Severe malaria is manifest by a variety of clinical syndromes dependent on properties of both the host and the parasite. In young infants, severe malarial anemia (SMA) is the most common syndrome of severe disease and contributes substantially to the considerable mortality and morbidity from malaria. There is now growing evidence, from both human and mouse studies of malaria, to show that anemia is due not only to increased hemolysis of infected and clearance of uninfected red blood cells (RBCs) but also to an inability of the infected host to produce an adequate erythroid response. In this review, we will summarize the recent clinical and experimental studies of malaria to highlight similarities and differences in human and mouse pathology that result in anemia and so inform the use of mouse models in the study of severe malarial anemia in humans.
Malaria in humans
The vast majority of morbidity and mortality from malaria is caused by infection with Plasmodium falciparum, although P vivax, P ovale, and P malariae also are responsible for human infections. The total burden of disease has recently been estimated to 515 million episodes annually and malaria is responsible for 18% of all childhood deaths in sub-Saharan Africa, equivalent to 800 000 deaths each year.1,2
The study of malarial anemia has somewhat belatedly excited academic and professional interest. Severe malarial anemia (SMA) certainly merits concern as a major public health problem because of the very large numbers of children affected, and it is likely that these numbers may increase as drug resistance spreads. Concerns have also been raised by data from recent vaccine studies, which suggest that monkeys immunized with erythrocytic-stage antigens, and that have acquired protection from acute infection, may succumb to severe anemia during a subacute or chronic phase of infection.3,4 Moreover, there is increasing awareness of the difficulty of satisfactory treatment by blood transfusion outside specialist centers in many endemic areas as a result of the limited availability of a rapid and safe supply of blood.1,5
SMA is seen most frequently in areas of very high malaria transmission and most commonly in young children and pregnant women.6 The prevalence of anemia, defined as a hematocrit (Hct) level higher than 0.33, in malaria-endemic areas of Africa, varies between 31% and 91% in children, and between 60% and 80% in pregnant women.7,8 Given the high degree of morbidity that is associated with SMA in children, this term will be used to refer to severe anemia in this group, unless stated otherwise.
It is very difficult to determine the number of cases of severe anemia attributable to malaria as the WHO definition of SMA (a hemoglobin [Hb] concentration of < 50 g/L [5 g/dL], or a Hct < 0.15, in the presence of a parasitemia > 10 000 parasites per microliter [μL], and a normocytic blood film) may exclude the considerable proportion of children admitted with severe anemia that has a blood smear negative for malaria parasites but that responds to antimalarial treatment.9,10 It may be difficult to attribute anemia to a single cause since the background to malarial anemia is often complex in endemic areas and hematinic deficiencies, genetic traits, and intercurrent infection all may contribute to anemia (see Roberts et al11 for review). Nevertheless, a randomized placebo-controlled trial of malaria chemoprophylaxis and iron supplementation in infants, from an endemic area, has shown that malaria infection was the main etiologic factor underlying anemia.9,12
The pathology of malaria is associated with the blood stage of infection (for overview of life cycle see Figure 1).13 P falciparum infections have high multiplication rates while also expressing clonally variant antigens at the surface of infected erythrocytes (Pf-EMP-1). Pf-EMP-1 binds to ligands on the surface of endothelial cells and mediates sequestration of infected erythrocytes in postcapillary venules. Both of these characteristics allow the P falciparum parasite to evade the host immune system, which results in the occurrence of high parasitemias with repeated infections that contribute to the chronic nature of this disease.14 In P vivax and P ovale malaria, high parasitemias are rare as invasion of erythrocytes is limited to reticulocytes. However, P vivax can, on occasion, cause severe disease including anemia with severe hemolysis.15,–17
Plasmodia life cycle. (A) The asexual life cycle begins when sporozoites from a female mosquito taking a blood meal enter the circulation and invade hepatocytes. (B) Up to 10 000 merozoites are formed. Following rupture of the hepatocyte, infective merozoites are released and invade erythrocytes (RBCs). (C) Within RBCs, the parasite develops through the stages of rings, trophozoites, and schizonts. Mature schizonts burst to release erythrocytic merozoites that invade new RBCs. (D) A small proportion of merozoites in RBCs transform into male and female gametocytes that are ingested by the mosquito. (E) The male and female gametes fuse and transform into an öocyst that divides asexually into many sporozoites that migrate to the salivary glands from where they are released during the next blood meal. Reproduced from Ocana-Morgher et al13 with permission.
Plasmodia life cycle. (A) The asexual life cycle begins when sporozoites from a female mosquito taking a blood meal enter the circulation and invade hepatocytes. (B) Up to 10 000 merozoites are formed. Following rupture of the hepatocyte, infective merozoites are released and invade erythrocytes (RBCs). (C) Within RBCs, the parasite develops through the stages of rings, trophozoites, and schizonts. Mature schizonts burst to release erythrocytic merozoites that invade new RBCs. (D) A small proportion of merozoites in RBCs transform into male and female gametocytes that are ingested by the mosquito. (E) The male and female gametes fuse and transform into an öocyst that divides asexually into many sporozoites that migrate to the salivary glands from where they are released during the next blood meal. Reproduced from Ocana-Morgher et al13 with permission.
The spectrum of the clinical presentation and severity of P falciparum infection is broad. In endemic areas, many infections in semi-immune and immune children and adults present as uncomplicated febrile illness. In more severe disease, nonimmune individuals may exhibit a number of syndrome(s) including anemia, coma, respiratory distress, and hypoglycemia, and have a high frequency of concurrent bacteremia.18,–20 Many children present with mild, moderate, and even severe anemia without other syndromes of severe disease. However, severe anemia may be accompanied by other syndromes of severe disease.18 For example, children with anemia may also present with malaise, fatigue, dyspnoea, or respiratory distress as metabolic acidosis supervenes18,21,22 (for review see English et al23 ). The age distribution of the syndromes of severe disease is striking, but poorly understood. Children born in endemic areas are largely protected from severe malaria in the first 6 months of life by the passive transfer of maternal immunoglobulins and by fetal hemoglobin. The presentation of disease changes from severe anemia in children aged between 1 and 3 years in areas of high transmission to cerebral malaria in older children in areas of lower transmission.24 As transmission intensity declines, severe malaria is most frequently found in older age groups.
The anemia of P falciparum malaria is typically normocytic and normochromic, with a notable absence of reticulocytes, although microcytosis and hypochromia may be present due to the very high frequency of alpha and beta thalassemia traits and/or iron deficiency in many endemic areas25,26 (for a general review of the hematologic features of malaria infection see Abdalla et al27 and Roberts et al28 ). The exact differences in the pathophysiology of anemia in the various clinical settings, ages, and geographic areas are poorly defined and surely represent a fruitful field for further study. A less common form of anemia in malaria is “blackwater fever” characterized by the sudden appearance of hemoglobin in the urine associated with irregular use of quinine.29,30
The clinical setting of severe anemia is therefore varied and complex: acute infection may lead to anemia and/or cerebral malaria, respiratory distress, and hypoglycemia; and chronic, repeated infection may lead to severe anemia. In addition, there may be a background of normal or low Hb. As such, any understanding of the pathophysiological processes has ultimately to be linked to the diverse clinical contexts.
Malaria in mice
In searching for tractable model systems, we are fortunate that laboratory mice and rats can be infected with natural species of rodent malaria. Strictly speaking the rodent host-parasite systems are not “models” (“representation on a smaller scale” or “an example”) since the progression of disease may be quite different than that seen in P falciparum malaria. A detailed comparative description of the features of the rodent “models” of malaria is therefore a prerequisite to improving the use of these systems in understanding severe anemia in human disease.
The scope of mouse models of malaria is enhanced by the availability of differentially susceptible inbred mouse strains in which infections can be lethal or nonlethal. The course of infection and immunologic response of infection is variable but specific for each host-parasite combination, and always results in anemia (Table 1; and see Lamb et al31 for review). There are several strains of Plasmodia that cause infection in mice, such as P chabaudi, P yoelii, P berghei, and P vinckei. As for P falciparum infections, P chabaudi invades RBCs of all ages,32,–34 while P yoelii has a preference for reticulocytes35 and therefore may serve better as a model for P vivax. However, the majority of these parasite-mouse models demonstrate an acute malaria infection with parasitemias often exceeding 20%, which is in contrast to severe anemia in humans where acute malaria frequently occurs with a lower parasitemia (Table 2).
Key rodent malaria infections with features of anemia
| Plasmodium species/parasite strain/clone . | Mouse strain . | Features of infection . | Features of anemia . | Reference no. . |
|---|---|---|---|---|
| P chabaudi chabaudi | ||||
| AS, CB, AJ, ER | C57BL/6 mice | Nonlethal Sequestration of mature iRBCs | Acute anemia with extravascular hemolysis followed by reticulocytosis after peak parasitemia | 36, 66, 82 |
| A/J | Lethal Sequestration of mature iRBCs | Acute and rapidly progressive anemia | 66 | |
| P berghei | ||||
| ANKA | C57BL/6 and CBA/J | Lethal Cerebral syndrome Some sequestration of mature iRBCs | Acute anemia Inadequate reticulocyte response | 63, 65 |
| ANKA | BALB/c and DBA/2 | Lethal, but also drug induced chronic infection No cerebral syndrome | Chronic anemia in semi-immune mice | 57 |
| P berghei | Rat | Lethal | Anemia in splenectomized rats | 119 |
| P yoelii | ||||
| 17X | BALB/c SCID | Nonlethal in BALB/c Sequestration of iRBCS in cerebral vessel | Invades only reticulocytes Premature removal of RBC Increased parasitized RBC rigidity | 56, 120 |
| 17XL | BALB/c | Lethal cerebral syndrome sequestration of iRBCS in cerebral vessel | Can invade RBCs of all ages; acute anemia | 112 |
| P vinckei | BALB/c | Lethal | Inhibition of erythropoiesis | 121 |
| Plasmodium species/parasite strain/clone . | Mouse strain . | Features of infection . | Features of anemia . | Reference no. . |
|---|---|---|---|---|
| P chabaudi chabaudi | ||||
| AS, CB, AJ, ER | C57BL/6 mice | Nonlethal Sequestration of mature iRBCs | Acute anemia with extravascular hemolysis followed by reticulocytosis after peak parasitemia | 36, 66, 82 |
| A/J | Lethal Sequestration of mature iRBCs | Acute and rapidly progressive anemia | 66 | |
| P berghei | ||||
| ANKA | C57BL/6 and CBA/J | Lethal Cerebral syndrome Some sequestration of mature iRBCs | Acute anemia Inadequate reticulocyte response | 63, 65 |
| ANKA | BALB/c and DBA/2 | Lethal, but also drug induced chronic infection No cerebral syndrome | Chronic anemia in semi-immune mice | 57 |
| P berghei | Rat | Lethal | Anemia in splenectomized rats | 119 |
| P yoelii | ||||
| 17X | BALB/c SCID | Nonlethal in BALB/c Sequestration of iRBCS in cerebral vessel | Invades only reticulocytes Premature removal of RBC Increased parasitized RBC rigidity | 56, 120 |
| 17XL | BALB/c | Lethal cerebral syndrome sequestration of iRBCS in cerebral vessel | Can invade RBCs of all ages; acute anemia | 112 |
| P vinckei | BALB/c | Lethal | Inhibition of erythropoiesis | 121 |
Characteristics of disease during infection with P falciparum– and plasmodia-infecting mouse strains
| Parameter . | Human . | Mouse . | ||
|---|---|---|---|---|
| P bergeii/Balb/c* . | P chabaudi/A/J . | |||
| Parasitemia, % | 2.672 | 15.7106 | 0.957 | 50–60109 |
| Hematocrit level, % | 12.972 | NR | NR | 10–15109 |
| Hemoglobin concentration, g/dL | 472 | < 5106 | 55% of baseline57 | NR |
| Erythropoietin, mU/mL | 503372 | 1000106 | NR | 1000–3000109 |
| Parameter . | Human . | Mouse . | ||
|---|---|---|---|---|
| P bergeii/Balb/c* . | P chabaudi/A/J . | |||
| Parasitemia, % | 2.672 | 15.7106 | 0.957 | 50–60109 |
| Hematocrit level, % | 12.972 | NR | NR | 10–15109 |
| Hemoglobin concentration, g/dL | 472 | < 5106 | 55% of baseline57 | NR |
| Erythropoietin, mU/mL | 503372 | 1000106 | NR | 1000–3000109 |
Representative values for parameters measured in patients who present with SMA are given for comparison with values obtained from a sample of studies in mice. For a more detailed study on the changes in erythropoietin and severity of anemia during infection with P falciparum see Casals-Pascual.72
NR indicates not reported.
Hb levels reported in this study were given as a percent of baseline values determined in noninfected mice.
Pathophysiology of malarial anemia
In attempting to develop an in vivo model of SMA, it may not be necessary to demonstrate all features of severe falciparum anemia but to represent a component of this syndrome that may help to identify factors that contribute to its severity. These should then be considered in the context of concurrent infections that may exacerbate the anemia.36 The examples of possible causes of SMA given in this article will be from clinical studies in which participants with coinfections, hemoglobinopathies, or folate or G6PD deficiencies were excluded from the study.
The underlying causes of SMA in humans may include one or more of the following mechanisms: (1) the clearance and/or destruction of infected RBCs, (2) the clearance of uninfected RBCs, (3) erythropoietic suppression and dyserythropoiesis. Each of these mechanisms has been implicated in both human and mouse malarial anemias (Table 3), but their relative contribution between the species is likely to differ. In the remainder of this review, the pathophysiological mechanisms of human and mouse malarial anemia will be discussed, highlighting similarities and differences.
Pathological features of P falciparum and mouse malarial anemia
| Mechanism . | Parameters . | References . | |
|---|---|---|---|
| Human . | Mouse . | ||
| Clearance of iRBCs | |||
| Rigidity | Parasite Ag in iRBCs | 122 | 120 |
| Opsonization | Parasite specific Abs and complement | 52, 123, 124 | 128,–130 |
| Band 3–specific Abs | 125 | ||
| Nonopsonization | Macrophage activation | 126 | 131 |
| Pitting | Deformability of RBC membrane by RESA | 127 | 132 |
| Clearance of uninfected RBCs and erythroblasts | |||
| Opsonization | Immune complexes and CD35 | 133,,–136 | Not determined |
| Nonopsonization | Macrophage activation | 44 | 57 |
| Rigidity | RSP-2 antibodies | 53 | Not identified |
| Oxidative stress | 47, 137, 138 | 56 | |
| Intravascular hemolysis | |||
| Hemoglobinemia and Hemoglobinuria | Quinine, mefloquine G6PD deficiency | 29, 30 | 57 |
| Suppression of erythropoiesis | |||
| Host genotype | Haptoglobin | 139, 140 | 40 |
| Erythroid genes | 115 | 116 | |
| Cytokines | High TNF:IL-10 | 78, 79 | 81 |
| High MIF | 88 | 87 | |
| Low IL-12 | 89 | 85 | |
| Parasite products | GPI | 98 | 97 |
| Hz | 71 | 88 | |
| Dyserythropoiesis | |||
| Abnormal precursor morphology | Eg, cytoplamsic bridging | 59 | Not observed |
| Insufficient reticulocytosis | Cell cycle arrest | 61 | 63 |
| Changes in BM precursor numbers | Decreased BFU-Es | 141 acute disease | 63 |
| Decreased CFU-Es | None reported | 142 | |
| Extramedullary erythropoiesis | |||
| Splenic erythropoiesis | None reported | 65, 66, 112 | |
| Hepatic erythropoiesis | None reported | 143 | |
| Mechanism . | Parameters . | References . | |
|---|---|---|---|
| Human . | Mouse . | ||
| Clearance of iRBCs | |||
| Rigidity | Parasite Ag in iRBCs | 122 | 120 |
| Opsonization | Parasite specific Abs and complement | 52, 123, 124 | 128,–130 |
| Band 3–specific Abs | 125 | ||
| Nonopsonization | Macrophage activation | 126 | 131 |
| Pitting | Deformability of RBC membrane by RESA | 127 | 132 |
| Clearance of uninfected RBCs and erythroblasts | |||
| Opsonization | Immune complexes and CD35 | 133,,–136 | Not determined |
| Nonopsonization | Macrophage activation | 44 | 57 |
| Rigidity | RSP-2 antibodies | 53 | Not identified |
| Oxidative stress | 47, 137, 138 | 56 | |
| Intravascular hemolysis | |||
| Hemoglobinemia and Hemoglobinuria | Quinine, mefloquine G6PD deficiency | 29, 30 | 57 |
| Suppression of erythropoiesis | |||
| Host genotype | Haptoglobin | 139, 140 | 40 |
| Erythroid genes | 115 | 116 | |
| Cytokines | High TNF:IL-10 | 78, 79 | 81 |
| High MIF | 88 | 87 | |
| Low IL-12 | 89 | 85 | |
| Parasite products | GPI | 98 | 97 |
| Hz | 71 | 88 | |
| Dyserythropoiesis | |||
| Abnormal precursor morphology | Eg, cytoplamsic bridging | 59 | Not observed |
| Insufficient reticulocytosis | Cell cycle arrest | 61 | 63 |
| Changes in BM precursor numbers | Decreased BFU-Es | 141 acute disease | 63 |
| Decreased CFU-Es | None reported | 142 | |
| Extramedullary erythropoiesis | |||
| Splenic erythropoiesis | None reported | 65, 66, 112 | |
| Hepatic erythropoiesis | None reported | 143 | |
The etiology of anemia based on observations made during the course of disease is compared for P falciparum and mouse malaria infections.
RBC indicates red blood cell; iRBC, infected red blood cell; Ag, antigen; Abs, antibodies; RESA, ring-infected erythrocyte surface antigen; RSP-2, ring surface protein 2; TNF, tumor necrosis factor α; IL, interleukin; MIF, macrophage inhibitory factor; GPI, glycophosphatidylinositol; HZ, hemozoin; BM, bone marrow; BFU-E, burst forming unit erythroid; and CFU-E, colony forming unit erythroid.
Loss of infected erythrocytes
During infection there is obvious loss of infected erythrocytes through parasite maturation as well as through recognition by macrophages. The pathways of phagocytic clearance for both humans and mice are summarized in Table 3 (see Casals-Pascual and Roberts37 for further discussion).
It is clear that similar mechanisms exist for the clearance of infected erythrocytes in humans and mice. However, the removal of infected erythrocytes in humans with parasitemias of less than 1% is unlikely to have a significant impact on the degree of anemia. This removal, therefore, may prove more pertinent for the onset of anemia in individuals suffering from an acute infection, in particular children where parasitemias are frequently greater than 10%. Since malaria infections in mice often attain high parasitemias with intravascular hemolysis of infected erythrocytes,38 they may provide suitable models to study the factors that contribute to the pathology of anemia during acute falciparum infection.
Loss of uninfected erythrocytes
During human malaria infection, many uninfected red cells are destroyed, in the spleen and quite possibly the liver, and their destruction has been identified as the major contributor to malarial anemia.39,–41 Both mathematical modeling and clinical observations suggest that 10 times as many uninfected RBCs are removed from the circulation for each infected erythrocyte.40 Although few direct measurements of red cell survival have been made in human infections, reduced half-life of normal erythrocyte and increased clearance of heat-treated erythrocytes have been demonstrated in patients with malaria, consistent with these observations.39,42
The activity and the number of macrophages are also increased during human malarial infection, and may therefore contribute to the increased removal of uninfected cells.43,,–46 The increased clearance of uninfected erythrocytes is due not only to the activation of splenic macrophages but also to extrinsic and intrinsic changes to the red blood cells that enhance their recognition and phagocytosis. First, uninfected RBCs have a reduced deformability leading to enhanced clearance in the spleen. The mechanism responsible for the loss of deformability is not completely understood. Increased oxidation of membranes in uninfected erythrocytes has been shown in children with severe P falciparum malaria, and the ongoing inflammatory insults associated with acute malaria (proinflammatory cytokines), or direct effect of parasite products have been shown to cause loss of RBC deformability.44,47,48 Intriguingly, a severe reduction in red cell deformability is also a strong predictor for mortality measured on admission, both in adults and children with severe malaria.49,50 Second, the deposition of immunoglobulin and complement on uninfected RBCs may enhance receptor-mediated uptake by macrophages (Table 3).
Parasite products that may be part of the immunoglobulin-antigen complexes deposited on uninfected RBCs include the P falciparum ring surface protein 2 (RSP-2). This protein, expressed shortly after merozoite invasion of RBCs, mediates adhesion of iRBCs to endothelial cells.51,52 RSP-2 is also deposited on uninfected RBCs and the opsonization of these RSP-2–bearing uninfected RBCs provides a mechanism of removing uninfected RBCs. Indeed high levels of these antibodies that facilitate complement-mediated phagocytosis of cells expressing RSP-2 are found in sera from immune adults and children with severe anemia.53 This antigen is also present on the surface of erythroblasts in bone marrows of P falciparum–infected patients, indicating that clearance or damage to circulating or developing erythroid cells by RSP-2 and anti–RSP-2 could contribute to the development of SMA.
Due to the high level of parasitemia, it may appear that the clearance of uninfected RBCs for the development of anemia is less significant in mice than in the majority of human malaria infections. However, in P chabaudi and P berghei infections the degree of anemia is not always related to peak parasitemia,54,55 and premature removal of uninfected RBCs has been shown in P yoelii infection. Interestingly, immunoglobulin-mediated removal of erythrocytes in this infection was not implicated, but rather it was thought that abnormalities in the erythrocytes themselves was responsible.56 It remains to be determined whether removal of RBCs via immune complex deposition takes place in mouse infection, and the parasite product RSP-2 has not yet been identified in rodent parasites. So far it seems that rodent models in this respect may be representative of acute human infections only where removal of uninfected RBCs is independent of opsonization. However, this is clearly an area where further research is needed. More recently, a model of chronic anemia has been developed where P berghei ANKA–infected semi-immune rodents show that, although parasitemia remains less than 1%, there is a 5- to 10-fold increase in the clearance of uninfected RBCs.57 In this study, clearance of uninfected RBCs was delayed in mice depleted of macrophages supporting the view that removal of uninfected RBCs is an important factor in anemia. Moreover, macrophage activation was dependent on CD4+ T cells. This model could therefore represent one feature of chronic human infection where removal of uninfected erythrocytes contributes to the severity of anemia.
Erythropoietic suppression and dyserythropoiesis
Normal erythropoiesis (as described in Figure 2) is perturbed during malaria infection. The earliest observations of reduced erythropoiesis in acute human malaria were made more than 60 years ago where reticulocytopenia was observed in P vivax and P falciparum malaria infection followed by reticulocytosis after parasite clearance.58 Later, it was demonstrated that low reticulocyte counts in Thai patients with malaria was accompanied by suppression of erythropoiesis (reviewed in Casals-Pascual and Roberts37 ).
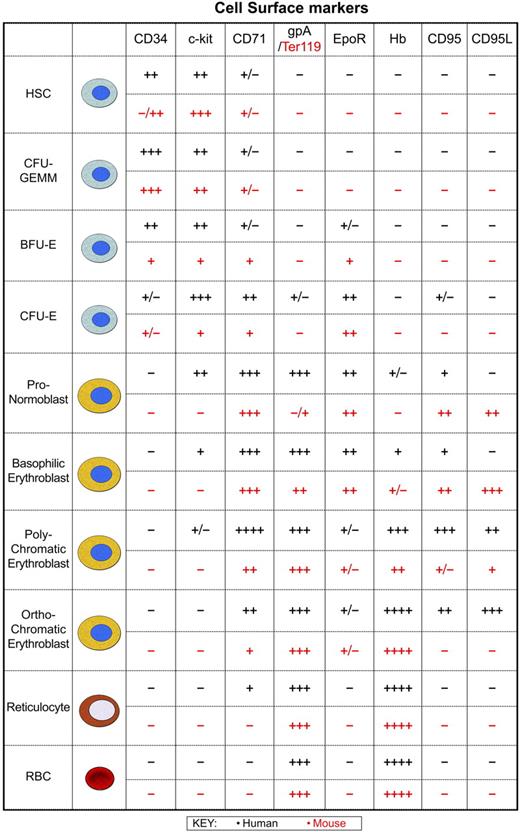
Human and murine erythropoiesis. At birth, erythropoiesis occurs throughout the human skeleton, although over time hematopoietic activity is confined to the sternum and pelvic region of most adults. Likewise, in adult mice the bone marrow is the major organ, with the majority of hematopoietically active cells typically present in the femur and tibia, though this is dependent on the age and strain of the mouse. Erythropoiesis from the multipotent hematopoietic stem cell (HSC) through to the mature red blood cell is shown to allow comparison of human and mouse cell surface phenotypes. The time at which these markers are expressed and lost at the cell surface is very similar between species indicating that as a first approximation the mechanisms used to control cell expansion, differentiation, and removal of precursors may not differ greatly. Developing erythroid cells respond to signals from stromal cells of the bone marrow or spleen. Erythroblasts are organized into erythroblastic islands that consist of macrophages surrounded by developing erythroid precursors.144,145 Macrophages provide many of the cellular mediators that control erythropoietic activity: GM-CSF, IL-3, and stem cell factor (SCF) generate colony-forming unit erythroid macrophage-granulocyte megakaryocyte (CFU-GEMM) and burst-forming unit erythroid (BFU-E), whereas TGF-β, TNF-α, and MIP1-α inhibit cell cycle activity146 and BFU-E development. Other factors that negatively regulate numbers include proapoptotic activity initiated by interaction of the Fas receptor (CD95) with Fas ligand (CD95L).147,148 Expression of caspases also results in cleavage of GATA-1 and loss of its antiapoptotic activity.148 Secretion of Epo induces the expansion of colony-forming unit erythroid (CFU-E) and initiates differentiation through a number of erythroid-specific events. SCF and Epo synergize to drive the proliferation of human erythroid progenitors and precursors150,151 and induce anti-apoptotic activity.152,153 In human and mouse erythropoiesis, levels of the transferrin receptor (CD71) peak when the highest transport of transferrin-bound iron is required for synthesis of heme from protoporphyrin. During erythroid precursor maturation an increase in expression of glycophorin A (gpA) in humans or Ter119 in mice is coupled with a drop in CD71 expressed at the cell surface. This loss of CD71 indicates reduced proliferation and heme synthesis with continued differentiation into reticulocytes.
Human and murine erythropoiesis. At birth, erythropoiesis occurs throughout the human skeleton, although over time hematopoietic activity is confined to the sternum and pelvic region of most adults. Likewise, in adult mice the bone marrow is the major organ, with the majority of hematopoietically active cells typically present in the femur and tibia, though this is dependent on the age and strain of the mouse. Erythropoiesis from the multipotent hematopoietic stem cell (HSC) through to the mature red blood cell is shown to allow comparison of human and mouse cell surface phenotypes. The time at which these markers are expressed and lost at the cell surface is very similar between species indicating that as a first approximation the mechanisms used to control cell expansion, differentiation, and removal of precursors may not differ greatly. Developing erythroid cells respond to signals from stromal cells of the bone marrow or spleen. Erythroblasts are organized into erythroblastic islands that consist of macrophages surrounded by developing erythroid precursors.144,145 Macrophages provide many of the cellular mediators that control erythropoietic activity: GM-CSF, IL-3, and stem cell factor (SCF) generate colony-forming unit erythroid macrophage-granulocyte megakaryocyte (CFU-GEMM) and burst-forming unit erythroid (BFU-E), whereas TGF-β, TNF-α, and MIP1-α inhibit cell cycle activity146 and BFU-E development. Other factors that negatively regulate numbers include proapoptotic activity initiated by interaction of the Fas receptor (CD95) with Fas ligand (CD95L).147,148 Expression of caspases also results in cleavage of GATA-1 and loss of its antiapoptotic activity.148 Secretion of Epo induces the expansion of colony-forming unit erythroid (CFU-E) and initiates differentiation through a number of erythroid-specific events. SCF and Epo synergize to drive the proliferation of human erythroid progenitors and precursors150,151 and induce anti-apoptotic activity.152,153 In human and mouse erythropoiesis, levels of the transferrin receptor (CD71) peak when the highest transport of transferrin-bound iron is required for synthesis of heme from protoporphyrin. During erythroid precursor maturation an increase in expression of glycophorin A (gpA) in humans or Ter119 in mice is coupled with a drop in CD71 expressed at the cell surface. This loss of CD71 indicates reduced proliferation and heme synthesis with continued differentiation into reticulocytes.
Bone marrow aspirates taken from Gambian children with acute anemia revealed that despite an increase in cellularity no significant difference in the total number of erythroblasts was observed when compared with uninfected patients, providing evidence for a suppressed erythroid response. Children who presented with chronic anemia (parasitemia < 1%) had higher levels of erythroid hyperplasia and dyserythropoiesis59,60 Dyserythropoiesis or morphologically and/or functionally abnormal production of RBCs was demonstrated by cytoplasmic vacuolation, stippling, fragmentation, intercytoplasmic bridges, nuclear fragmentation, and multinuclearity. This coincided with reduced reticulocytosis indicating functional disruption of RBC output from the bone marrow59,60 (Figure 3). In a smaller study of 6 children with chronic disease, an increased proportion of polychromatic erythroblasts in the G2 phase of division was observed.61 Treatment of these patients with antimalarial drugs increased reticulocyte numbers, which pointed to P falciparum as the cause of dyserythropoiesis and ineffective erythropoiesis.
Direct and indirect effects of parasite on the development of malarial anemia. Severe malarial anemia is characterized by destruction of infected red blood cell (iRBC) following schizogony and clearance of both iRBCs and uninfected RBCs. During malarial infection, changes in membrane protein composition occur and the resultant immune complexes of RBCs, Ag, and immunoglobulin (Ig) (eg, RBC:RSP2:Ig) are cleared by macrophages to the spleen where they become activated (see Table 3 for more examples). Pigment-containing macrophages may release inflammatory cytokines and other biologically active mediators such as hydroxy-nonenal (HNE). It is possible that malarial pigment or other parasite products may have a direct inhibitory effect on erythropoiesis. Inhibition of erythropoiesis may be at one or more sites in the growth and differentiation of hematopoietic progenitors. Both indirect and direct effects may cause suppression of the bone marrow and spleen resulting in inadequate reticulocyte counts for the degree of anemia. The mechanisms of insufficient erythropoiesis in murine malaria have been summarized in Chang and Stevenson.154 Blue box indicates demonstrated in human infection; pink box, demonstrated in mouse infection; yellow box, demonstrated in both human and mouse infections. Hz indicates hemozoin; GPI, glycophosphatidylinositol anchors of merozoite proteins; Epo, erythropoietin; Epo-R, erythropoietin receptor; MΦ, macrophage; RSP-2, ring surface protein-2; and Ig, immunoglobulin. Figure modified with permission from Springer Science Business Media, Heidelberg, Germany.
Direct and indirect effects of parasite on the development of malarial anemia. Severe malarial anemia is characterized by destruction of infected red blood cell (iRBC) following schizogony and clearance of both iRBCs and uninfected RBCs. During malarial infection, changes in membrane protein composition occur and the resultant immune complexes of RBCs, Ag, and immunoglobulin (Ig) (eg, RBC:RSP2:Ig) are cleared by macrophages to the spleen where they become activated (see Table 3 for more examples). Pigment-containing macrophages may release inflammatory cytokines and other biologically active mediators such as hydroxy-nonenal (HNE). It is possible that malarial pigment or other parasite products may have a direct inhibitory effect on erythropoiesis. Inhibition of erythropoiesis may be at one or more sites in the growth and differentiation of hematopoietic progenitors. Both indirect and direct effects may cause suppression of the bone marrow and spleen resulting in inadequate reticulocyte counts for the degree of anemia. The mechanisms of insufficient erythropoiesis in murine malaria have been summarized in Chang and Stevenson.154 Blue box indicates demonstrated in human infection; pink box, demonstrated in mouse infection; yellow box, demonstrated in both human and mouse infections. Hz indicates hemozoin; GPI, glycophosphatidylinositol anchors of merozoite proteins; Epo, erythropoietin; Epo-R, erythropoietin receptor; MΦ, macrophage; RSP-2, ring surface protein-2; and Ig, immunoglobulin. Figure modified with permission from Springer Science Business Media, Heidelberg, Germany.
In the mouse, both lethal and nonlethal malaria infections induce ineffective erythropoiesis, with alterations in erythropoietic progenitor and precursor populations, as well as in the sites of erythropoiesis (Figure 3). In contrast to the human studies cited, a decrease in mouse bone marrow cellularity of between 40% to 75% of normal levels is seen at peak parasitemia or death, whichever is reached first,62,,–65 with reductions in cellularity proportional to the severity of infection.54,63,66 However, despite these discrepancies with the reported pathology in human infection, reports of only a minimal reduction in the total number of BFU-Es, and at best only a modest increase in CFU-Es in mice,63,,–66 suggest that the bone marrow is unable to compensate for RBC loss in both humans and mice through increased erythropoiesis during the acute phase of infection. At present there are no studies reporting the production of dyserythropoietic erythroblasts during mouse malaria infection, most probably because there are few chronic infection models. Dyserythropoiesis has been observed only in chronic human infections, thus future studies on the production of abnormal erythroblasts in the newly described chronic model of P berghei57 may help elucidate the mechanism by which this occurs.
A parasite by-product of hemoglobin digestion, hemozoin, may have a role in the impaired erythroid development through its effects on human monocyte function. Hemozoin reduces human macrophage oxidative burst activity, prevents up-regulation of activation markers,67,68 and also stimulates the secretion of biologically active endoperoxides from monocytes, such as 15(S)-hydroxyeicosatetraenoic (HETE) and 4-hydroxy-nonenal (4-HNE) through oxidation of membrane lipids,69,70 which may effect erythroid growth.71 Macrophage dysfunction could also disrupt the function of erythroblastic islands in which macrophages support terminal differentiation of erythroblasts in the bone marrow.
Hemozoin and TNF-α also have additive effects on erythropoiesis in vitro, and in a clinical study hemozoin-containing macrophages and plasma hemozoin were associated with anemia and reticulocyte suppression.72 Moreover, bone marrow sections from children who died from severe malaria show a significant association between the quantity of hemozoin (located in erythroid precursors and macrophages) and the proportion of erythroid cells that was abnormal. These findings are consistent with a direct inhibitory effect of hemozoin on erythropoiesis and therefore warrant further investigation. Disappointingly, however, there has been little focus on the role of hemozoin on erythropoiesis during mouse malaria infection, where effects of hemozoin-induced suppression of erythropoiesis could be dissected in an in vivo setting. Clearly hemozoin present in bone marrow may have a role in mice, as the efficient reticulocyte response is not observed until there is parasite clearance.55,66
Cytokine suppression of erythropoiesis.
During the acute phase of both human and mouse infections there is a strong inflammatory response, which results in increases in TNF-α and IFN-γ.73,–75 TNF-α inhibits all stages of erythropoiesis,76 and IFN-γ works with TNFα to inhibit erythroid growth and differentiation by up-regulating expression of TRAIL, TWEAK, and CD95L in developing erythroblasts.77 However, in a nonlethal P chabaudi (AS) infection of C57BL/6 mice, neutralization of TNF-α or IFN-γ has no effect on in vitro erythropoiesis.74 While severe disease in children is associated with elevated levels of proinflammatory and anti-inflammatory cytokines, the severity of anemia seems to be dependent on levels of TNF-α relative to its regulator, the potent anti-inflammatory cytokine IL-10. Several clinical studies have demonstrated that a low ratio of plasma IL-10/TNF-α is associated with SMA in young children.78,79 Furthermore, a number of polymorphisms in the human TNF-α promoter show greater association with anemia than with cerebral malaria.80 The IL-10/TNF-α ratio is also important in mice where IL-10 knock-out mice infected with P chabaudi display increased anemia,81 which is reversed following TNF-α neutralization in vivo.82 It is therefore conceivable that in both humans and mice, IL-10 may protect against bone marrow suppression and erythrophagocytic activity induced by TNF-α and/or mitigate other proinflammatory stimuli.
Many other proinflammatory cytokines such as IL-12, IL-18, and migration inhibitory factor (MIF) have also been implicated in the pathogenesis of anemia in malaria. In humans, the secretion of IL-12 and IL-18 from macrophages induces production of IFN-γ from natural killer (NK), B cells, and T cells,83 while MIF is produced by activated T cells and macrophages and inhibits the anti-inflammatory activity of glucocorticoids (for review see Clark and Cowden84 ).
IL-12 is present at higher levels in nonlethal, compared with lethal, infections of P chabaudi, suggesting that this cytokine may be a stimulator of erythropoiesis.85,86 Conversely, with elevated levels found during infection, MIF has been shown to suppress hematopoiesis,87 and more recently P chabaudi infection of MIF knock-out mice resulted in a less severe anemia with improved development of erythroid progenitors in the bone marrow.88 In this latter study, a hyperparasitemic model of malaria (P chabaudi AS in Balb/c) was used. However the levels of parasitemia, TNF-α, and IFN-γ in wild-type and knock-out mice were equally raised, therefore indicating a direct effect of MIF on erythroid development during the acute phase of disease.
The association of IL-12 with severe falciparum malaria is less clear. While some studies observe moderate increases in IL-12 and IL-18 in patients with severe anemia,83,89 others report decreases in IL-12 in patients with severe malaria (Hb of < 75 g/L [7.5 g/dL]) compared with uncomplicated controls (Hb of > 100 g/L [10 g/dL]), or no significant increases in patients with severe disease compared with uncomplicated malaria.90,91 In these last 2 examples, anti-inflammatory cytokines such as TGF-β or IL-10 were also reduced in patients with severe disease. In contrast, patients with acute disease and elevated levels of IL-12 had marked increases in IL-10.83 Since the majority of patients with anemia in this last study had average Hb levels of 90 g/L (9 g/dL) it is possible that, as with mice, increases in IL-12 are associated with reduced severity of SMA. The data on serum levels of MIF in patients with malaria are, however, consistent with its role as a hematopoietic suppressor in mice: MIF concentrations in those with moderate anemia are decreased,89 and MIF is elevated in patients with more severe anemia.90
These observations, in both human and mouse infections, show the complexity of cytokine responses, and also highlight the importance of a balance between proinflammatory and anti-inflammatory cytokines, which can either be protective or detrimental to the host. Understanding the role of these cytokines will require more data from adequately powered studies to enable use of more sophisticated multivariate analyses that may allow for intricate interactions between each factor. In addition, significant similarities do indeed exist between humans and mice, and the ready availability of gene knock-out mouse models, for example, will allow for more in-depth analysis of proinflammatory and anti-inflammatory mechanisms without the confusion of human genetic variability.
A parasite product found in plasma during human and mouse infections that may be implicated in the proinflammatory cytokine effects on SMA is the glycophosphatidylinositol (GPI) anchor of the merozoite proteins, MSP-1, MSP-2, and MSP-4.92 GPIs are likely to contribute to malarial anemia since they can induce the release of TNF-α from human macrophages,93 which could contribute to the pathology of SMA. More specifically, it has recently been demonstrated that the proinflammatory response from human monocytes is through interaction of GPIs with TLR2, and to a lesser extent TLR4.94 The role of GPI-induced anemia in mice has not been studied directly. The injection of crude infected RBC lysate into mice does result in a transient decrease in the number of circulating RBCs,95 which is probably through the induction of host inflammatory responses,96 however this could be the effect of a range of other parasite products as well as GPI. Purified GPI immunization of mice in one study did result in reduced cerebral pathology and fatality, however effects on SMA were not reported.97 Further analysis of GPI-induced effects in mouse models of malaria to investigate a similar mechanism in humans would be of both academic and therapeutic interest because antibodies specific to GPIs are found in sera of adults from endemic regions in Kenya, but at reduced levels in children who, in general, have more severe disease and malarial anemia.98
A product discussed earlier, hemozoin, may also be more intimately linked to an innate immune response, and thus proinflammatory cytokine release, than previously thought. In humans, synthetic pigment induces expression of TNF-α, which has been linked to the ability of hemozoin to induce the metalloproteinase MMP-9,99,–101 and relatively recent studies in mice have suggested that hemozoin stimulates an innate proinflammatory response102 via a MyD88-dependent TLR9 pathway.103
Erythropoietin.
A fall in Hb and subsequent reduction in oxygen tension should stimulate elevated levels of erythropoietin (Epo) in patients with SMA. The clinical evidence for appropriately raised levels of Epo in malaria is somewhat contradictory. Studies in adults from Thailand and Sudan have suggested that the Epo concentration, although raised, was inappropriate for the degree of anemia.104,105 However, several studies of malaria in African children suffering from malarial anemia have shown appropriately raised Epo concentrations.25,106,–108 In fact, the Epo levels in malarial anemia are more than 3-fold higher when compared with anemic children without malaria.72 It is possible that ineffective or inadequate Epo synthesis does contribute to malarial anemia in some settings, possibly related to age, ethnic origin, or presentation of the patient. However, in African children with malaria, Epo synthesis is indeed elevated more than expected and it is more likely that a reduced response to Epo, not an inappropriately low level of Epo, is the more significant contribution to pathology.
Similarly, in mice, P chabaudi AS infection of resistant C57BL/6 and susceptible A/J strains results in anemia despite increases in serum Epo.109 However, the increase in Epo, albeit driving a somewhat blunted reticulocyte response, is a crucial determinant of the outcome of infection. Neutralization of Epo transforms P chabaudi AS infection in normally resistant C57BL/6 mice into a fatal infection. Intriguingly, induction of reticulocytosis by exogenous Epo prior to infection enhanced parasite multiplication and resulted in lethal infection. However, timely induction of reticulocytosis shortly after infection could alleviate malarial anemia and increase survival of A/J (susceptible) mice.55
Thus, in both human and mouse malaria infections, increased serum Epo appears essential for recovery, despite the possibility of an attenuated bone marrow response to this growth factor. Clearly, more extensive human studies are required to investigate the kinetics of erythrocyte production in response to elevated Epo during infection. However, a detailed phenotypic analysis of the abnormalities in erythropoiesis during the rodent P chabaudi infection may provide an insight to this mechanism, which has revealed that not only is Epo-induced proliferation of early erythroblasts suppressed, but also terminal differentiation and maturation of TER119+ erythroblasts is impaired.110 Perhaps surprisingly, these abnormalities of erythropoiesis were accompanied by neither an increase in apoptosis nor dysregulation of the cell cycle, suggesting that serum Epo is increased appropriately but that a reduced response to Epo is responsible for an insufficient erythrocyte output.
Hematinic deficiency.
Although dietary deficiencies are widespread in malaria-endemic regions, the influence of reduced folate and iron levels is not thought to be a major contributor to dyserythropoiesis seen during SMA.60 However dysregulation of iron metabolism may contribute to the severity of disease in children that present with SMA. The peptide hormone hepcidin has been implicated in mediating anemia of chronic disease or inflammation by reducing the availability of iron stores for erythropoiesis.111 Hepcidin is regulated by proinflammatory mediators such as IL-6, which is elevated in both murine infections and in patients who present with severe falciparum malaria.38,91 To date, there are no reports that describe the association of elevated hepcidin with SMA.
Extramedullary hematopoiesis.
One substantial difference in human and mouse responses to malarial anemia is that in mice the marked decrease in bone marrow cellularity appears to be compensated by increased erythropoietic activity in the spleen (Figure 3). However, to date there are no studies that have investigated extramedullary erythropoiesis during P falciparum infection. In mice, massive splenomegaly is observed with cellularity increasing 20-fold at peak parasitemia compared with that of naive animals.64,81 This rise is reflected in greater erythroid progenitor populations with up to an 8-fold increase of BFU-E and 100-fold increase of CFU-E total numbers in the spleen.65,66 Interestingly, there is a strong correlation between the severity of disease and the observed increases in splenic erythropoiesis. Lower rises in BFU-E and CFU-E populations are seen in fatal infections, when compared with nonfatal infections, which strongly suggests that a marked splenic erythropoietic response is required for survival in mice.54,112 It is unknown whether this is relevant to human studies because although splenomegaly is also observed in human patients with severe anemia, this has been primarily attributed to increased sequestration and erythrophagocytosis of infected and uninfected RBCs113,114 (Figure 3). However, extramedullary hematopoiesis commonly occurs in other conditions such as thalassemia in both humans and mice, and it would therefore be important to explore the erythropoietic role of the spleen in future human malaria studies, since if this is the case then the mouse model gains in relevance.
Finally, it should be noted that microarray analysis of peripheral responses to human and mouse malaria infection could prove extremely useful. One human study has allowed for some validation of clinical observations through the description of the gene expression profiles in children with severe malaria and during their convalescence.115 In this case, a positive correlation between neutrophil counts and increased expression of genes activated in the innate immune response as well as an inverse relationship between reticulocyte count and expression of genes associated with hemoglobin synthesis was observed. The advantage of a rodent model for SMA would be that a more complete study of bone marrow and splenic erythrocytic responses to malaria infection is possible, and several studies analyzing different organs from infected mice have described some promising results, such as a reduction in the level of gene transcripts involved in erythropoiesis and erythroid cell survival, during the early stages of infection.116,–118 These preliminary observations reveal that this technology is capable of extensive analyses in both humans and mice. Future studies should be considered because, as well as being able to identify the similarities and differences of the human and mouse peripheral response, microarrays may also facilitate the development of therapeutics.
Summary
In summary, it is possible to identify specific similarities and differences between disease pathology during P falciparum infection in humans, and infection with specific strains of Plasmodia in mice. The mouse is particularly useful for studying anemia associated with the acute phase of infection. One of the key similarities at this stage is that the erythropoietic response to Epo does not correct the deficit in hematocrit caused by hemolysis and sequestration of RBCs and that this is associated with abnormalities in the bone marrow. In acute disease the imbalance between proinflammatory and anti-inflammatory mediators may be the main cause of dyserythropoiesis. Mouse models that may represent this etiology of disease include those that use P chabaudi and P yoelii. However, the causative factors of SMA in chronic infection in humans are less clear and have not been extensively investigated in mice. In addition to the chronic P bergei model of rodent malaria mentioned previously, more chronic models of malaria infection with persistent but low parasitemias need to be developed. During the course of a chronic infection, there would be impaired clearance of parasite products (eg, RSP2, Hz, and GPI), and their accumulation may together or individually contribute to the chronic nature of SMA. Future studies of the longer term effects of parasite products on cytokine regulation and hematopoiesis in mice may be valuable in elucidating the mechanism involved in suppression of RBC production. Such studies coupled with in vitro investigations of the effects of parasite products on human hematopoietic cells will allow us to determine the stages of erythropoiesis involved and will increase our understanding of the etiology of SMA. However, it will be essential to link these animal and experimental data with studies in patients with malaria aimed at understanding not only the mechanisms leading to severe anemia but also their relative importance in different clinical settings. Already, from the studies summarized in this review on both mouse and human SMA, several interesting hypotheses await further clinical investigation and may lay the foundation for novel interventions to prevent or treat severe malarial anemia.
Acknowledgments
This work was supported by the National Blood Service, the Howard Hughes Medical Institute (A.A.L. and D.J.R.), the MRC (D.B., A.P., and J. L.), and the EU BioMalPar NoE (A.A.L., D.J.R., D.B., A.P., and J. L.).
We would like to thank Kevin Marsh, Paulo Arese, Suzanne Watt, and Bill Wood for helpful discussions and critical thoughts. The research was carried out in part at the NBS-Oxford Center and benefits from NHS R&D funding.
Authorship
Contribution: A.A.L. and D.B. contributed to design and content of paper, to review of literature, and to writing the draft; A.P. and C.C.-P. contributed to content of the review; J.L. and D.J.R. critically reviewed text and edited tables and figures.
A.A.L. and D.B. contributed equally to the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean Langhorne, National Institute for Medical Research, The Ridgeway, London, NW7 1AA; e-mail: jlangho@nimr.mrc.ac.uk; and David J. Roberts, Nuffield Department of Clinical Laboratory Sciences and National Blood Service Oxford Centre, Oxford OX3 9BQ; e-mail: david.roberts@ndcls.ox.ac.uk.