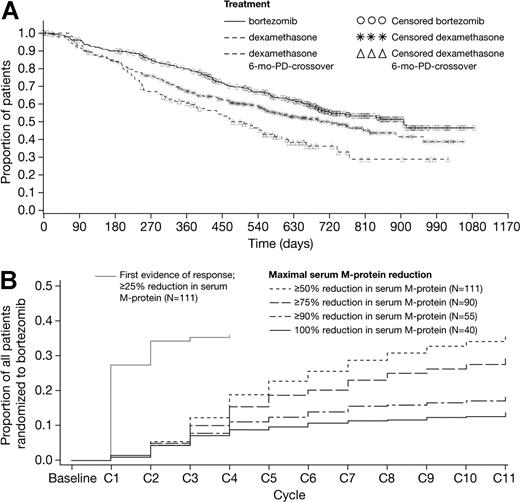
Median follow-up was 22 months in surviving patients (44% of patients have died), an additional 14 months of follow-up compared with the initial analysis. Median OS was 29.8 months (95% CI: 23.2–not evaluable) in the bortezomib arm versus 23.7 months (95% CI: 18.7-29.1) in the dexamethasone arm (hazard ratio = .77, P = .027), despite more than 62% of dexamethasone patients crossing over to receive bortezomib (Figure 1A). The 1-year survival rates were 80% and 67%, respectively (P = .001). These results show that, with longer follow-up and despite substantial crossover from the high-dose dexamethasone arm, bortezomib continues to demonstrate improved survival compared with dexamethasone. Figure 1A also shows survival for a subset of dexamethasone patients who crossed over to bortezomib after PD within 6 months. Because baseline characteristics are not matched with bortezomib patients, the exploratory analysis was conducted to compare earlier versus later bortezomib.
Bortezomib continues to demonstrate improved survival compared with high-dose dexamethasone, and overall quality of response continues to improve with longer bortezomib treatment beyond initial response. (A) Updated overall survival in the bortezomib (n = 333) and high-dose dexamethasone (n = 336) arms, plus overall survival in the subset of dexamethasone patients who crossed over to receive bortezomib following PD within 6 months of study start (n = 113); the baseline characteristics of these poorer-prognosis patients are not matched with those of patients on the bortezomib arm. (B) Time to maximal serum M-protein reduction plus time to first evidence (≥ 25% serum M-protein reduction) of response in patients responding to bortezomib in whom baseline serum M-protein data were available (n = 111 of 135 responders), as a proportion of all patients randomized to bortezomib.
Bortezomib continues to demonstrate improved survival compared with high-dose dexamethasone, and overall quality of response continues to improve with longer bortezomib treatment beyond initial response. (A) Updated overall survival in the bortezomib (n = 333) and high-dose dexamethasone (n = 336) arms, plus overall survival in the subset of dexamethasone patients who crossed over to receive bortezomib following PD within 6 months of study start (n = 113); the baseline characteristics of these poorer-prognosis patients are not matched with those of patients on the bortezomib arm. (B) Time to maximal serum M-protein reduction plus time to first evidence (≥ 25% serum M-protein reduction) of response in patients responding to bortezomib in whom baseline serum M-protein data were available (n = 111 of 135 responders), as a proportion of all patients randomized to bortezomib.
Overall response rate (complete response [CR] + partial response [PR]) in the bortezomib arm improved from 38% at initial analysis to 43%; CR rate improved from 6% to 9%; median TTP, TTR, and DOR remained unchanged (Table 1). Median DOR was longer in patients achieving 100% M-protein reduction (n = 40, 11.5 months) compared with patients achieving 50% or more but less than 100% reduction (n = 71, 7.6 months). Notably, DOR was not associated with TTR; rapid achievement of first response did not predict longer DOR (r = .03), suggesting comparable clinical benefit irrespective of time taken to respond.
Median time to progression, response rate, median time to first response, and median duration of response in patients on the bortezomib arm in this updated analysis versus the initial analysis of bortezomib and high-dose dexamethasone
| . | Bortezomib update . | Bortezomib initial . | High-dose dexamethasone initial . |
|---|---|---|---|
| no. | 333 | 333 | 336 |
| Median TTP, mo* | 6.2 | 6.2 | 3.5 |
| Response rate CR + PR, % (n/N) | 43 (135/315) | 38 (121/315) | 18 (56/312) |
| CR | 9 (27/315) | 6 (20/315) | <1 (2/312) |
| PR† | 34 (108/315) | 32 (101/315) | 17 (54/312) |
| nCR | 7 (21/315) | 7 (21/315) | <1 (3/312) |
| Median TTR, mo† | 1.4 | 1.4 | 1.4 |
| CR | 0.8 | 0.8 | 0.8 |
| PR† | 1.4 | 1.4 | 1.4 |
| nCR | 0.8 | 0.8 | 0.8 |
| Median DOR, mo† | 7.8 | 8.0 | 5.6 |
| 100% M-protein reduction, CR/nCR; n = 40‡ | 11.5 | NE | NE |
| 50% or more but less than 100% M-protein | 7.6 | NE | NE |
| reduction, PR; n = 71‡ |
| . | Bortezomib update . | Bortezomib initial . | High-dose dexamethasone initial . |
|---|---|---|---|
| no. | 333 | 333 | 336 |
| Median TTP, mo* | 6.2 | 6.2 | 3.5 |
| Response rate CR + PR, % (n/N) | 43 (135/315) | 38 (121/315) | 18 (56/312) |
| CR | 9 (27/315) | 6 (20/315) | <1 (2/312) |
| PR† | 34 (108/315) | 32 (101/315) | 17 (54/312) |
| nCR | 7 (21/315) | 7 (21/315) | <1 (3/312) |
| Median TTR, mo† | 1.4 | 1.4 | 1.4 |
| CR | 0.8 | 0.8 | 0.8 |
| PR† | 1.4 | 1.4 | 1.4 |
| nCR | 0.8 | 0.8 | 0.8 |
| Median DOR, mo† | 7.8 | 8.0 | 5.6 |
| 100% M-protein reduction, CR/nCR; n = 40‡ | 11.5 | NE | NE |
| 50% or more but less than 100% M-protein | 7.6 | NE | NE |
| reduction, PR; n = 71‡ |
TTP indicates time to progression; CR, complete response; PR, partial response; nCR, near complete response; TTR, time to response; DOR, duration of response; and NE, not evaluated.
Median length of bortezomib therapy was 6 cycles, while responding patients received a median of 10 cycles (protocol-specified 8 cycles plus 2 additional maintenance cycles).
Based on responders in each group.
‡Based on patients with measurable baseline serum M-protein (n = 111).
Among 135 patients responding to bortezomib, 73 (54%) achieved their first response (CR, PR, or minor response [MR]) after treatment cycle 2. First response was achieved on or after cycle 4 in 39 (29%) patients, and on or after cycle 6 in 10 (7%) patients. In total, 76 (56%) patients had improved response after first response; 20 (15%) improved from MR or PR to CR, and 56 (41%) from MR to PR. Best response was achieved in cycle 1 or 2 in 47 (35%) patients. When best response was CR, it occurred on or after cycle 8 in 22% of patients. Similarly, approximately 20% of patients who responded achieved maximal M-protein reduction in cycle 8 or later (Figure 1B). Therefore, longer therapy beyond rapid initial responses led to continued improvement in overall quality of response. This, combined with the finding that higher quality of response was associated with longer duration of response, supports extended treatment with bortezomib beyond initial response in patients tolerating therapy.
Where they occurred, only slight differences were seen in incidences of commonly reported adverse events between updated and initial analyses.1 Optimal duration of therapy is determined by the risk-benefit ratio of continuing treatment. No new safety concerns were identified in this updated analysis compared with the initial report1 or 2 phase 2 trials of bortezomib in relapsed and/or refractory MM.3-5 In addition, data from an extension study6 of the phase 2 trials3,4 indicate that prolonged therapy is associated with a manageable safety profile, and no evidence of new, cumulative toxicity.6
In the exploratory analysis of 118 matched pairs of patients receiving bortezomib earlier (on APEX) compared with later (on companion study following PD on high-dose dexamethasone), median OS was 23.2 months (95% CI: 20.1–not evaluable) versus 16.3 months (95% CI: 14.5–not evaluable; hazard ratio = .67, P = .047), respectively; one-year survival probability was 75% versus 65% (P = .095). Response rate (CR + PR) was 44% versus 34% (P = .153). The longer survival seen in patients receiving bortezomib earlier, together with data from additional APEX analyses showing greater efficacy with bortezomib at first relapse versus later salvage therapy,1,7,8 indicates that, as might be expected, earlier use of bortezomib in relapsed MM may offer greater benefit. Bortezomib-based combinations are also being investigated with the aim of improving outcomes, with promising results to date.9-24
In conclusion, bortezomib continues to demonstrate superior survival to high-dose dexamethasone in patients with relapsed MM following 1 to 3 prior therapies, confirming the substantial activity of bortezomib as a single agent and further supporting its study both earlier in the disease course and in combination regimens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Millennium Pharmaceuticals and Johnson & Johnson Pharmaceuticals Research & Development.
The study investigators thank the patients for their participation in this trial, as well as the study nurses and clinical research associates for their important contributions to the implementation and management of the study. A complete list of the APEX Investigators may be found in Document S2.
Authorship
Contribution: A.B., J.B., D.S., J.S.M., P.G.R., S.V.R., D.-L.E., K.C.A., and W.D. were involved in conception and design of the study; J.C., A.B., D.B.-Y., M.A., H.G., P.G.R., D.R., M.B., A.J., J.-L.H., D.I., E.S., K.C.A., S.L., T.F., and M.S. performed the research; J.C., J.B., M.A., H.G., J.S.M., P.G.R., D.R., M.L., A.J., D.I., K.C.A., and M.S. collected data; A.B., P.S., D.S., P.G.R., D.-L.E., K.C.A., W.D., and M.S. were involved in data analysis; P.G.R., D.-L.E., and K.C.A. were involved in writing the paper; J.C., J.B., D.B.-Y., P.S., J.S.M., P.G.R., D.R., S.V.R., J.-L.H., D.-L.E., D.I. E.S., K.C.A., S.L., M.B., and M.S. reviewed and gave final approval of the paper.
Conflict-of-interest disclosure: A.B. and D.S. have declared a financial interest in Millennium Pharmaceuticals, whose product was studied in the present work. A.B., D.S., and D.-L.E. are employed by Millennium Pharmaceuticals, whose product was studied in the present work. D.R., S.V.R., J.-L.H., M.S., and K.A. have received research funding from Millennium Pharmaceuticals. E.S. has received research funding from Celgene.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Institute, 44 Binney St, Dana 1B02, Boston, MA 02115; e-mail:paul_richardson@dfci.harvard.edu.