Hepcidin is a liver-made peptide proposed to be a central regulator of intestinal iron absorption and iron recycling by macrophages. In animal models, hepcidin is induced by inflammation and iron loading, but its regulation in humans has not been studied. We report that urinary excretion of hepcidin was greatly increased in patients with iron overload, infections, or inflammatory diseases. Hepcidin excretion correlated well with serum ferritin levels, which are regulated by similar pathologic stimuli. In vitro iron loading of primary human hepatocytes, however, unexpectedly down-regulated hepcidin mRNA, suggesting that in vivo regulation of hepcidin expression by iron stores involves complex indirect effects. Hepcidin mRNA was dramatically induced by interleukin-6 (IL-6) in vitro, but not by IL-1 or tumor necrosis factor α (TNF-α), demonstrating that human hepcidin is a type II acute-phase reactant. The linkage of hepcidin induction to inflammation in humans supports its proposed role as a key mediator of anemia of inflammation.
Introduction
The recently discovered peptide hepcidin1 may be the key mediator of anemia of inflammation.2,3 It is a conserved 25–amino acid peptide produced in the liver and detectable in blood and urine.1,4 Mice lacking hepcidin mRNA developed iron overload affecting the liver and pancreas, with iron deficit in the macrophage-rich spleen.5 Transgenic mice overexpressing hepcidin died at birth of severe iron deficiency.6 These studies suggested that hepcidin inhibits iron absorption in the small intestine, the release of recycled iron from macrophages,2and transport of iron across the placenta.6 In agreement with the animal studies, patients with large hepatic adenomas and otherwise unexplained iron-refractory anemia overexpressed hepcidin mRNA in their tumors.3 Studies of hepcidin mRNA regulation showed increase in iron-overloaded mice7 and decrease in mice with anemia from bleeding or hemolysis.8 Mice injected with lipopolysaccharide7 or turpentine,8 and fish with bacterial infection9 also had elevated hepcidin mRNA in the liver. Together, the data suggest hepcidin is induced by iron stores and inflammation, and functions as a signal inhibiting iron absorption in the small intestine and sequestering iron in macrophages.2However, all findings on hepcidin regulation have come from animal models. We explored the regulation of hepcidin synthesis in human patients and tissues.
Study design
Subjects
Approval was obtained from the UCLA institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. The patients' total iron-binding capacity (TIBC), and levels of serum iron, ferritin, hemoglobin, hematocrit, and urinary creatinine were determined at the UCLA Hospital Laboratory.
Reagents
Hepcidin antibody was prepared by rabbit immunization with synthetic hepcidin1 conjugated to keyhole limpet hemocyanin (KLH, no. 7762; Pierce, Rockford, IL).
Urinary hepcidin assay
Cationic peptides were extracted from urine using CM Macroprep (Bio-Rad, Hercules, CA).1 Urine extracts equivalent to 4 mg of creatinine were analyzed along with synthetic hepcidin standards (0.05, 0.15, 0.5, and 1.5 μg) by sodium dodecyl sulfate (SDS)–Tricine–polyacrylamide gel electrophoresis (PAGE) and Western blotting. Hepcidin was detected on the blots using rabbit antihuman hepcidin antibody.
Hepatocyte culture
Human hepatocytes (Liver Tissue Procurement and Distribution System, Minneapolis, MN) were cultured in human hepatocyte maintenance medium (Clonetics, San Diego, CA) at 37°C, 5% CO2. Hepatocyte treatments included 10 μM ferric-ammonium citrate (FAC; Sigma, St Louis, MO), 30 μM diferric transferrin (Sigma), 20 ng/mL interleukin-1α (IL-1α; R&D Systems, Minneapolis, MN), 20 ng/mL IL-6 (PeproTech, Rocky Hill, NJ), 20 ng/mL tumor necrosis factor α (TNF-α; R&D Systems), 100 ng/mL lipopolysaccharide (LPS;Escherichia coli 055:B5), medium conditioned by monocytes incubated with LPS (Mo-LPS; final concentration 12.5%), and 200 ng/mL IL-1 receptor antagonist (R&D Systems).
Monocyte culture
Blood monocytes were isolated by centrifugation at 400g for 20 minutes through Ficoll-Paque (Amersham Pharmacia Biotech, Piscataway, NJ) and at 550g for 30 minutes in 46% isoosmotic Percoll (Sigma) gradient. Monocyte purity was 80% by Wright stain. Monocytes were cultured at 1 × 106/mL in Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum and 20% autologous serum. The Mo-LPS conditioned medium (CM) was prepared by incubating monocytes with LPS for 4 days and collecting the cell-free supernatant.
RNA isolation and Northern blot analysis
Hepatocyte RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA (15 μg per lane) was separated on a 1% agarose formaldehyde gel, transferred, and ultraviolet (UV) light–crosslinked. Hepcidin probe (173–base pair [bp]) was generated by polymerase chain reaction (PCR).1 A 218-bp probe for ferritin heavy chain was generated by reverse transcriptase (RT)–PCR from hepatic RNA with the following primers: forward, 5′-CTGTCCATGTCTTACTACTTTGACC-3′ and reverse, 5′-TCCAAATGTAATGCACACTCC-3′. Hybridization was performed at 42°C in Ultrahybe (Ambion, Austin, TX) with random-primed,32P-labeled cDNA probes for hepcidin, ferritin, and glyceraldehyde-3-phosphate dehydrogenase (G3PD).
Results and discussion
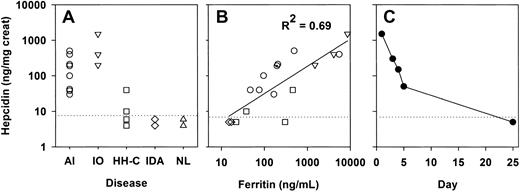
Patients with anemia of inflammation, diagnosed by elevated serum ferritin and compatible clinical history, had elevated urinary hepcidin excretion compared with healthy subjects and patients with iron deficiency anemia or well-controlled hereditary hemochromatosis (Figure 1A). Patients with transfusion-induced iron overload (2 sickle-cell anemia, 1 myelodysplasia) identified by compatible clinical history, Fe/TIBC higher than 50% and ferritin levels higher than 300 ng/mL, also showed greatly increased urinary hepcidin. Urinary hepcidin excretion correlated with serum ferritin levels (R2 = 0.69, Figure 1B). In a patient with epididymitis and sepsis (Figure 1C), Western blotting showed very high urinary hepcidin excretion on day 1 (1.5 mg/d), which gradually decreased over a period of days to undetectable levels (day 25) as the infection resolved with treatment. Because of its small size (approximately 2 kDa) and disulfide crosslinking,10hepcidin is expected to be filtered into the urine, where it apparently escapes tubular proteolysis and recycling. Unless circulating hepcidin is variably degraded by as yet unknown metabolic pathways, its urinary excretion should closely reflect production rates. This is the first direct evidence that infection and inflammation induce hepcidin production in humans.
Hepcidin excretion is increased in patients with anemia of inflammation, iron overload, or infection.
(A) Urinary hepcidin excretion in patients with anemia of inflammation (AI, ○), iron overload (IO, ▿), compensated hereditary hemochromatosis (HH-C, ■), and iron deficiency anemia (IDA, ⋄); urinary hepcidin excretion in healthy donors (NL, ▵). (B) Urinary hepcidin excretion in the same patients and its correlation with serum ferritin. (C) Urinary hepcidin excretion in response to acute infection in a single donor with epididymitis and sepsis, treated with antibiotics from day 1. Dotted line indicates detection limit. Creat indicates creatinine.
Hepcidin excretion is increased in patients with anemia of inflammation, iron overload, or infection.
(A) Urinary hepcidin excretion in patients with anemia of inflammation (AI, ○), iron overload (IO, ▿), compensated hereditary hemochromatosis (HH-C, ■), and iron deficiency anemia (IDA, ⋄); urinary hepcidin excretion in healthy donors (NL, ▵). (B) Urinary hepcidin excretion in the same patients and its correlation with serum ferritin. (C) Urinary hepcidin excretion in response to acute infection in a single donor with epididymitis and sepsis, treated with antibiotics from day 1. Dotted line indicates detection limit. Creat indicates creatinine.
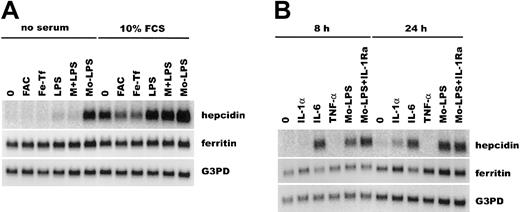
We next explored the molecular basis of hepcidin regulation in primary human hepatocytes. These were incubated either in serum-free medium, or with 10% fetal calf serum (FCS), with iron loading or inflammatory stimuli (Figure 2A). Surprisingly, iron loading of hepatocytes resulted in a 50% decrease in hepcidin mRNA. When higher concentrations of iron were used (up to 10 mM, not shown), hepcidin mRNA decreased even more. In contrast, ferritin H expression increased with higher doses of iron (not shown). Since hepcidin expression was induced in mice fed or injected with iron,7 our data raise the possibility that other iron-sensing cells signal to hepatocytes to induce the production of hepcidin during iron overload. An indirect link between iron and hepcidin induction in hepatocytes is also supported by studies in a mouse model of anemia where hepcidin mRNA was down-regulated in the liver, despite normal or even increased hepatic iron.8
Hepcidin mRNA is induced by IL-6.
Northern blots of human hepatocyte RNA were probed for hepcidin, ferritin H, and glyceraldehyde-3-phosphate-dehydrogenase (G3PD) mRNAs. (A) Hepatocytes were treated for 24 hours in the presence or absence of 10% FCS, with serum-free medium (0), ferric ammonium citrate (FAC), iron-saturated ferritin (Fe-Tf), lipopolysaccharide (LPS), 12.5% monocyte medium + LPS (M+LPS), and 12.5% monocyte medium conditioned by monocytes exposed to LPS (Mo-LPS). (B) Hepatocytes were treated for the indicated time with cytokines or Mo-LPS (12.5% monocyte medium conditioned by monocytes exposed to LPS), without or with IL-1 receptor antagonist (IL-1Ra).
Hepcidin mRNA is induced by IL-6.
Northern blots of human hepatocyte RNA were probed for hepcidin, ferritin H, and glyceraldehyde-3-phosphate-dehydrogenase (G3PD) mRNAs. (A) Hepatocytes were treated for 24 hours in the presence or absence of 10% FCS, with serum-free medium (0), ferric ammonium citrate (FAC), iron-saturated ferritin (Fe-Tf), lipopolysaccharide (LPS), 12.5% monocyte medium + LPS (M+LPS), and 12.5% monocyte medium conditioned by monocytes exposed to LPS (Mo-LPS). (B) Hepatocytes were treated for the indicated time with cytokines or Mo-LPS (12.5% monocyte medium conditioned by monocytes exposed to LPS), without or with IL-1 receptor antagonist (IL-1Ra).
Treatment of hepatocytes with Mo-LPS CM increased hepcidin mRNA up to 25-fold, but LPS alone caused only a small (2- to 3-fold) increase (Figure 2B). Monocytes/macrophages exposed to LPS secrete cytokines that mediate acute-phase response. Of the 2 major patterns of acute-phase response in hepatocytes,11 12 type I response is induced by IL-1–like cytokines (IL-1α, IL-1β, TNF-α, and TNF-β) and increases the production of serum amyloid A, C-reactive protein, and complement C3; whereas type II response is induced by IL-6–like cytokines and results in increased synthesis of fibrinogen, haptoglobin, and α1-antitrypsin. Hepcidin was induced within 8 hours by IL-6 (25-fold) and Mo-LPS CM, but not by IL-1α or TNF-α, indicating that induction of hepcidin is a type II acute-phase response. After 24 hours of cytokine treatment, hepcidin mRNA was also induced by IL-1α, but this was probably an indirect effect of IL-1 via induction of IL-6 synthesis in hepatocytes. Also, addition of IL-1 receptor antagonist to LPS-conditioned monocyte medium did not reduce hepcidin expression, supporting the finding that IL-1 is not an important direct mediator of hepcidin induction.
Ferritin H mRNA showed a weak type I pattern of induction in hepatocytes: IL-1α and TNF-α, but not IL-6, increased ferritin mRNA, and addition of IL-1Ra to Mo-LPS CM decreased ferritin mRNA. Although measurements of urinary hepcidin and serum ferritin correlate in patients with different iron disorders, and although both are shown to be regulated by iron and inflammation,7,8 13 the respective molecular pathways regulating their expression appear to be distinct.
Hepcidin is thus a type II acute-phase protein that provides a molecular link between inflammation, resulting anemia, and the regulation of iron metabolism. Additional studies will be necessary to explain the mechanism of hepcidin effect on iron transport and the more complex indirect effects that appear to mediate the regulation of hepcidin production by iron stores.
We thank Audrea Troutman for her contribution to this project, Lide Liu for advice and reagents, and Stephen Strom for providing human hepatocytes and advice.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood-2002-10-3235.
Supported by the Will Rogers Fund (T.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomas Ganz, 37-055 CHS, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA 90095-1690; e-mail:tganz@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal