Abstract
Although imatinib is clearly the treatment of choice for FIP1L1/PDGFRA-positive chronic eosinophilic leukemia (CEL), little is known about optimal dosing, duration of treatment, and the possibility of cure in this disorder. To address these questions, 5 patients with FIP1L1/PDGFRA-positive CEL with documented clinical, hematologic, and molecular remission on imatinib (400 mg daily) and without evidence of cardiac involvement were enrolled in a dose de-escalation trial. The imatinib dose was tapered slowly with close follow-up for evidence of clinical, hematologic, and molecular relapse. Two patients with endomyocardial fibrosis were maintained on imatinib 300 to 400 mg daily and served as controls. All 5 patients who underwent dose de-escalation, but neither of the control patients, experienced molecular relapse (P < .05). None developed recurrent symptoms, and eosinophil counts, serum B12, and tryptase levels remained suppressed. Reinitiation of therapy at the prior effective dose led to molecular remission in all 5 patients, although 2 patients subsequently required increased dosing to maintain remission. These data are consistent with suppression rather than elimination of the clonal population in FIP1L1/PDGFRA-positive CEL and suggest that molecular monitoring may be the most useful method in determining optimal dosing without the risk of disease exacerbation. This trial was registered at http://www.clinicaltrials.gov as no. NCT00044304.
Introduction
A subset of patients presenting with hypereosinophilic syndrome (HES), as defined by persistent and unexplained eosinophilia count of 1.5 × 109/L or higher with evidence of eosinophil-mediated end organ pathology, has clinical features consistent with a myeloproliferative disorder. These patients have aggressive disease characterized by tissue fibrosis, including endomyocardial fibrosis and myelofibrosis and, prior to the availability of imatinib, a poor prognosis with 5-year mortality rates as high as 50%. Despite normal cytogenetics, an interstitial deletion in chromosome 4 leading to the formation of the imatinib-sensitive FIP1L1/PDGFRA fusion gene can be demonstrated in the peripheral blood mononuclear cells of the overwhelming majority of these patients by molecular testing, fulfilling the WHO criteria for a diagnosis of chronic eosinophilic leukemia (CEL)
The tyrosine kinase inhibitor, imatinib mesylate, was originally developed for the treatment of chronic myelogenous leukemia (CML) and has revolutionized the therapy for this disorder. Nevertheless, despite cytogenetic remission and significant reduction of bcr/abl transcripts in response to imatinib mesylate therapy, most studies have failed to demonstrate complete molecular remission in a majority of patients with CML.1 This is thought to be due to the persistence of a small population of BCR/ABL-positive hematologic progenitors that do not undergo apoptosis in the presence of imatinib.2-4 Although pre-existent bcr/abl kinase mutations in primitive CD34+ cells may explain relapse in some cases,5 this mechanism does not appear to be widespread.6
The FIP1L1/PDGFRA fusion gene is markedly more sensitive to imatinib than BCR/ABL,7 and patients with this mutation respond dramatically to therapy with clinical, hematologic, and molecular remission documented in nearly all patients described to date.7-9 Furthermore, disease progression while on imatinib appears to be uncommon in patients with FIP1L1/PDGFRA-positive CEL. Although the response to imatinib in these patients is dramatic and appears to be sustained, the optimal dose and duration of imatinib treatment have not been defined. In fact, clinically successful treatment regimens reported in the literature range from 400 mg daily to 100 mg weekly.
In CML, the magnitude of the molecular response is a predictor of relapse and disease progression,10,11 and molecular monitoring has been recommended every 3 months. In part due to the lack of commercially available testing for FIP1L1/PDGFRA, few studies have monitored the molecular response to treatment in FIP1L1/PDGFRA-positive CEL except to document initial remission. Finally, although lineage studies suggest the involvement of an early hematopoietic progenitor cell,12 making the prospect of imatinib cure unlikely in FIP1L1/PDGFRA-positive CEL, this possibility has not been systematically explored.
To address these issues, 5 patients with FIP1L1/PDGFRA-positive CEL with documented clinical, hematologic, and molecular remission on an imatinib dose of 400 mg daily and without evidence of cardiac involvement were enrolled in a dose de-escalation trial. The imatinib dose was tapered slowly with close follow up for evidence of clinical, hematologic, and molecular relapse. Two patients with a history of life-threatening cardiac involvement prior to institution of imatinib therapy were maintained on 300 to 400 mg imatinib daily and served as a control population.
Patients and methods
Patient population
Seven subjects enrolled in a previously described ongoing study of imatinib therapy for hypereosinophilic syndrome8 were eligible for the present study based on documented presence of the FIP1L1/PDGFRA fusion gene prior to initiation of imatinib therapy and complete clinical, hematologic, and molecular remission in response to imatinib. The study was approved by the Institutional Review Board of the National Institutes of Allergy and Infectious Diseases, and informed consent was obtained from all study participants in accordance with the Declaration of Helsinki.
Study design
The initial imatinib regimen was selected based on data from phase 2 studies of chronic myelogenous leukemia (CML), which showed a decreased rate of cytogenetic remission at doses less than 300 mg/day despite decreased circulating blasts.13 Imatinib therapy was interrupted and restarted at a lower dose for neutropenia (absolute neutrophil count < 1.0 × 109/L), thrombocytopenia (platelet count < 10 × 109/L, or 50 × 109/L with clinical evidence of bleeding), transaminase or bilirubin elevation (> 5 or > 3 times the upper limit of normal, respectively), according to the guidelines proposed for the treatment of CML.14 Adverse events were graded using the Common Toxicity Criteria, version 2, of the National Cancer Institute (Bethesda, MD).
All study participants received imatinib mesylate in a single daily oral dose of 300 to 400 mg for a minimum of 1 year before beginning the dose de-escalation portion of the study. A comprehensive clinical evaluation, including complete blood count (CBC) with differential, routine chemistries, serum IgE, B12, and tryptase levels, electrocardiogram (EKG), echocardiogram, pulmonary function tests (PFTs), and bone marrow aspirate and biopsy, was performed prior to and at 1 month following the initiation of imatinib therapy. CBC and liver function tests were followed weekly for one month after the initiation of therapy, biweekly for 2 additional months, and monthly thereafter. Clinical evaluation, including EKG, echocardiogram, PFTs, and molecular testing for the fusion gene, was performed every 3 months.
If molecular testing remained negative (molecular remission) and the subject had no history of endomyocardial fibrosis or other life-threatening complications of HES, the imatinib dose was decreased by 100 mg every 3 months to a dose of 100 mg daily. After a minimum of 6 months at 100 mg daily without evidence of molecular relapse, imatinib was discontinued. At this point, the frequency of molecular testing was increased to monthly. In the event of molecular relapse (as defined by 2 consecutive positive tests), imatinib therapy was reinstituted at 100 mg daily and/or the dose was increased incrementally until molecular remission was documented in 2 consecutive monthly blood samples or a dose of 600 mg daily was reached.
All laboratory testing reported in this study was performed in the Department of Laboratory Medicine at the NIH Clinical Center, with the exception of serum total tryptase levels, which were measured at the Mayo Medical Labs (Rochester, MN). The normal range for serum tryptase levels (< 11.5 ng/mL) was provided by Mayo Medical Labs.
Detection of the FIP1L1-PDGFRA fusion gene
RNA was isolated from peripheral blood mononuclear cells using RNA STAT-60 (Tel-Test, Friendswood, TX) and/or the RNAEasy RNA isolation kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from 2 μg total RNA using Superscript first-strand synthesis system (Invitrogen, Frederick, MD) with random hexamer primers. Fusion of FIP1L1 to PDGFRA was detected by nested polymerase chain reaction (PCR) using primers FIP1L1-F1 (5′-acctggtgctgatctttctgat) and PDGFRA-R1 (5′-tgagagcttgtttttcactgga) during the first PCR, and primers FIP1L1-F2 (5′-aaagaggatacgaatgggacttg) and PDGFRA-R2 (5′-gggaccggcttaatccatag) for the second PCR. Control reverse-transcription (RT)–PCR for GAPDH was performed using the primers GAPDH-F (5′-tggaaatcccatcaccatct) and GAPDH-R (5′-gtcttctgggtggcagtgat).
Statistical analysis
A 2-sided Fisher exact test was used to test for association between de-escalating dose or not and molecular relapse. A P value of less than .05 was considered statistically significant.
Results
Patient characteristics
Between January 2003 and February 2005, 7 patients with the myeloproliferative variant of HES were enrolled in a study of imatinib mesylate therapy (Table 1). All 7 demonstrated a rapid and complete resolution of clinical symptoms and laboratory abnormalities. The FIP1L1/PDGFRA fusion gene was detected by RT-PCR in all 7 prior to imatinib treatment and became undetectable by 12 months (Figure 1). Two of the patients were excluded from the dose de-escalation portion of the study because of cardiac involvement (endomyocardial fibrosis with mitral and tricuspid regurgitation). The remaining 5 patients underwent a slow taper of their imatinib dose.
Clinical characteristics of patient population
| Pt no. . | Age at dx, y . | Peak eos/mm3 . | End organ manifestations . | Prior therapy* . | Initial imatinib dose, mg . | Months to imatinib de-escalation . | Months to imatinib interruption . |
|---|---|---|---|---|---|---|---|
| 1 | 35 | 18 877 | Mucosal ulcerations, dermatitis, anemia, thrombocytopenia, splenomegaly | Pred, HU, IFN, SCH55700 | 400 | 21 | NA |
| 2 | 47 | 7 220 | Mucosal ulcerations, dermatitis, splenomegaly | Pred, HU, IFN, SCH55700 | 400 | 14 | 29 |
| 3 | 35 | 24 500 | Fatigue, splenomegaly | HU | 400 | 9 | 25 |
| 4 | 45 | 24 000 | Restrictive lung disease, dermatitis, splenomegaly | None | 400 | 10 | 28 |
| 5 | 34 | 5 395 | Lymphomatoid papulosis, dermatitis | None | 400 | 7 | 19 |
| 6 | 32 | 7 400 | Endomyocardial fibrosis, anemia, thrombocytopenia, hepatosplenomegaly | None | 300 | NA | NA |
| 7 | 44 | 200 000 | Endomyocardial fibrosis, anemia, thrombocytopenia, splenomegaly, dermatitis, restrictive pulmonary disease | Pred, HU, IFN, CyA | 400 | NA | NA |
| Pt no. . | Age at dx, y . | Peak eos/mm3 . | End organ manifestations . | Prior therapy* . | Initial imatinib dose, mg . | Months to imatinib de-escalation . | Months to imatinib interruption . |
|---|---|---|---|---|---|---|---|
| 1 | 35 | 18 877 | Mucosal ulcerations, dermatitis, anemia, thrombocytopenia, splenomegaly | Pred, HU, IFN, SCH55700 | 400 | 21 | NA |
| 2 | 47 | 7 220 | Mucosal ulcerations, dermatitis, splenomegaly | Pred, HU, IFN, SCH55700 | 400 | 14 | 29 |
| 3 | 35 | 24 500 | Fatigue, splenomegaly | HU | 400 | 9 | 25 |
| 4 | 45 | 24 000 | Restrictive lung disease, dermatitis, splenomegaly | None | 400 | 10 | 28 |
| 5 | 34 | 5 395 | Lymphomatoid papulosis, dermatitis | None | 400 | 7 | 19 |
| 6 | 32 | 7 400 | Endomyocardial fibrosis, anemia, thrombocytopenia, hepatosplenomegaly | None | 300 | NA | NA |
| 7 | 44 | 200 000 | Endomyocardial fibrosis, anemia, thrombocytopenia, splenomegaly, dermatitis, restrictive pulmonary disease | Pred, HU, IFN, CyA | 400 | NA | NA |
All patients were male.
dx indicates diagnosis; eos, eosinophil count; HU, hydroxyurea; IFN, interferon; SCH55700, monoclonal antibody to interleukin 5; CyA, cyclosporin A; and NA, not applicable.
Molecular relapse in the setting of imatinib dose de-escalation in FIP1L1/PDGFRA-positive CEL. Results of molecular testing for FIP1L1/PDGFRA by nested RT-PCR (rectangular bar beneath graph) are shown for 7 subjects as a function of time. The subject number is indicated by the white numeral. Each rectangle represents a 1-month period. Black rectangles indicate the presence of the fusion gene, and gray rectangles indicate the absence of the fusion gene. White rectangles represent months during which testing was not performed. Imatinib dose (y-axis) as a function of time (x-axis) is indicated by the gray shaded area. The dashed line in graph 1 represents the eosinophil count over time for this subject. All 5 subjects who underwent dose de-escalation (A), and neither subject who was maintained on stable high-dose imatinib (B), experienced molecular relapse.
Molecular relapse in the setting of imatinib dose de-escalation in FIP1L1/PDGFRA-positive CEL. Results of molecular testing for FIP1L1/PDGFRA by nested RT-PCR (rectangular bar beneath graph) are shown for 7 subjects as a function of time. The subject number is indicated by the white numeral. Each rectangle represents a 1-month period. Black rectangles indicate the presence of the fusion gene, and gray rectangles indicate the absence of the fusion gene. White rectangles represent months during which testing was not performed. Imatinib dose (y-axis) as a function of time (x-axis) is indicated by the gray shaded area. The dashed line in graph 1 represents the eosinophil count over time for this subject. All 5 subjects who underwent dose de-escalation (A), and neither subject who was maintained on stable high-dose imatinib (B), experienced molecular relapse.
Imatinib dose de-escalation
Molecular relapse occurred in all 5 of the patients on the tapering dose schedule; relapse did not occur in either of the patients maintained on 300 to 400 mg imatinib daily (P < .05; Figure 1). Molecular relapse occurred in patient 1 after 5 months on a daily dose of 100 mg. The remaining 4 patients were able to discontinue imatinib with a median time from imatinib initiation to interruption of 25.5 months (range, 19-31 months). In 3 patients, the fusion gene became detectable 2 to 3 months after discontinuation of drug therapy. A fourth patient was not tested until 5 months after stopping imatinib, at which time the fusion gene was detectable.
None of the patients developed recurrent symptoms and their eosinophil counts, serum B12, and tryptase levels remained suppressed at the point of initial molecular relapse (Table 2). The 2 patients with cardiac involvement (patients 6 and 7) who remained on 300 to 400 mg imatinib daily have remained in clinical, hematologic, and complete molecular remission for 51 and 56 months, respectively. Bone marrow examination was performed in 2 patients at the time of molecular relapse and was unchanged from the bone marrow obtained at 1 month after treatment (Figure 2). Notably, there was no increase in eosinophilia, cellularity, atypical mast cells, or reticulin fibrosis.
Laboratory parameters during the course of imatinib treatment and at the time of molecular relapse
| Pt no. . | Eosinophils/mm3 . | Serum B12, ng/mL . | Serum tryptase, ng/mL . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Disc . | Relapse . | Before . | After . | Disc . | Relapse . | Before . | After . | Disc . | Relapse . | |
| 1 | 5993 | 107 | NA | 150 | 5488 | 761 | NA | 269 | 22.6 | 2.35 | NA | 3.35 |
| 2 | 1906 | 109 | 157 | 224 | 1385 | 440 | 322 | 279 | 17.9 | 1.49 | 2.05 | 2.86 |
| 3 | 8352 | 157 | 88 | 155 | 6995 | 729 | 356 | 356 | 19 | <1 | <1 | 1.21 |
| 4 | 16228 | 68 | 57 | 147 | 7085 | 570 | 337 | 338 | 29.4 | 2.3 | 4.08 | 8.76 |
| 5 | 4027 | 71 | 96 | 90 | 1443 | 906 | 924 | 1301 | 14.3 | 2.08 | 2.83 | 2.79 |
| 6 | 43 | 107 | NA | NA | 3889 | 926 | NA | NA | 13.7 | 4.11 | NA | NA |
| 7 | 758 | 1376 | NA | NA | 1192 | 882 | NA | NA | 49.5 | 3.35 | NA | NA |
| Pt no. . | Eosinophils/mm3 . | Serum B12, ng/mL . | Serum tryptase, ng/mL . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | After . | Disc . | Relapse . | Before . | After . | Disc . | Relapse . | Before . | After . | Disc . | Relapse . | |
| 1 | 5993 | 107 | NA | 150 | 5488 | 761 | NA | 269 | 22.6 | 2.35 | NA | 3.35 |
| 2 | 1906 | 109 | 157 | 224 | 1385 | 440 | 322 | 279 | 17.9 | 1.49 | 2.05 | 2.86 |
| 3 | 8352 | 157 | 88 | 155 | 6995 | 729 | 356 | 356 | 19 | <1 | <1 | 1.21 |
| 4 | 16228 | 68 | 57 | 147 | 7085 | 570 | 337 | 338 | 29.4 | 2.3 | 4.08 | 8.76 |
| 5 | 4027 | 71 | 96 | 90 | 1443 | 906 | 924 | 1301 | 14.3 | 2.08 | 2.83 | 2.79 |
| 6 | 43 | 107 | NA | NA | 3889 | 926 | NA | NA | 13.7 | 4.11 | NA | NA |
| 7 | 758 | 1376 | NA | NA | 1192 | 882 | NA | NA | 49.5 | 3.35 | NA | NA |
Before indicates before treatment; after, 1 month after treatment; disc, at the time treatment was discontinued; relapse, at the time of molecular relapse; NA, not applicable.
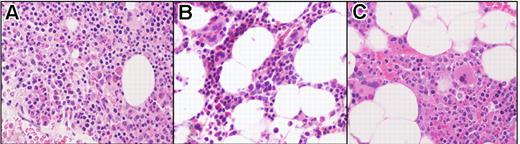
Bone marrow biopsy findings in a FIP1L1/PDGFRA-positive subject (subject 3). Before imatinib treatment (A), at 1 month following initiation of treatment (B), and at the time of molecular relapse (C). Posttreatment resolution of eosinophilia and hypercellularity was maintained despite molecular relapse. Sections were stained with hematoxylin and eosin (H&E), magnification 400×, and images were obtained by digital microscopy using an Olympus BH-Z microscope (Olympus America, Melville, NY) equipped with a DPlan 40 × 10.65 numeric aperture objective. Images were captured using an Olympus DP12 digital camera system and recorded on a 3.3V Smart Media (SSFDC) card. Imaging software was Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA).
Bone marrow biopsy findings in a FIP1L1/PDGFRA-positive subject (subject 3). Before imatinib treatment (A), at 1 month following initiation of treatment (B), and at the time of molecular relapse (C). Posttreatment resolution of eosinophilia and hypercellularity was maintained despite molecular relapse. Sections were stained with hematoxylin and eosin (H&E), magnification 400×, and images were obtained by digital microscopy using an Olympus BH-Z microscope (Olympus America, Melville, NY) equipped with a DPlan 40 × 10.65 numeric aperture objective. Images were captured using an Olympus DP12 digital camera system and recorded on a 3.3V Smart Media (SSFDC) card. Imaging software was Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA).
Reinitiation of imatinib therapy
Imatinib was restarted at the most recent prerelapse dose in all patients within 2 months of the confirmation of molecular relapse. Although a second molecular remission was achieved in all patients, 2 patients (1 and 2) relapsed a second time on the prerelapse dose. In patient 1, whose initial molecular relapse occurred on 100 mg daily, the fusion gene remained detectable despite an increase in the imatinib dose to 200 mg daily. At this point, imatinib was discontinued briefly when he developed angioedema and abdominal pain that proved to be secondary to lisinopril. Two weeks following imatinib discontinuation, his eosinophil count began to rise peaking at 814/mm3. Imatinib was restarted at 400 mg daily with a decrease in his eosinophil count to his baseline (133/mm3) 2 weeks later, and a second molecular remission. He remained in molecular remission for 4 months before relapsing again likely due to noncompliance with the drug regimen. With resumption of 400 mg daily, he has again achieved molecular remission. Of note, he remained asymptomatic with a normal eosinophil count throughout this entire period.
Patient 2 became negative by molecular testing 1 month after restarting imatinib at 100 mg daily, but had intermittently positive tests beginning 2 months later. His dose was increased to 200 mg daily approximately 1 year after his initial positive test with molecular remission documented after 1 month on the increased dose. He has been entirely asymptomatic with a normal eosinophil count since beginning imatinib therapy 5 years ago and remains in molecular remission at this time (6 months after the last relapse).
Discussion
Although the exquisite sensitivity of FIP1L1/PDGFRA-positive CEL to imatinib mesylate has been well documented in numerous studies, little is known about the durability of the response and the potential for cure with imatinib therapy in this disorder. Imatinib therapy has been discontinued after molecular remission in patients with CML.15-18 Although most patients relapse, some experience durable remissions (9-24 months), and reinitiation of imatinib therapy has been followed by a new molecular response in all cases reported to date. A similar picture has emerged in gastrointestinal stromal tumors (GISTs), where 9 of 14 patients developed progressive disease after stopping imatinib therapy with a median progression-free survival of 10 months and no increase in imatinib resistance.19 A recent report of clinical relapse in 4 FIP1L1/PDGFRA-positive CEL patients who initially responded to imatinib and discontinued therapy for a variety of reasons suggests that relapse occurs with discontinuation of therapy; however, it is unclear whether the patients were in molecular remission at the time of treatment interruption.9 A single case of molecular relapse after molecular remission has been documented in the context of a multicenter study of real-time quantitative PCR monitoring in FIP1L1/PDGFRA-positive CEL, although few clinical details are provided.20
In the present study, 5 patients who had been in stable clinical, hematologic, and molecular remission for a minimum of 1 year, as documented by repeated clinical assessments and molecular testing conducted at regular intervals, underwent dose de-escalation and/or interruption. Molecular relapse occurred in all 5. Importantly, this was not likely due to the development of imatinib resistance as a function of duration of therapy, since neither of the control patients who remained on a stable dose of 300 to 400 mg daily has had evidence of molecular relapse despite comparable duration of therapy and frequency of molecular testing. Rather, the kinetics of the molecular relapse and the rapid reinduction of remission with resumption of imatinib are consistent with the presence of a residual population of mutation-positive stem cells, as have been described in CML. The lack of clinical breakthrough is likely due to the close monitoring and early identification and treatment of molecular relapse.
The most appropriate dose of imatinib in FIP1L1/PDGFRA-positive CEL remains controversial, as systematic dose comparison studies have not been performed. In the current study, the dose necessary to suppress the presence of the fusion gene below the level of detection by nested RT-PCR was 100 to 400 mg daily. Potential reasons for this variability include differences in drug absorption or drug metabolism, noncompliance, differences in disease burden, and/or susceptibility of different fusion breakpoints to imatinib. As we did not have access to testing for drug levels during this study, it is not possible to address the first 3 possibilities directly. It is interesting to note, however, that although 2 of the subjects (subjects 1 and 5) smoke cigarettes, a known inducer of cytochrome P-450, only one of them (subject 1) required increased dosing. With respect to disease burden, we have previously examined lineage involvement in 5 of the 7 subjects involved in the present study.12 Although the numbers are small, there was no association between the number of cell lineages involved and either the time to molecular remission or the imatinib maintenance dose (data not shown).
The magnitude of the molecular response has been shown to be a predictor of relapse and disease progression in CML.10,11 This is likely to be the case in FIP1L1/PDGFRA-positive CEL; however, the low prevalence of this disorder precludes the large treatment studies needed to address this question. Thus, although quantitative PCR monitoring for FIP1L1/PDGFRA-positive CEL has recently been described,20 whether this is more useful than the currently available techniques (nested RT-PCR and fluorescent in situ hybridization [FISH]) in predicting relapse and disease progression is likely to remain unknown.
In summary, we have demonstrated that despite the dramatic clinical, hematologic, and molecular response to imatinib in FIP1L1/PDGFRA-positive HES, this therapy appears to suppress rather than eliminate the clonal population. The relatively rapid molecular relapse observed in all patients suggests that interruption of therapy is not indicated in this disorder. Furthermore, although 100 mg daily appears to be sufficient to maintain molecular remission in most patients, some patients appear to require higher doses. Although assessment of the predictive value of molecular remission in preventing disease progression in FIP1L1/PDGFRA-positive CEL is not likely to be possible due to the low prevalence of the disorder, it would seem prudent to follow patients with periodic molecular testing (RT-PCR or FISH) and to increase the dose of imatinib until the fusion gene is no longer detectable. The utility of quantitative PCR monitoring, as has been recommended in CML, remains to be determined.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Intramural Research program of the NIH and NIAID.
National Institutes of Health
Authorship
Contribution: A.D.K. participated in all aspects of the trial, including the design, patient care, analysis, and writing of the paper; J.R. participated in the design and analysis of the trial, performed the bone marrow biopsies, and helped in the preparation of the paper; I.M. reviewed the bone marrow biopsies, supervised the molecular studies, and participated in the preparation of the paper; W.F. and S.L. performed the majority of the molecular studies; L.S. participated in the collection and analysis of the clinical data; M.A.L., C.T.-W., and M.H. participated in the study design and provided clinical support for the study; M.P.F. served as statistician and helped with study design and analysis; P.N., C.E.D., and T.B.N. participated in the study design and analysis and in the preparation of the paper.
Conflict-of-interest disclosure: P.N.'s spouse is an employee of Novartis and has stock options in Novartis as part of the employee pension plan. All other authors declare no competing financial interests.
Correspondence: Amy D. Klion, Bldg 4, Rm 126, National Institutes of Health, Bethesda, MD 20892; e-mail:aklion@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal