Introduction
The disease we now call diffuse large B-cell lymphoma has gone by many names in the past. These have included reticulum cell sarcoma,1 diffuse histiocytic lymphoma,2 and, more recently, diffuse mixed lymphoma, diffuse large cell lymphoma, or immunoblastic lymphoma—terms from the Working Formulation.3 Early studies of therapy for patients with diffuse large B-cell lymphoma contained some patients with aggressive T-cell lymphoma, as these were lumped together in the Working Formulation and some older classifications. The correct diagnosis today is diffuse large B-cell lymphoma, as used in the World Health Organization (WHO) classification (Table 1).4 However, we know that this is still a heterogenous group that includes lymphomas with a wide variety of morphologic appearances (Table 2), protein-expression patterns, and gene-expression patterns. For example, patients with diffuse large B-cell lymphoma can be divided into at least 3 clinically relevant groups using gene-expression profiling.5,–7 These include the germinal-center type, the activated B-cell type, and mediastinal large B-cell lymphoma (Table 3). A few patients will not easily be classified into these categories.8 Mediastinal large B-cell lymphoma represents less than 10% of all large B-cell lymphomas, occurs primarily in young women, and always presents with a mediastinal mass. The gene-expression profile is similar to that seen in classical Hodgkin disease.7,9 The other 2 types of diffuse large B-cell lymphoma, and those not easily classified, have a median age at presentation in the 60s, a male predominance, and can present at essentially any site in the body.8 They will be the major focus of this paper.
WHO histologic classification of lymphoid neoplasms
| Precursor B-cell and T-cell neoplasms |
| Precursor B-lymphoblastic leukemia/lymphoblastic lymphoma (precursor B-cell acute lymphoblastic leukemia) |
| Precursor T-lymphoblastic leukemia/lymphoblastic lymphoma (precursor T-cell acute lymphoblastic leukemia) |
| Mature B-cell neoplasms |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| B-cell prolymphocytic leukemia |
| Lymphoplasmacytic lymphoma |
| Splenic marginal zone lymphoma |
| Hairy-cell leukemia |
| Plasma-cell myleoma |
| MGUS |
| Solitary plasmactyoma of bone |
| Extraosseous plasmacytoma |
| Primary amyloidosis |
| Heavy-chain diseases |
| MALT lymphoma |
| Nodal marginal zone B-cell lymphoma |
| Follicular lymphoma |
| Mantle-cell lymphoma |
| Diffuse large B-cell lymphoma |
| Mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| Primary effusion lymphoma |
| Burkitt lymphoma/leukemia |
| Mature T-cell and NK-cell neoplasms |
| Leukemic/disseminated |
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Aggressive NK-cell leukemia |
| Adult T-cell leukemia/lymphoma |
| Cutaneous |
| Mycosis fungoides |
| Sezary syndrome |
| Primary cutaneous anaplastic large-cell lymphoma |
| Lymphomatoidpapulosis |
| Other extranodal |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-type T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis–like T-cell lymphoma |
| Nodal |
| Angioimmunoblastic T-cell lymphoma |
| Peripheral T-cell lymphoma, unspecified |
| Anaplastic large-cell lymphoma |
| Neoplasm of uncertain lineage and stage of differentiation |
| Blastic NK-cell lymphoma |
| Precursor B-cell and T-cell neoplasms |
| Precursor B-lymphoblastic leukemia/lymphoblastic lymphoma (precursor B-cell acute lymphoblastic leukemia) |
| Precursor T-lymphoblastic leukemia/lymphoblastic lymphoma (precursor T-cell acute lymphoblastic leukemia) |
| Mature B-cell neoplasms |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| B-cell prolymphocytic leukemia |
| Lymphoplasmacytic lymphoma |
| Splenic marginal zone lymphoma |
| Hairy-cell leukemia |
| Plasma-cell myleoma |
| MGUS |
| Solitary plasmactyoma of bone |
| Extraosseous plasmacytoma |
| Primary amyloidosis |
| Heavy-chain diseases |
| MALT lymphoma |
| Nodal marginal zone B-cell lymphoma |
| Follicular lymphoma |
| Mantle-cell lymphoma |
| Diffuse large B-cell lymphoma |
| Mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| Primary effusion lymphoma |
| Burkitt lymphoma/leukemia |
| Mature T-cell and NK-cell neoplasms |
| Leukemic/disseminated |
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Aggressive NK-cell leukemia |
| Adult T-cell leukemia/lymphoma |
| Cutaneous |
| Mycosis fungoides |
| Sezary syndrome |
| Primary cutaneous anaplastic large-cell lymphoma |
| Lymphomatoidpapulosis |
| Other extranodal |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-type T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis–like T-cell lymphoma |
| Nodal |
| Angioimmunoblastic T-cell lymphoma |
| Peripheral T-cell lymphoma, unspecified |
| Anaplastic large-cell lymphoma |
| Neoplasm of uncertain lineage and stage of differentiation |
| Blastic NK-cell lymphoma |
MGUS indicates monoclonal gammopathy of undetermined significance; MALT lymphoma, extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue; and NK, natural killer.
Adapted with permission from Jaffe et al.4
Morphologic subtypes of diffuse lymphocytic B-cell lymphoma
| Centroblastic |
| Immunoblastic |
| Anaplastic |
| Plasmablastic |
| Intravacular |
| Multi-lobulated |
| T-cell rich |
| Lymphomatoid granulomatosis type |
| Primary effusion |
| Centroblastic |
| Immunoblastic |
| Anaplastic |
| Plasmablastic |
| Intravacular |
| Multi-lobulated |
| T-cell rich |
| Lymphomatoid granulomatosis type |
| Primary effusion |
Clinically relevant molecular subtypes of diffuse large B-cell lymphoma
| . | Germinal-center B-cell . | Activated B-cell . | Mediastinal . |
|---|---|---|---|
| Median age, y | 58 | 66 | 35 |
| Age older than 60 y, % | 52 | 66 | 9 |
| Female, % | 50 | 40 | 70 |
| Female younger than 35 y, % | 3 | 2 | 35 |
| 5-year survival, #% | 59 | 30 | 64 |
| . | Germinal-center B-cell . | Activated B-cell . | Mediastinal . |
|---|---|---|---|
| Median age, y | 58 | 66 | 35 |
| Age older than 60 y, % | 52 | 66 | 9 |
| Female, % | 50 | 40 | 70 |
| Female younger than 35 y, % | 3 | 2 | 35 |
| 5-year survival, #% | 59 | 30 | 64 |
Lymphomas are the fifth most common systemic cancer, with the most common subtype being diffuse large B-cell lymphoma followed by follicular lymphoma and Hodgkin lymphoma. Diffuse large B-cell lymphoma represents approximately 30% of all lymphomas and is the most common subtype throughout the world. This is in contrast to many other types of lymphoma, which have striking geographic variation in frequency of occurrence.10
Diffuse large B-cell lymphoma can be seen after histologic transformation of most other types of B-cell lymphoma. This is particularly frequent in patients with follicular lymphoma and is recognized clinically in up to 50% of patients.11,12 Although much less frequent, this transformation occurring in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma has the eponym Richter transformation.13,14 In general, patients with diffuse large B-cell lymphoma seen after histologic transformation have a poorer response to therapy and prognosis than those with a de novo appearance, particularly if the patient were treated for the preceding lymphoma. This manuscript will focus on the treatment of patients with primary diffuse large B-cell lymphoma.
Diagnosis
The first step in treating any cancer is an accurate histologic diagnosis. For non-Hodgkin lymphomas in general, and diffuse large B-cell lymphoma in particular, the initial diagnosis should be based on an adequate sample of tissue preferably obtained with an excisional biopsy of an abnormal lymph node or a generous incisional biopsy of an involved organ. In some cases, cutting-needle biopsies can provide adequate tissue for diagnosis; however, the diagnosis of lymphoma based on fine-needle aspirates should be discouraged. One of the most frustrating situations in the care of a patient with lymphoma arises when the diagnosis was based on inadequate material, the patient does not respond to therapy as expected, and to obtain another biopsy has become difficult or impossible.
The diagnosis of lymphoma and its subtype is best made by a hematopathologist with experience in diagnosing lymphomas. Expert hematopathologists using the WHO classification can make highly reproducible diagnoses for most subtypes of lymphoma.15 However, this depends upon adequate tissue and the availability of immunohistochemistry.15 On occasion, cytogenetics or fluorescent in situ hybridization (FISH) may help clarify a difficult diagnosis.
At the present time, gene-expression profiling is not part of routine clinical practice. This may be partly because of the technical difficulties in performing the arrays. The assignment of germinal-center–type versus non–germinal-center–type diffuse large B-cell lymphoma seems to be able to be reproduced by studying the expression of 3 proteins using immunohistochemistry.16 Although the germinal-center B-cell type and the activated B-cell type of diffuse large B-cell lymphoma do not have the same prognosis with anthracycline-containing chemotherapy regimens,8 they are still treated in a similar way in the absence of studies showing superiority of specific regimens for each subtype. The poorer prognosis of patients with primary brain diffuse large B-cell lymphoma and the better outcome in pediatric patients might be partly explained by the predominance of activated B-cell type in the former17,18 and germinal-center B-cell type in the latter.19,20 The one time that gene-expression profiling may be important in current clinical practice is in the distinction between diffuse large B-cell lymphoma and Burkitt lymphoma. A recent study showed that gene-expression profiling might be able to make this distinction more accurately than other studies.21 This is important because patients with Burkitt lymphoma should be treated with different regimens from those used for diffuse large B-cell lymphoma, and their survival is dramatically better when they receive appropriate regimens.
In my practice I am extremely reluctant to treat a patient for diffuse large B-cell lymphoma in the absence of an adequate biopsy reviewed by experienced hematopathologists. I prefer rebiopsy to guessing about the correct diagnosis.
Staging/restaging
As would be true for any type of cancer, after the diagnosis of diffuse large B-cell lymphoma a patient must be evaluated to determine sites of involvement by the lymphoma and the presence or absence of key prognostic factors to complete a staging evaluation.22 As currently used in practice today, the process of staging accomplishes several important tasks. These include allowing the choice of the most appropriate therapy, providing the most accurate possible prognosis for the patient and their family, and making clinical research and quality assessment possible by allowing patients to be stratified into prognostic groups. In potentially curable diseases such as diffuse large B-cell lymphoma, this initial evaluation will be the basis for “restaging” that will be done after some or all of the patient's treatment. This restaging will document the presence or absence of a complete response to treatment. Obviously, cure of the disease requires a complete response to therapy, but not all complete responders will be cured, as our current tests cannot always find minimal residual lymphoma. Conversely, some patients with apparent incomplete responses due to residual masses on computed tomograms might be cured if the residual masses contain no active lymphoma. This problem is at least partially addressed by the use of functional imaging such as positron emission tomography (PET) scans.
The initial evaluation of a patient with diffuse large B-cell lymphoma should include a careful history and physical examination and laboratory studies including hematologic parameters, screening chemistry studies, and, specifically, a serum lactate dehydrogenase level. Imaging studies should include at least computed tomograms of the chest, abdomen, and pelvis and a PET scan if available. An adequate bone marrow biopsy should be performed. Other laboratory studies, images, and biopsies might be appropriate for specific patients. For example, I perform lumbar puncture to rule out meningeal involvement in patients presenting with testicular, epidural, or sinus involvement.
PET scanning is an increasingly important tool in the care of patients with diffuse large B-cell lymphoma. However, the best use of this technology is still in flux and basic issues such as what represents a negative PET scan after treatment does not have general agreement. Whether scans need to be done before treatment in a disease with a high likelihood of positivity such as diffuse large B-cell lymphoma has been debated.23 The appropriate timing for follow-up scans has also been a point for controversy. A recent consensus report on the use of PET scanning in lymphomas is a step toward trying to resolve these areas of uncertainty.24
Patients with diffuse large B-cell lymphoma are stratified into prognostic groups based on the International Prognostic Index (IPI).25 This system uses anatomic stage, performance status, the number of extranodal sites, serum lactate dehydrogenase level, and age to predict treatment outcome (Table 4). The IPI remains our most useful prognostic tool and should be applied to all patients with diffuse large B-cell lymphoma (Table 5). However, the improvement in treatment response associated with the addition of the antibody rituximab to treatment regimens seems to have altered the survival of prognostic groups using the IPI (Table 6).26
International Prognostic Index
| Full index |
| Prognostic factors |
| Age older than 60 y |
| Performance status of 2 or higher |
| LDH level greater than 1× normal |
| Extranodal sites of 2 or more |
| Stage III or IV |
| Risk category and factors |
| Low, factor 0 or 1 |
| Low-intermediate, factor 2 |
| High-intermediate, factor 3 |
| High, factor 4 or 5 |
| Age adjusted |
| Prognostic factors |
| Performance status higher than 1 |
| LDH level greater than 1× normal |
| Stage III or IV |
| Risk category and factors |
| Low, factor 0 |
| Low-intermediate, factor 1 |
| High-intermediate, factor 2 |
| High, factor 3 |
| Full index |
| Prognostic factors |
| Age older than 60 y |
| Performance status of 2 or higher |
| LDH level greater than 1× normal |
| Extranodal sites of 2 or more |
| Stage III or IV |
| Risk category and factors |
| Low, factor 0 or 1 |
| Low-intermediate, factor 2 |
| High-intermediate, factor 3 |
| High, factor 4 or 5 |
| Age adjusted |
| Prognostic factors |
| Performance status higher than 1 |
| LDH level greater than 1× normal |
| Stage III or IV |
| Risk category and factors |
| Low, factor 0 |
| Low-intermediate, factor 1 |
| High-intermediate, factor 2 |
| High, factor 3 |
LDH indicates lactate dehydrogenase.
Adapted with permission.25
Outcome for patients with diffuse aggressive lymphoma after anthracycline-containing chemotherapy International Index
| Risk category . | No. of risk factors . | % cases . | CR rate, % . | 5-y RFS of CRs, % . | 5-y survival, % . |
|---|---|---|---|---|---|
| Low | 0, 1 | 35 | 87 | 70 | 72 |
| Low-intermediate | 2 | 27 | 67 | 50 | 50 |
| High-intermediate | 2 | 22 | 55 | 48 | 43 |
| High | 4, 5 | 16 | 44 | 40 | 26 |
| Risk category . | No. of risk factors . | % cases . | CR rate, % . | 5-y RFS of CRs, % . | 5-y survival, % . |
|---|---|---|---|---|---|
| Low | 0, 1 | 35 | 87 | 70 | 72 |
| Low-intermediate | 2 | 27 | 67 | 50 | 50 |
| High-intermediate | 2 | 22 | 55 | 48 | 43 |
| High | 4, 5 | 16 | 44 | 40 | 26 |
CR indicates complete remission.
Adapted with permission.25
Results with a revised IPI when CHOP-R is given for diffuse large B-cell lymphoma
| Group and factors . | % patients . | % 4-y overall survival . |
|---|---|---|
| Standard IPI | ||
| 0, 1 | 28 | 86 |
| 2 | 27 | 81 |
| 3 | 21 | 54 |
| 4,5 | 24 | 58 |
| Revised IPI | ||
| 0 | 10 | 92 |
| 1.2 | 45 | 82 |
| 3.4.5 | 45 | 58 |
| Group and factors . | % patients . | % 4-y overall survival . |
|---|---|---|
| Standard IPI | ||
| 0, 1 | 28 | 86 |
| 2 | 27 | 81 |
| 3 | 21 | 54 |
| 4,5 | 24 | 58 |
| Revised IPI | ||
| 0 | 10 | 92 |
| 1.2 | 45 | 82 |
| 3.4.5 | 45 | 58 |
Adapted with permission from Sehn et al.26
Restaging is a process of repeating all previously abnormal tests to see if the patient has achieved a remission. Although this is often done at the completion of a planned course of therapy, I perform restaging after 4 cycles of treatment for diffuse large B-cell lymphoma, with the intention of treating patients 2 cycles past documented complete remission.27 Thus, if the patient is in remission at 4 cycles, they receive a total of 6. If they do not achieve remission until after 6 cycles, they would receive a total of 8 cycles. If they have not achieved a remission by 6 cycles, then I would switch to alternate therapies.
Therapy
The discussion of management of patients with diffuse large B-cell lymphoma can be conveniently divided into 3 groups: those presenting with localized disease, those presenting with disseminated disease, and those patients whose lymphoma recurs after an initial remission. In each group, patients who are elderly might not be managed in exactly the same way as young patients. Also, patients who have the disease involving specific organs might require special treatment approaches.
Localized disease
Patients with stage I diffuse large B-cell lymphoma (ie, involvement of only one lymph-node region or isolated organ involvement) fit into this category of localized disease; however, selected patients with stage II (ie, 2 adjacent lymph-node regions involved or organ involvement with involvement of regional lymph nodes) who could have their disease encompassed in one radiotherapy port might be approached in a similar manner. These patients were once treated with radiotherapy alone and a few were cured.28 The addition of adjuvant chemotherapy following the radiation improved treatment outcome,29 but an abbreviated course of chemotherapy followed by radiation became the most popular treatment.30 A study done by the Southwest Oncology Group in the United States showed superiority of an abbreviated course of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) followed by radiation over a complete course of CHOP alone.31 This became and has remained the standard treatment in the United States. A more recent Eastern Cooperative Oncology Group study suggested benefit of adjuvant radiation after 8 cycles of CHOP.32 However, selected patients in whom radiation might be unusually problematic (eg, young women in whom the treatment field would involve the breast or patients of any age in whom salivary gland treatment might lead to a dry mouth and loss of teeth) are often treated with a complete course of chemotherapy alone.
Studies from Europe have challenged this treatment approach. A Groupe d'Etude des Lymphomes de l'Adulte (GELA) study in elderly patients compared 4 cycles of CHOP alone with 4 cycles of CHOP followed by radiotherapy.33 There was no advantage to the radiation and, in fact, a possible disadvantage in patients older than 70 years of age. In younger patients, the same group compared an intensive chemotherapy regimen (ACVBP [doxorubicin, cyclophosphamide, vindesine, bleomycin and prednisone]) to 3 cycles of CHOP followed by involved field radiotherapy.34 There was a significant advantage to the ACVBP arm. The Mabthera International (MINT) trial included some patients with minimal disease and compared a CHOP-like chemotherapy regimen to the same regimen with the addition of rituximab in young good-prognosis patients.35 For the most favorable patients (ie, those without bulky disease), the results with a complete course of chemotherapy plus rituximab alone and no radiation led to a survival in excess of 90%. The Southwest Oncology Group in the United States reported a pilot study of an abbreviated course of CHOP plus the antibody rituximab followed by radiation and also showed progression-free and overall survival in excess of 90%.36
My personal approach to patients with localized diffuse large B-cell lymphoma involves the use of CHOP plus rituximab for 4 cycles. If the patient is in remission at that point, either involved field radiotherapy or 2 more cycles of the chemotherapy regimen would be administered based on the patient's preferences and the site of the disease. I would generally not recommend radiation to young women in whom the breast would have to be irradiated and would offer drugs alone to patients in whom radiation to the salivary glands might lead to loss of their teeth. For the patient with very bulky (ie, > 10-cm mass) but localized lymphoma, I would favor CHOP plus rituximab for 6 cycles followed by involved field irradiation.
Patients with localized disease involving certain organs need modifications of the general plan. Patients with testicular lymphoma have a predilection for the disease to spread to the opposite testis and to the central nervous system.37 Central nervous system involvement can be meningeal or parenchymal. In addition, patients with testicular involvement, who are usually elderly men, have a higher than anticipated risk of late relapse. They would usually be treated with a complete course of chemotherapy such as CHOP plus rituximab accompanied by intrathecal treatment with methotrexate and/or cytarabine. After treatment, these patients should have scrotal irradiation.37,,–40 Primary brain lymphoma is an increasing problem.41 While often accompanied by HIV infection, an increasing number of patients without HIV are developing the disease. Current treatment regimens are built around high-dose methotrexate.41 The use of whole-brain radiotherapy as part of the initial treatment is controversial and frequently associated with the development of dementia, particularly in elderly patients. Patients with epidural or sinus presentations and those with circulating tumor cells seem especially likely to develop meningeal metastasis. These patients should be treated with intrathecal methotrexate and/or cytarabine along with their initial chemotherapy regimen.
Disseminated disease
The potential for cure using chemotherapy alone in patients with disseminated diffuse aggressive lymphoma was first reported in the early 1970s by Levitt et al42 and DeVita et al.43 In both studies, some patients with documented complete remissions achieved long-term disease-free survival. Shortly after these reports, the CHOP regimen became popular in the United States and became the standard treatment regimen for patients with diffuse aggressive lymphoma.44 However, in the subsequent decade, the development of new treatment regimens including M-BACOD (methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone),45 MACOP-B (methotrexate with leucovorin rescue doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin),46 and ProMACE/CytaBOM (cyclophosphamide, doxorubicin, etoposide cytozar, bleomycin, vincristine, methotrexate prednisone)47 were reported to achieve results that seemed much better than had been observed with CHOP. These so-called third-generation regimens appeared to represent an important advance in therapy until an intergroup trial carried out in the United States demonstrated no superiority over CHOP.48 This surprising result was almost certainly related to treating a better group of patients (ie, probably younger, lower stage, with a lower IPI score) in the studies of the new very intensive regimens and assuming that the results would apply to all patients with the disease. This example illustrates the importance of the randomized trial in documenting moderate improvements in therapy in this and any other disease.
After the disappointing results with the third-generation chemotherapy regimens in the United States, there was a lull in developing new regimens and CHOP was the standard therapy. However, research continued, particularly in countries other than the United States, and a number of new treatment approaches have been developed. Currently, the search for the optimal chemotherapy regimen for treating diffuse large B-cell lymphoma continues.
The GELA developed the ACVBP regimen (ie, that involves very intensive chemotherapy for 4 courses followed by an intensive consolidation), which was shown to be superior to CHOP in subgroups of patients.49 In Germany, national trials found that the addition of etoposide to CHOP improved results in young patients,50 whereas CHOP administered at 14- rather than 21-day intervals seemed to improve the results in elderly patients.51 An infusional chemotherapy regimen developed at the United States National Cancer Institute referred to as EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) had very encouraging results.52 This regimen plus rituximab is currently in a randomized trial in the United States.
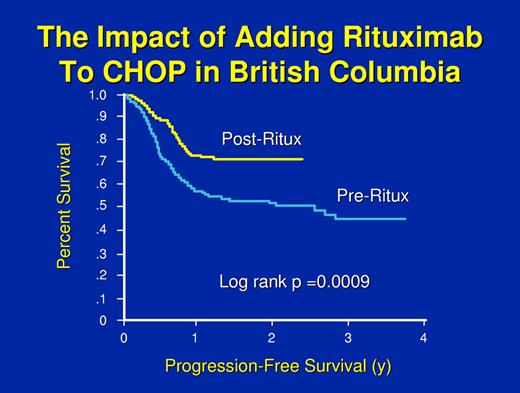
However, the study that changed practice throughout the world was performed by the GELA and compared CHOP versus the same regimen plus the antibody rituximab in elderly patients.53,54 A highly significant advantage in response rate, failure-free survival, and overall survival was seen with the addition of the antibody. In the United States, a trial comparing CHOP with or without rituximab administered in a different schedule and with or without maintenance rituximab generally confirmed the GELA results, with a significant advantage for receiving rituximab either during induction or maintenance but no advantage to getting both.55 An international study called the MINT trial compared chemotherapy with CHOP or a “CHOP-like” regimen with or without rituximab in younger good-prognosis patients (ie, both the GELA and the America Intergroup trials were done in patients older than 60 years of age).35 The MINT trial demonstrated a significant advantage in response, failure-free survival, and overall survival with the addition of the antibody. The German High-Grade Lymphoma Study Group studied the utility of 6 versus 8 cycles of CHOP at 14-day intervals with or without rituximab in elderly patients with diffuse large B-cell lymphoma. Again, this demonstrated the importance of rituximab but also hinted that 8 cycles of treatment might be deleterious in very elderly patients.56 Finally, investigators from the Cancer Institute in British Columbia had the opportunity to do a population-based study of the impact of adding rituximab to CHOP.57 Because cancer drugs are paid for by the government in British Columbia and the addition of rituximab to CHOP was approved at a particular point in time, they used that point in time as the variable to see if approval of the drug improved treatment outcome. This was despite the fact that a few patients received the drug before the date of approval and not all patients received the drug after the date of approval. However, with only approval of the drug and its general availability as a variable, survival for diffuse large B-cell lymphoma in British Columbia increased by about 20% (Figure 1).57
The progression-free survival of patients treated in British Columbia before or after the approval of rituximab for general use. A few patients received rituximab before the date of approval and some patients did not receive the drug after the date of approval; however, all patients are included. Adapted with permission from Sehn et al.57
The progression-free survival of patients treated in British Columbia before or after the approval of rituximab for general use. A few patients received rituximab before the date of approval and some patients did not receive the drug after the date of approval; however, all patients are included. Adapted with permission from Sehn et al.57
The addition of rituximab to CHOP or other chemotherapy regimens has been a major improvement in our ability to treat patients with diffuse large B-cell lymphoma. An important question has been whether or not all patients need the rituximab. Studies from France and the American National Cancer Institute suggested that the improvement with rituximab was largely seen in patients with tumors overexpressing the Bcl-2 protein,58,59 although not all groups found the same results.60 This might relate to the Bcl-2 protein expression having prognostic significance in the activated B-cell type but not the germinal-center B-cell type of diffuse large B-cell lymphoma.61 A recent report from the French group using the method of competing risks suggested that benefit was seen in both Bcl-2–positive and Bcl-2–negative lymphomas but that the benefit was more striking in those patients whose tumors were Bcl-2 positive.62
Autologous hematopoietic stem cell transplantation has been shown to be an effective therapy for patients with diffuse large B-cell lymphoma who relapsed from complete remission and whose lymphoma still responded to standard-dose salvage chemotherapy.63 In a randomized trial comparing DHAP (dexamethasone, cytarabine, and cisplatin) plus radiotherapy to autologous hematopoietic stem cell transplantation, patients who underwent transplantation had a superior disease-free and overall survival.64 Benefit from transplantation was seen in patients with an IPI score of 1 or higher.65
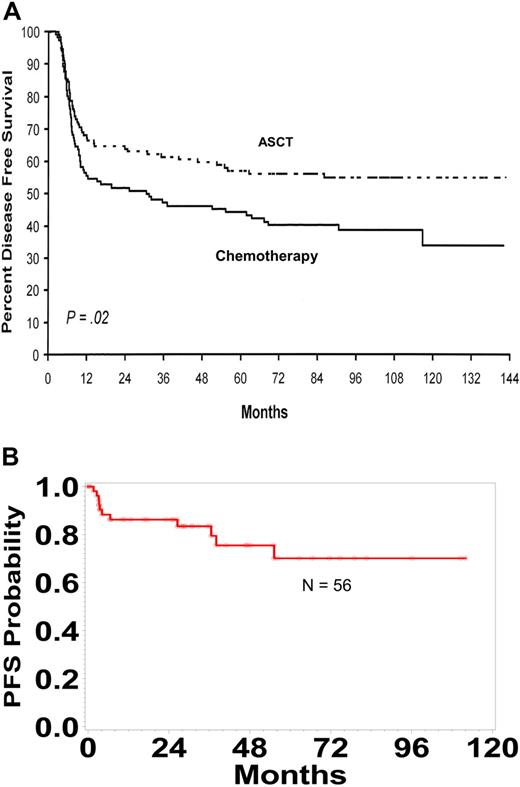
Because of these encouraging data, a large number of studies have tested the value of incorporating autologous hematopoietic stem cell transplantation into the primary therapy of patients with diffuse large B-cell lymphoma.66,,,,,,,,,–76 These studies were not all comparable with some studies testing transplantation early in the course of therapy with an abbreviated standard treatment regimen: some have used novel treatment regimens, some have studied complete and partial responders, some but not all have incorporated only high-risk patients, and some have tested transplantation as an adjuvant treatment following complete remission. Although the interpretation of these data remains a point for controversy, the conclusion that I draw is that transplantation is only likely to benefit high-risk patients who achieve a complete remission after a complete course of a standard chemotherapy regimen. The results from a randomized French trial that restricted analysis to patients with an age-adjusted IPI score of 2 or 3 are presented in Figure 2A.70 The progression-free survival curve for 53 high-risk patients who received a transplant in first complete remission following a complete course of anthracycline-based chemotherapy treated at the University of Nebraska Medical Center is presented in Figure 2B. One important issue to consider is that the definition of high risk may change as new treatment approaches are developed. For example, a recent report from the University of British Columbia in Vancouver suggested that the results by IPI score have “shifted” with the addition of rituximab (Table 6).26
Autologous Transplantation in High Risk Patients with DLBCL. (A) The disease-free survival of patients who had 2 or 3 adverse risk factors in the age-adjusted IPI who responded to ACVBP and were randomly allocated to autologous transplant or consolidation with further chemotherapy. Adapted with permission from J Clin Oncol. Copyright 2000.70 (B) Progression-free survival (PFS) for 56 high-risk patients in first remission after an anthracycline-containing chemotherapy regimen undergoing autotransplantation at the University of Nebraska Medical Center.
Autologous Transplantation in High Risk Patients with DLBCL. (A) The disease-free survival of patients who had 2 or 3 adverse risk factors in the age-adjusted IPI who responded to ACVBP and were randomly allocated to autologous transplant or consolidation with further chemotherapy. Adapted with permission from J Clin Oncol. Copyright 2000.70 (B) Progression-free survival (PFS) for 56 high-risk patients in first remission after an anthracycline-containing chemotherapy regimen undergoing autotransplantation at the University of Nebraska Medical Center.
My treatment approach for patients with disseminated diffuse large B-cell lymphoma who are not participating in a clinical trial is to initiate CHOP plus rituximab after starting the patient on allopurinol, as tumor lysis syndrome can be seen in this disease. Patients received concomitant intrathecal methotrexate if the testis, sinus, or epidural area were involved at presentation. After 4 cycles of treatment, I reevaluate the patient with history and physical examination, laboratory studies, and images including PET scan. If the patient has achieved a complete remission, I give 2 more cycles of therapy and discontinue treatment. If the patient presented with a very bulky (ie, > 10 cm) mass at any site, I would consider adjuvant radiotherapy to that site if it could be administered safely. If the patient were 60 years of age or younger and had a high serum lactate dehydrogenase level, poor performance status, multiple extranodal sites of disease, and Ann Arbor stage III or IV (ie, or at least 2 of these findings), I would discuss adjuvant autologous hematopoietic stem cell transplantation in complete remission as an option. I do not believe there is any evidence to support maintenance therapy in patients with this disease.
Follow-up
After a patient has completed planned therapy and is in complete remission, there is still a significant chance for recurrence. Most patients who are going to relapse will do so in the first 2 or 3 years, but we have seen patients relapse more than 13 years after completing therapy. Follow-up is aimed at identifying relapse but also at managing the complications that might develop related to the treatment and at helping patients deal with the diagnosis and their concerns about possible relapse. I see patients at 2-month intervals for the first year, 3-month intervals for the second year, 4-month intervals for the third year, twice a year for the fourth and fifth years, and then annually indefinitely. While this follow-up pattern is arbitrary, I believe that seeing the patient more often early after treatment is useful to the patient.
Follow-up visits include interval history, careful physical examination, and laboratory studies including a complete blood count, chemistry screen, and serum lactate dehydrogenase level. Once a complete remission is documented, I would do no more images in the absence of some abnormality hinting at relapse or at the patient's request. I know it is standard care in much of the United States to do routine images in complete remission, but this approach cannot be supported with data. There is no convincing evidence that routine images in remission accomplish their goal of improving survival by finding early relapse, although this could be tested in a prospective trial. While there is at best minimal evidence that routine images in remission could improve survival,77 it is certain that they are expensive. Whether these studies make a patient less anxious because a negative test is reassuring, or make them more anxious by reminding them that they should be afraid of relapsing, is a point that could be argued. However, given the specificity and sensitivity of the tests and the chances of relapse at any particular point in time, it can be shown that abnormal findings on routine images are much more likely (ie, > 80% of the time) to represent false positives and lead to inappropriate further evaluation or, even worse, instituting inappropriate therapy.78
A major mistake to avoid in following patients with diffuse large B-cell lymphoma in complete remission is to initiate therapy for apparent relapse without a biopsy. While most patients with new lymphadenopathy will have recurrent lymphoma, it is certainly not true for all. Table 7 lists the diagnoses that my colleagues and I have found on biopsy in patients with “obvious” recurrent lymphoma. Patients who have never been diagnosed with lymphoma would not be treated without a biopsy and neither should patients who are being followed in documented complete remission.
Diagnoses from biopsies in “obviously” relapsed patients
| Follicular hyperplasia |
| Thymic rebound |
| A different lymphoma |
| Carcinoma |
| Desmoid tumor |
| Glioblastoma |
| Nonspecific inflammatory process |
| Tuberculosis |
| Fungal infection |
| Sarcoidosis |
| Follicular hyperplasia |
| Thymic rebound |
| A different lymphoma |
| Carcinoma |
| Desmoid tumor |
| Glioblastoma |
| Nonspecific inflammatory process |
| Tuberculosis |
| Fungal infection |
| Sarcoidosis |
Salvage therapy
Unfortunately, some patients with diffuse large B-cell lymphoma will not respond to their initial treatment, not achieve an initial complete remission, or relapse from remission. True, primarily refractory patients occasionally benefit from alternate chemotherapy regimens but, in general, have very poor outlook. Partial responders will sometimes benefit further from an alternate chemotherapy regimen and might undergo autologous hematopoietic stem cell transplantation. Some of these patients will be long-term, disease-free survivors. Patients who relapse from complete remission and are younger than 60 to 65 years of age are usually offered hematopoietic stem cell transplantation, and a significant subset of these patients can be cured.63,64 If patients respond to an alternate chemotherapy regimen and achieve a complete remission, approximately 50% of these patients will be long-term disease-free survivors, with a smaller proportion surviving free of disease after a partial response. Salvage chemotherapy regimens today often include a platinum-containing agent, but it is unclear that one regimen is distinctly superior to others. Patients who relapse after an autologous hematopoietic stem cell transplantation can occasionally be rescued with allogeneic hematopoietic stem cell transplantation.79,–81 Some patients relapsing after autologous transplantation seem unusually responsive to rituximab and can have prolonged survival. I have seen a small number of patients have prolonged survival using rituximab and [alpha] interferon.82 For patients with localized relapse, involved field radiotherapy can be used but durable responses are the exception rather than the rule.
The future
The future for treating patients with diffuse large B-cell lymphoma is likely to be exciting. Advances in functional imaging will change staging and restaging and may make other tests obsolete. Further understanding of the genetic subtypes and the associated patterns of protein expression is likely to lead to individualized therapy based on knowing that lymphomas expressing certain proteins (ie, associated with activation of specific metabolic pathways) are particularly likely to respond to specific agents. Among the first hints at this approach are the apparent disproportionate benefit of patients with the activated B-cell type of diffuse large B-cell lymphoma from treatment with rituximab83 and the rare patient with a durable response to a salvage regimen after failing CHOP plus rituximab. We already cure a significant proportion of patients with diffuse large B-cell lymphoma. Almost certainly this proportion will continue to rise.
Authorship
Conflict-of-interest disclosure: The author has given lectures funded by Roche and Biogen IDEC.
Correspondence: James O. Armitage, 987680 Nebraska Medial Center, Omaha, NE 68198-7680; e-mail: joarmita@unmc.edu.