Abstract
Long-term survival and outcome were determined for 80 patients with immunoglobulin light chain (AL) amyloidosis treated with high-dose melphalan and stem cell transplantation (HDM/SCT) more than 10 years ago. Seventeen (21%) patients died within the first year of treatment, of treatment-related complications (14%) or progressive disease (8%). Of the 63 surviving evaluable patients at one year, 32 (51%) achieved a complete hematologic response (CR). For all 80 patients, the median survival was 57 months (4.75 yrs). The median survival exceeds 10 years for patients achieving a CR after HDM/SCT, compared with 50 months for those not achieving a CR (P < .001). In conclusion, HDM/SCT leads to durable remissions and prolonged survival, particularly for those patients who achieve a hematologic CR.
Introduction
Immunoglobulin light chain (AL) amyloidosis is a plasma cell dyscrasia in which clonal immunoglobulin light chains misfold, forming amyloid fibrils that are deposited in tissues and vital organs, leading to organ dysfunction and failure.1 Median survival of untreated patients is 10 to 14 months from the time of diagnosis and is marginally prolonged to 16 to 18 months with oral cyclic melphalan and prednisone regimen.2,3 Moreover, this form of treatment rarely results in hematologic complete response (CR)
High-dose intravenous melphalan and autologous stem cell transplantation (HDM/SCT) has become a first-line treatment for patients with multiple myeloma because of high hematologic response rates and survival benefits when compared with conventional chemotherapy regimens.4,5 Promising treatment outcomes observed with HDM/SCT in myeloma provided a rationale for evaluating this aggressive treatment approach in AL amyloidosis. HDM/SCT has been shown to induce both hematologic and clinical remissions in AL amyloidosis, and it appears to prolong survival substantially when hematologic remissions are achieved.6,7
At Boston University Medical Center, we began treating patients with AL amyloidosis with high-dose melphalan and autologous stem cell transplantation (HDM/SCT) in 1994.8 The hematologic relapse rate and long-term survival have not been studied in AL amyloidosis, but short-term results of hematologic responses and survival are promising. To address the durability and long-term results of treatment with HDM/SCT, here we report on the outcome for AL amyloidosis patients treated with HDM/SCT more than 10 years ago.
Patients and methods
Patients with AL amyloidosis undergoing HDM/SCT from July 1994 to July 1997 were studied with the approval of the Institutional Review Board of Boston University Medical Center. Informed consent was obtained in accordance with the Declaration of Helsinki. All patients had a histologic diagnosis of amyloidosis with evidence of a plasma cell dyscrasia and met eligibility criteria for HDM/SCT treatment in clinical protocols. Patients received 100 mg/m2 to 200 mg/m2 intravenous melphalan, followed by stem cell transplant 24 to 72 hours after completion of chemotherapy. The dose of intravenous melphalan was selected as described in our previous report.7 All patients were followed for hematologic and clinical responses at 3 and 6 months, and annually thereafter. At these visits, immunofixation electrophoresis (IFE) of serum and urine was performed, and bone marrow biopsies for immunohistochemical analysis of plasma cell numbers and κ and λ light chain expression were performed. Hematologic complete responses (CR) following treatment required a normalization of all these studies, as reported previously. Since 2003, we also have used the measurement of serum-free light chains (FreeLite, the Binding Site) to assess plasma cell dyscrasia, but this was not available at the time for these patients. Hematologic relapses were defined as reappearance of monoclonal protein in serum and/or urine by IFE or clonal plasma cell expression in the bone marrow biopsy. All patients were followed for survival and for clinical improvement of the amyloid-related organ dysfunction. Kaplan-Meier estimates were obtained for all patients and for patients with hematologic CR and for patients without hematologic CR. We used the log-rank test to compare the survival distributions in patients who achieved CR and patients without CR. Patients who survived through the end of the study period were considered censored observations. All deaths were classified as either related or unrelated to progressive amyloid disease. Treatment-related mortality was defined as death occurring within 100 days of HDM/SCT. Deaths during stem cell mobilization and collection phase of treatment also were reported.
Results and discussion
Eighty patients with AL amyloidosis received HDM/SCT from July 1994 to July 1997. Their median age was 56 years (range, 29-71), performance status was Southwest Oncology Group (SWOG) 1 (range, 0-3), and number of organ involvement was 2 (range, 1-5). Thirty-eight (48%) had cardiac involvement. Forty-three (54%) patients received full high-dose melphalan at 200 mg/m2, while 37 (46%) received modified high-dose melphalan at 100 mg/m2 to 140 mg/m2. All patients underwent stem-cell mobilization with G-CSF (granulocyte colony stimulating factor) alone. The treatment-related mortality in these earlier patients was 14% (n = 11/80). In addition, 5 more patients died during the mobilization and collection phase of treatment, prior to initiating HDM. Furthermore, 6 (8%) patients died of complications related to amyloidosis prior to 1-year follow-up evaluation.
Hematologic responses were assessed in 63 (79%) patients at one year following treatment. Of evaluable patients, 32 (51%) achieved a complete hematologic response, and of these, 19 patients had received high-dose melphalan at 200 mg/m2. Hematologic relapses occurred in 34% (n = 11/32) patients at a median time of 2.5 years (range, 2-8). Patients with hematologic relapses were treated with a second line of treatment if there was evidence of clinical disease progression.
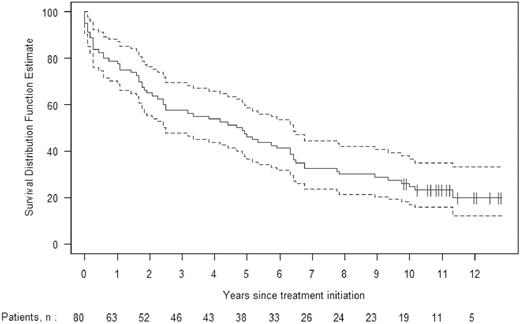
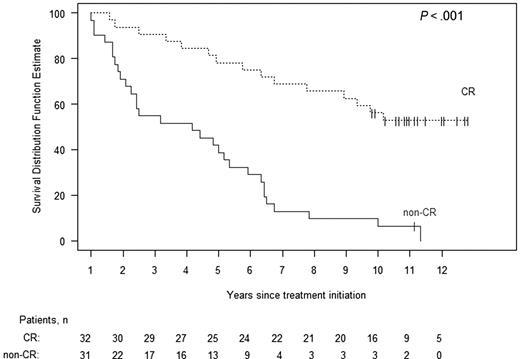
The median survival for all 80 patients is 57 months. Kaplan-Meier estimates of survival with 95% confidence bands are shown in Figure 1. Eighteen (23%) of these patients are alive at present, 10 or more years after undergoing HDM/SCT. In Figure 2, Kaplan-Meier survival is plotted separately for those patients who achieved a hematologic CR and for those who did not. The median survival for patients achieving a hematologic CR has not yet been reached; the survival at 10 years is 53%. In contrast, the median survival for patients failing to achieve a hematologic CR is 50 months (95% confidence interval, 27-64), and the estimated probability of survival at 10 years is 6% (P < .001). The median survival for the patients receiving full HDM is 71 months, compared with 38 months for those receiving modified HDM (P = .20, log-rank test).
Kaplan-Meier estimate of overall survival, with 95% confidence intervals, for all 80 patients treated with HDM/SCT more than 10 years ago (1994-1997).
Kaplan-Meier estimate of overall survival, with 95% confidence intervals, for all 80 patients treated with HDM/SCT more than 10 years ago (1994-1997).
Kaplan-Meier estimates of survival according to hematologic response, comparing those patients who achieved a hematologic complete response at 1 year (dashed upper plot) to those who did not (solid lower plot). Surviving patients are indicated with vertical lines. The median survival of the CR patients has not been reached but is 50 months for the non-CR patients (P < .001).
Kaplan-Meier estimates of survival according to hematologic response, comparing those patients who achieved a hematologic complete response at 1 year (dashed upper plot) to those who did not (solid lower plot). Surviving patients are indicated with vertical lines. The median survival of the CR patients has not been reached but is 50 months for the non-CR patients (P < .001).
Sixty-two (78%) patients have died. Seventeen patients died within 1 year of HDM/SCT; 11 from treatment-related mortality (TRM) and 6 due to disease progression. Of 11 patients dying from TRM, 6 (16%, 6/37) had received modified HDM, and 5 (12%, 5/43) had received full HDM. Fifteen patients died subsequent to achieving a hematologic CR, and 30 patients died without achieving a CR. Of note, only 1 patient who did not achieve a hematologic CR is alive (3%), while 17 patients (53%) of those who did achieve a hematologic CR are still alive. Of the 15 patients who died after achieving a hematologic CR, 6 patients developed hematologic relapses and died of progressive clinical disease, 1 patient died with therapy-related myelodysplastic syndrome and acute myelogenous leukemia, 2 with metastatic solid tumors (lung and renal), and 6 patients with severe organ dysfunction despite a sustained hematologic CR.
In summary, long-term survival beyond 10 years was achieved in 23.5% (95% CI, 15% and 33%) of patients with AL amyloidosis treated with HDM/SCT. Only 2% of patients treated with oral melphalan and prednisone survive more than 10 years.3 While some of this difference reflects a selection bias in patients undergoing HDM/SCT, the fact that the outcome is so strongly dependent upon achieving a hematologic CR indicates that treatment response plays an important role in long-term survival. An oral regimen substituting dexamethasone for prednisone has produced a hematologic CR rate of 33%,9 and survival appears also to be strongly dependent upon achievement of CR; while the 10-year results with this regimen are not known, recent correspondence from the authors indicate that with a median follow-up of 5 years, median survival is 5.1 years.17 Thus, efforts should continue to be directed at increasing the hematologic CR rate. Strategies to accomplish this include the use of tandem transplantation10 and the use of new agents, for example, thalidomide,11-13 lenalidomide,14,15 or bortezomib.16 The optimal timing and sequencing of regimens containing these agents, and comparison to or combination with one or 2 cycles of HDM/SCT, will be determined in future trials.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the numerous colleagues in the Amyloid Treatment and Research Program, Clinical Trials Office, and Center for Cancer and Blood Disorders at Boston University Medical Center, who assisted with the multidisciplinary evaluation and treatment of the patients with AL amyloidosis.
This research was supported in part by the National Institutes of Health (P01 HL68705) and the Amyloid Research Fund at Boston University School of Medicine.
National Institutes of Health
Authorship
Contribution: V.S. designed and performed research, analyzed data, and wrote the manuscript. M.S. edited the manuscript with critical review. K.Q. designed and performed research, analyzed data, and critically reviewed the manuscript. K.T.F. collected and analyzed data, and designed research. G.D. performed statistical analysis. D.S. designed and performed research, analyzed data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, Section of Hematology/Oncology, F 302, 732 Harrison Ave, Boston, MA 02118, e-mail: vaishali.sanchorawala@bmc.org.