Abstract
A characteristic feature of tumors is high production of lactic acid due to enhanced glycolysis. Here, we show a positive correlation between lactate serum levels and tumor burden in cancer patients and examine the influence of lactic acid on immune functions in vitro. Lactic acid suppressed the proliferation and cytokine production of human cytotoxic T lymphocytes (CTLs) up to 95% and led to a 50% decrease in cytotoxic activity. A 24-hour recovery period in lactic acid–free medium restored CTL function. CTLs infiltrating lactic acid–producing multicellular tumor spheroids showed a reduced cytokine production. Pretreatment of tumor spheroids with an inhibitor of lactic acid production prevented this effect. Activated T cells themselves use glycolysis and rely on the efficient secretion of lactic acid, as its intracellular accumulation disturbs their metabolism. Export by monocarboxylate transporter-1 (MCT-1) depends on a gradient between cytoplasmic and extracellular lactic acid concentrations and consequently, blockade of MCT-1 resulted in impaired CTL function. We conclude that high lactic acid concentrations in the tumor environment block lactic acid export in T cells, thereby disturbing their metabolism and function. These findings suggest that targeting this metabolic pathway in tumors is a promising strategy to enhance tumor immunogenicity.
Introduction
One of the paradoxes in tumor immunology is the fact that, although the patient mounts a specific T-cell response against tumor-associated antigens, these T cells are unable to control tumor growth. There is increasing evidence that the reason for this is a functional impairment of T cells in the tumor micromilieu. For malignant melanoma, it has been shown that T cells infiltrating metastatic lymph nodes exhibit a reduced cytokine production and availability of perforin and granzyme-B molecules.1 Here, we addressed the question of whether tumor cell–derived lactic acid is one of the causes of the suppressed phenotype of T lymphocytes at the tumor site.
Tumor cells are characterized by an altered metabolic phenotype when compared with nonmalignant cells. As early as 1930, Otto Warburg demonstrated that cancer cells rely on glycolysis even in the presence of oxygen, a phenomenon known as aerobic glycolysis (“Warburg effect”2 ). In addition, hypoxia in the tumor environment up-regulates genes encoding glucose transporters and glycolytic enzymes such as lactate dehydrogenase (LDH).3,4 Accordingly, tumor cells produce and secrete lactic acid, which in turn lowers the pH in the tumor environment. Most likely hypoxia-inducible factors (HIF-1α, HIF-2α), transcription factors that are induced either under hypoxic conditions or by cell transformation, are involved in this regulatory process.5,6 HIF-1α and HIF-2α are expressed in the majority of human tumors7 and can also be induced by the glycolysis end products pyruvate and lactate.8 Gatenby and Gillies proposed that this “glycolytic phenotype” of tumor cells confers a growth advantage and is necessary for the evolution of invasive human cancers.9 This hypothesis was confirmed by Walenta et al who found a correlation between lactate concentration in tumor tissues and the incidence of metastases as well as a reduced overall survival of the cancer patients.10–12 Thus, the expression of LDH and the production of lactic acid by tumor cells may facilitate the escape from immune surveillance. LDH serum levels were used for a long time as a diagnostic and prognostic parameter for several malignancies.13 However, only few data exist concerning serum levels of lactate, the product of the LDH reaction, in cancer patients or tumor-bearing animals.14,15 Here, we show for the first time a possible correlation between lactate serum levels and tumor burden in 140 patients suffering from different tumor entities.
Cham and Gajewski have reported recently that activated T cells also depend on glycolysis and thus produce lactate16 due to a higher energetic demand during proliferation and cytokine production.17 To assure the continuation of glycolysis, lactic acid molecules have to be exported.18 As lactate anions cannot cross the plasma membrane by free diffusion, cells require a specific transport system provided by the proton-linked monocarboxylate transporters (MCTs), which cotransport protons and lactate anions following a concentration gradient.19 Expression of MCT-1, -2, and -4 has already been reported in lymphocytes.20 Our study clearly demonstrates that an inauspicious lactic acid gradient between the extracellular milieu and the cytoplasm caused by an accumulation of lactic acid in the tumor environment decelerates the energy metabolism and finally induces a suppressed phenotype in tumor-infiltrating T cells.
Patients, materials, and methods
Media, cytokines, and peptides
Cells were cultured in supplemented RPMI-1640 medium (Gibco, Karlsruhe, Germany) plus 10% human AB-serum (PAN Biotech, Aidenbach, Germany). The following recombinant human cytokines were used: 800 U/mL GM-CSF (Schering-Plough, Brussels, Belgium), 500 U/mL IL-4, 5 ng/mL TGF-β1 (both from Tebu, Offenbach, Germany), 10 ng/mL IL-1β, 1000 U/mL IL-6, 10 ng/mL TNF-α, 2000 U/mL IFN-γ (all from PromoCell, Heidelberg, Germany), and 1 μg/mL prostaglandin E2 (PGE2; Pharmacia, Erlangen, Germany). Preparation of T-cell growth factor (TCGF) was described previously.21 The HLA-A2–binding peptide Melan-A26-35 peptide (ELAGIGILTV) was prepared by Clinalfa (Laeufelfingen, Switzerland).
Patients
Approval was obtained from the University of Regensburg/Department of Hematology and Oncology institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. The study regarding lactate measurement in the serum of tumor patients was approved by the local ethics committee.
Lactic acid levels were measured in the serum of patients with malignant disease visiting our outpatient clinic (lymphoid malignancies: 58 pts, myeloid malignancies: 20 pts, breast cancer: 19 pts, gastrointestinal cancer: 15 pts, urogenital cancer: 15 pts, sarcoma: 4 pts, lung cancer: 3 pts, melanoma: 2 pts, and other types of cancer: 4 pts). Patients were grouped for disease burden by an experienced specialist for hematology and medical oncology who did not have knowledge of the results of lactate measurements. Patients were grouped either as (1) in remission (no evidence of active disease), (2) low tumor burden (only small lesions in diagnostic imaging procedures; no malignancy-induced general symptoms), or (3) high tumor burden (detected by imaging or clinical examination and/or considerable cancer-induced general symptoms).
Lactate quantification
Lactate was measured with a routine procedure using an ADVIA 1650 (Bayer, Tarrytown, NY) and specific reagents (Roche, Mannheim, Germany) at the Department of Clinical Chemistry at the University of Regensburg.
Generation of antigen-specific CD8+ CTLs
Melan-A–specific cytotoxic T lymphocyte (CTL) lines were generated as described previously.22 Briefly, CD8+ T cells were magnetically enriched from peripheral blood mononuclear cells (PBMCs) of healthy donors (Miltenyi Biotec, Bergisch-Gladbach, Germany). Mature dendritic cells (mDCs) were generated by incubating elutriated monocytes for 5 days in complete medium with 10% FCS (PAA, Pasching, Austria), GM-CSF, IL-4, and TGF-β1. On days 5 to 7, TNF-α, IL-6, IL-1β, and PGE2 were added to achieve maturation. mDCs (2 × 106) were pulsed for 2 hours at 37°C with 30 μg/mL Melan-A26-35 peptide and 10 μg/mL human β2-microglobulin (Scipac, Kent, United Kingdom) and used for weekly restimulation of 6 × 106 CD8+ T cells in complete medium (3% TCGF). CTL lines contained 60% to 95% Melan-A–specific cells, as determined by Melan-A–MHC tetramer staining (Beckman/Coulter, Fullerton, CA).
Flow cytometry
For staining of surface antigens, CTLs were stained with α–CD3-APC, α–CD4-PerCP, α–CD8-PerCP, α–CD25-PE, α–CD95-PE, α–HLA-DR-FITC (all from BD/Pharmingen, Heidelberg, Germany); α–ICAM1-FITC (Chemicon, Hofheim, Germany); α–CD45RO-APC, α–CD69-PE (both from Caltag, Hamburg, Germany); α–CD71-FITC, and α–TCR-αβ-chain-PE (both from Immunotech, Krefeld, Germany) mAbs. Appropriate fluorochrome-labeled isotypes were used as control. Prior to intracellular cytokine staining, CTLs were incubated for the indicated time periods with lactic acid, HCl, sodium lactate (all from Sigma, Taufkirchen, Germany), alpha-cyano-4-hydroxy-cinnamic acid (CINN; Sigma), or tumor cells. During the last 5 hours of treatment, cells were stimulated with 20 ng/mL PMA and 1 μM ionomycin (both from Sigma) in the presence of monensin (Golgistop; BD Lifesciences, Breda, the Netherlands). Cells were harvested, fixed, permeabilized (BD Cytofix/Cytoperm; BD Lifesciences), and stained with α–IL-2-PE and α–IFN-γ-FITC mAbs (both from BD/Pharmingen). Alternatively, CTLs were stained intracellularly with α–perforin-PE mAb (δG9) or α–granzyme-B-PE mAb (HC2; both from Hoelzel Diagnostika, Köln, Germany). After coculture with tumor cells, CTLs were stained with α–CD8-APC mAb (BD Lifesciences). Viability was determined via annexinV-FITC (BD/Pharmingen) and propidium iodide (Calbiochem, Darmstadt, Germany) staining. Flow cytometric analyses were performed on a FACSCalibur (BD, Immunocytometry Systems, San José, CA). For data analysis, we used CellQuestPro (BD/Pharmingen), FlowJo (Tree Star, Ashland, OR), or WinMDI free software.
CFSE assays
CTLs (10 × 106/mL) were stained with 4 μM CFSE (Fluka, Buchs, Switzerland) for 4 minutes in the dark. CFSE+ CTLs (8 × 104) were seeded in 200 μL/well in 96-well microtiter plates in the absence or presence of lactic acid at indicated concentrations. CTLs were stimulated from days 1 to 2 with 0.2 μg/well PHA-L (Sigma). On day 6, cells were stained with α–CD25-PE mAb and CFSE dilution was determined by fluorescence-activated cell sorting (FACS).
3H-thymidine assays
CTLs (8 × 104) were harvested on day 4 after antigen-specific restimulation, seeded in 96-well microtiter plates, and treated with lactic acid, HCl, or sodium lactate (all from Sigma). CTLs were pulsed in parallel for 6.5 hours with 0.037 MBq [methyl-3H]-thymidine/well (Amersham Pharmacia, Piscataway, NJ). Cells were harvested onto UniFilter plates using a Wallac harvester and incorporated 3H-thymidine was determined with a Wallac Betaplate counter (all from PerkinElmer, Gaithersburg, MD).
Cytotoxicity assay
CTL-mediated cytotoxicity was determined via the PKH26 assay.23 Briefly, Melan-A peptide–loaded T2 target cells were stained with membrane dye PKH26 (Sigma) and plated with CTLs at indicated effector-target ratios. During 3-hour coincubation, lactic acid or control substances were applied. Cells were harvested and stained with annexinV-FITC, and cell damage was determined by FACS.
Reverse transcription–quantitative LightCycler polymerase chain reaction (PCR)
Total RNA was isolated from CTLs using RNeasy Spin Columns from Qiagen (Hilden, Germany). Reverse transcription (RT) was performed using a SuperScript II RNase H− Reverse Transcriptase from Invitrogen (Karlsruhe, Germany). cDNA was amplified on a Roche Applied Science LightCycler using the QuantiTect SYBR Green PCR Kit (Qiagen). The sequences of the primers were as follows: IL-2: sense, 5′-CTCACCAGGATGCTCACATTTA-3′ and antisense, 5′-TCCAGAGGT TTGAGTTCTTCTTCT-3′; IFN-γ sense, 5′-CTAATTATTCGGTAACTGACTTGA-3′ and antisense, 5′-ACAGTTCAGCCATCACTTGGA-3′; 18S: sense, 5′-ACCGATTGGATGGTTTAGTGAG-3′ and antisense, 5′-CCTACGGAAACCTTGTTACGAC-3′; CD8: sense, 5′-CCCTGAGCAACTCCATCATGT-3′ and antisense, 5′-GTGGGCTTCGCTGGCA-3.
Multicellular spheroid coculture
Three-dimensional multicellular tumor spheroids (MCTSs) were generated using the liquid overlay culture technique as described previously.24 In brief, 5 × 103 suspended cells from exponentially growing tumor cell monolayers was cultured on 1% solid agarose in 96-well plates. On day 5, medium was replaced by CTLs suspended in fresh medium. After 1 day of coculture, spheroids were used for immunohistochemical experiments. Alternatively, MCTSs were harvested, washed, mechanically disintegrated, and stained for FACS analysis. MCTSs generated in the presence of oxamic acid (Sigma) were washed before coculture in order to avoid a direct effect of oxamic acid on CTLs. In a set of experiments, we harvested cell culture supernatants of MCTSs generated with or without oxamic acid for various time intervals and measured the cumulative lactate concentration.
Immunohistochemistry
MCTSs were fixed in 4% formalin, paraffin embedded, and serially sectioned (5 μm). Paraffin sections were processed using the NexEs/HC module (Ventana Medical Systems, Tucson, AZ), α-CD3 mAb, and the iVIEW Diaminobenzidine detection kit (Ventana Medical Systems). All sections were counterstained with hematoxylin. MCTSs were documented using an image processing system consisting of an AxioVert 200 inverted microscope equipped with an AxioCam MRc digital camera and KS300 software (all from Carl Zeiss, Göttingen, Germany). Images were taken at a magnification of 50× (objective 5×/0.12 NA, ocular 10×) and were further processed (cut out and white balanced) with Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA). Respective magnification bars are shown for each image.
Western blot
Either freshly isolated CD8+ cells, CTLs, or Hela cells (ATCC, Manassas, VA) were used for analysis. Probes were separated in 15% SDS gels and blotted on PVDF membranes (Millipore, Bedford, MA). After blocking with 5% dry milk in TBST, blots were incubated overnight (O/N) with α–MCT-1 or α–MCT-2 mAb (1:1000; Abcam, Cambridge, United Kingdom) or α–β-actin mAb (1:2000; Sigma). Secondary antibodies (Dako, Glostrup, Denmark) were given for 1 hour at room temperature. Blots were developed with enhanced chemiluminescent (ECL) detection reagents (Amersham, Freiburg, Germany).
Measurement of lactate uptake by mass spectrometry
CTLs (106/mL medium) were incubated for 30 minutes with 20 mM 13C-labeled lactate (Eurisotop, Saint-Aubin, France) with or without 30 μL 1% HCl to shift pH to approximately 6.6. Cells were washed with PBS, counted, and then snap frozen and stored at −80°C. After homogenization, metabolites were extracted by 80% methanol, and protein content was determined by the Bradford assay. Samples were analyzed by CE-ESI-MS, using PolyE-323–coated capillaries, 100 μM itaconic acid (Sigma), and 1,2,3 13C3-labeled lactate (Eurisotop) as internal standards for quantification.25 Detection was performed on a time of flight mass spectrometer (Agilent Technologies, Palo Alto, CA) in negative mode.26
Measurement of intracellular pH
CTLs were loaded with 10 μM pH-sensitive dye carboxy-SNARF-1/AM for 30 minutes at 37°C, washed, and incubated for 30 minutes with lactic acid in medium. Internal pH values were determined via the ratio mean FL-2/FL-3 by FACS. Standard curve was generated by treating CTLs with 10 μM nigericin in calibration buffers from pH 6.0 to 8.0.
Student t test
Statistical analysis was performed using an unpaired (only patient data) or paired 2-tailed Student t test. A value of P < .05 was considered statistically significant.
Results
Increased serum lactate levels in cancer patients
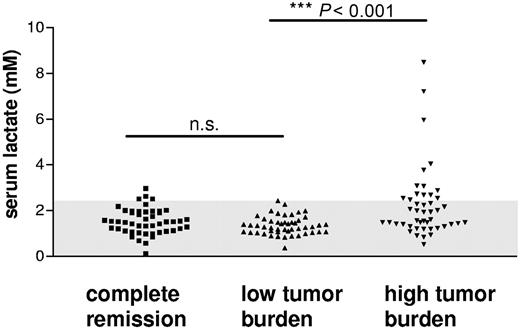
To demonstrate a possible correlation between serum lactate levels and patients' tumor burden, we collected the serum of 140 patients suffering from different malignancies and determined the lactate concentration. In the blood of about one third of the patients with a high tumor burden, a significantly elevated level of serum lactate could be detected. This led to a significant between-group difference compared with patients with low tumor burden or in complete remission (Figure 1; P < .001). This finding ascertains the fact that a multitude of established tumors are able to produce large amounts of lactic acid and shows for the first time that a high lactate concentration in the patient's serum may reflect the tumor burden.
Lactate levels measured in the serum of patients with different malignant diseases. Lactate serum concentrations were determined in 140 patients with different malignancies. Patients were grouped for disease burden into 3 groups describing the stage of disease (“Material and methods”). The normal range of lactate serum levels determined in healthy individuals (0-2.2 mM) is indicated (gray background).
Lactate levels measured in the serum of patients with different malignant diseases. Lactate serum concentrations were determined in 140 patients with different malignancies. Patients were grouped for disease burden into 3 groups describing the stage of disease (“Material and methods”). The normal range of lactate serum levels determined in healthy individuals (0-2.2 mM) is indicated (gray background).
Lactic acid inhibits T-cell proliferation
A characteristic feature of the tumor environment is local acidosis, which is attributed to the local increase of lactic acid production. To investigate the direct effect of lactic acid on T-cell proliferation, we incubated CTLs with lactic acid or sodium lactate, the sodium salt of lactic acid, and determined proliferation by 3H-thymidine uptake. Lactic acid clearly suppressed CTL proliferation after antigen-specific stimulation (Figure 2A) or mitogenic stimulation (Figure 2B) in a dose-dependent manner, whereas sodium lactate, which does not lead to an acidification of the medium, had no suppressive effect on CTL proliferation (Figure 2A). The administration of 20 mM lactic acid led to a pH decrease from pH 7.4 to approximately pH 6.5 to 6.8 (data not shown). To study the effect of acidification on proliferation, we cultured CTLs in an acidified medium and observed a moderate inhibition of proliferation after short-term culture but a significant inhibition after 24 hours (data not shown).
Lactic acid suppresses proliferation and partially induces cell death in CTLs. (A) 3H-thymidine uptake in CTLs during short-term (6.5 hours) incubation in 5 to 20 mM lactic acid or sodium lactate. Data are means ± SEM (n = 3). (B) CFSE-labeled CTLs were stimulated for 24 hours with PHA/ionomycin, and cell division was determined by FACS on day 6 in the absence or presence of 5, 10, and 20 mM lactic acid. Gated viable cells from a representative experiment are shown (n = 3). (C) CTLs were treated with 10 or 20 mM lactic acid or HCl (pH 6.5) for 6.5 hours or 24 hours, and viable cells were determined by flow cytometry as annexinV-FITC/PI-negative cells. Data are means ± SEM; n = 8; *P < .02; **P < .003.
Lactic acid suppresses proliferation and partially induces cell death in CTLs. (A) 3H-thymidine uptake in CTLs during short-term (6.5 hours) incubation in 5 to 20 mM lactic acid or sodium lactate. Data are means ± SEM (n = 3). (B) CFSE-labeled CTLs were stimulated for 24 hours with PHA/ionomycin, and cell division was determined by FACS on day 6 in the absence or presence of 5, 10, and 20 mM lactic acid. Gated viable cells from a representative experiment are shown (n = 3). (C) CTLs were treated with 10 or 20 mM lactic acid or HCl (pH 6.5) for 6.5 hours or 24 hours, and viable cells were determined by flow cytometry as annexinV-FITC/PI-negative cells. Data are means ± SEM; n = 8; *P < .02; **P < .003.
To analyze whether lactic acid or acidification of the culture medium induces cell death in CTLs, we stained the cells with annexinV and propidium iodide. Flow cytometric analyses revealed that short-term incubation with lactic acid (6.5 hours) had no effect on cell viability, whereas prolonged incubation (24 hours) with high amounts of lactic acid (20 mM) led to cell death of up to 60% of the CTLs. In contrast, treatment with HCl for up to 24 hours had no such effect (Figure 2C). This indicates that acidification alone cannot account for the suppressive effect of lactic acid.
Lactic acid does not modulate the surface antigen profile of CTLs
We next investigated whether lactic acid has a modulatory effect on CTL surface antigen expression. CTLs were incubated for 24 hours with or without lactic acid, washed, and stained with mAbs against surface markers known to be involved in T-lymphocyte activation and maturation such as CD71, CD69, T-cell receptor (TCR) β-chain, CD25, ICAM-1, HLA-DR, CD45RO, and CD95. No significant differences for any of these antigens were detected (Figure 3). Accordingly, we found no changes in CD25, CD71, and CD69 expression on mRNA level (data not shown). Thus, lactic acid does not seem to interfere globally with signal transduction or cellular activation of CTLs.
Lactic acid does not alter surface antigen profile in CTLs. CTLs were treated for 24 hours with 20 mM lactic acid or were left untreated. After staining with the indicated mAbs, the phenotype of CTLs was determined by FACS. Grey indicates isotype control of untreated cells; dotted line, control; and solid line, with lactic acid. Results are representative of 3 independent experiments.
Lactic acid does not alter surface antigen profile in CTLs. CTLs were treated for 24 hours with 20 mM lactic acid or were left untreated. After staining with the indicated mAbs, the phenotype of CTLs was determined by FACS. Grey indicates isotype control of untreated cells; dotted line, control; and solid line, with lactic acid. Results are representative of 3 independent experiments.
Lactic acid impairs cytokine production in CTLs
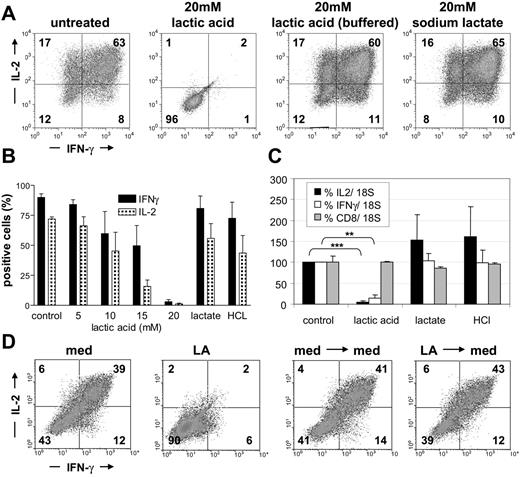
To study the influence of lactic acid on cytokine production of tumor-reactive T cells, we stimulated CTLs with PMA/ionomycin for 5 hours in the absence or presence of lactic acid to evoke cytokine production. CTLs treated with HCl and sodium lactate served as control. Intracellular levels of IL-2 and IFN-γ were determined by flow cytometry. CTLs treated with 10 mM or 15 mM lactic acid were strongly repressed in their cytokine production in comparison with untreated T cells, and lactic acid at a concentration of 20 mM completely abolished IL-2 and IFN-γ production (Figure 4A-B). The frequency of cells that produced IL-2 decreased from more than 70% in the untreated sample to 45%, 16%, and 1% in the cultures with 10, 15, and 20 mM lactic acid, respectively. The effect on IFN-γ expression was less pronounced but IFN-γ–positive cells decreased from 90% in the untreated sample to 60%, 50%, and 3% in the cultures with 10, 15, and 20 mM lactic acid, respectively. As expected, even a prolonged treatment (21 hours) of CTLs with sodium lactate did not affect cytokine production, and acidification alone (with HCl pH ∼ 6.5-6.8) had only a partial inhibitory effect (Figure 4B). These findings complement the proliferation data and clearly indicate that the inhibitory effect of lactic acid is linked to the presence of protons. To formally prove this, we buffered the lactic acid–containing culture to pH 7.4 and observed a complete reversion of the suppressive effect (Figure 4A). Next, we evaluated if the decreased ability of CTLs to produce cytokines in the presence of lactic acid is also reflected by a decreased IL-2 and IFN-γ transcription. Therefore, we stimulated CTLs for 3 hours with PMA/ionomycin in the presence or absence of 20 mM lactic acid. Quantitative RT-PCR revealed that lactic acid significantly diminished IL-2 and IFN-γ transcription, whereas incubation with sodium lactate or acidification of the medium had no effect (Figure 4C). In line with the protein data, down-regulation of IL-2 transcription was more profound than that of IFN-γ. No effect was determined regarding CD8 expression, indicating that lactic acid has no global effect on transcription.
Lactic acid strongly diminishes cytokine production in CTLs. (A) CTLs were incubated for 16 hours with or without lactic acid, lactic acid/buffered with NaOH, or sodium lactate and then stimulated for another 5 hours with PMA/ionomycin in the presence of the substances. Cells were stained intracellularly with mAbs for IL-2 and IFN-γ and analyzed by flow cytometry. Results are representative of 3 independent experiments. (B) CTLs were cultured in the absence or presence of 5 to 20 mM lactic acid, sodium lactate, or HCl (∼ pH 6.8). Data represent means ± SEM; n = 4. (C) IL-2, IFN-γ, and CD8 mRNA were determined by quantitative RT-PCR after stimulation for 3 hours with PMA/ionomycin in the presence of 20 mM lactic acid, 20 mM sodium lactate, or HCl (∼ pH 6.8). Data are means ± SEM; n = 4. (D) CTLs were treated for 24 hours with 20 mM lactic acid (LA), washed, and recovered for 24 hours in normal medium (med). Intracellular cytokines were determined by flow cytometry. Results are representative of 3 independent experiments. **P < .0016; ***P < .0001.
Lactic acid strongly diminishes cytokine production in CTLs. (A) CTLs were incubated for 16 hours with or without lactic acid, lactic acid/buffered with NaOH, or sodium lactate and then stimulated for another 5 hours with PMA/ionomycin in the presence of the substances. Cells were stained intracellularly with mAbs for IL-2 and IFN-γ and analyzed by flow cytometry. Results are representative of 3 independent experiments. (B) CTLs were cultured in the absence or presence of 5 to 20 mM lactic acid, sodium lactate, or HCl (∼ pH 6.8). Data represent means ± SEM; n = 4. (C) IL-2, IFN-γ, and CD8 mRNA were determined by quantitative RT-PCR after stimulation for 3 hours with PMA/ionomycin in the presence of 20 mM lactic acid, 20 mM sodium lactate, or HCl (∼ pH 6.8). Data are means ± SEM; n = 4. (D) CTLs were treated for 24 hours with 20 mM lactic acid (LA), washed, and recovered for 24 hours in normal medium (med). Intracellular cytokines were determined by flow cytometry. Results are representative of 3 independent experiments. **P < .0016; ***P < .0001.
Finally, we analyzed whether the inhibitory effect of lactic acid on cytokine production in CTLs is reversible. To this end, CTLs were treated for 24 hours with 20 mM lactic acid, washed, and plated for another 24 hours in fresh T-cell medium. Intracellular cytokines were determined as described above in “Flow cytometry” under “Patients, materials, and methods.” Indeed, this treatment was sufficient to revert the primary inhibition, and CTLs regained their capacity to produce IL-2 and IFN-γ (Figure 4D; LA → med). Together these data indicate that although some cytotoxicity occurs under the influence of lactic acid (Figure 2D), surviving cells recover and show normal cytokine production.
Lactic acid reduces cytotoxic activity of CTLs
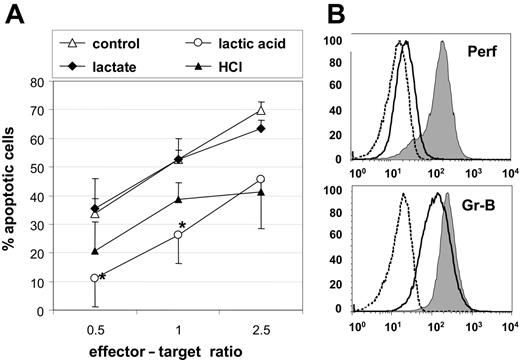
To study the effect of exogenous lactic acid on the cytotoxic capacity of Melan-A–specific CTLs, we cocultured CTLs and Melan-A–loaded T2 target cells at indicated ratios in the presence of 15 mM lactic acid and determined the target cell killing over a period of 3 hours. Untreated CTLs served as control. We found that the capability of lactic acid–treated CTLs to kill target cells was reduced by one half (Figure 5A). In contrast, CTLs treated with 15 mM sodium lactate were not impaired in their cytotoxicity, and CTLs treated with HCL showed a decrease in their cytotoxic function that was donor dependent and not statistically significant.
Lactic acid affects the cytotoxic activity and the availability of intracellular perforin and granzyme-B. (A) The cytotoxic activity of Melan-A–specific CTLs was determined by incubating CTLs with PKH26+ Melan-A peptide–loaded T2 target cells at indicated effector-target ratios. The cytotoxicity assay was performed for 3 hours in the presence of 15 mM lactic acid, 15 mM sodium lactate, HCL, or in normal medium. (B) Untreated CTLs (gray) or CTLs that were treated for 24 hours with 20 mM lactic acid (solid line) were analyzed for their intracellular content of human perforin and granzyme-B. Appropriate isotype control of untreated CTLs is shown (dashed line). Results are representative of 3 independent experiments. Mean of triplicate ± SEM. *P < .05.
Lactic acid affects the cytotoxic activity and the availability of intracellular perforin and granzyme-B. (A) The cytotoxic activity of Melan-A–specific CTLs was determined by incubating CTLs with PKH26+ Melan-A peptide–loaded T2 target cells at indicated effector-target ratios. The cytotoxicity assay was performed for 3 hours in the presence of 15 mM lactic acid, 15 mM sodium lactate, HCL, or in normal medium. (B) Untreated CTLs (gray) or CTLs that were treated for 24 hours with 20 mM lactic acid (solid line) were analyzed for their intracellular content of human perforin and granzyme-B. Appropriate isotype control of untreated CTLs is shown (dashed line). Results are representative of 3 independent experiments. Mean of triplicate ± SEM. *P < .05.
To discover possible reasons for the diminished cytotoxicity in lactic acid–treated CTLs, we determined the intracellular content of granzyme-B and perforin after 24-hour incubation with lactic acid. Lactic acid strongly derogated the content of perforin as reflected by a reduction of the mean fluorescence intensity from 191 (untreated CTLs) to 26, the level of the isotype control (Figure 5B). The amount of granzyme-B was reduced 1.7-fold after lactic acid treatment (Figure 5B).
Tumor cell–derived lactic acid inhibits CTLs
To study the effect of lactic acid in a more in vivo–like model, we used 3-dimensional MCTSs generated from MelIm melanoma cells as a source of lactic acid (Figure 6A). Supernatants of day-4 to day-7 MelIm MCTSs contain extracellular lactate concentrations in a range of 2.4 to 10.5 mM (n = 7; mean, 7.9 ± 1 mM); day-8 to day-11 MCTSs exhibit extracellular lactic acid concentrations in the range of 8.1 to 14.5 mM (n = 7; mean, 11.6 ± 0.9 mM). Addition of oxamic acid, an LDH inhibitor, results in lower lactate concentration at every time point, however in older spheroids the remaining levels are still in the range of 7 mM (Figure 6B).
Tumor spheroid–infiltrating CTLs exhibit a diminished ability for cytokine production, and oxamic acid is able to prevent this suppression. (A) MCTSs were generated in the presence or absence of oxamic acid from exponentially growing MelIm tumor cells. After 4 to 5 days, medium was replaced by a suspension containing 0.4 × 106/mL CTLs. Infiltrating CTLs were visualized in 5-μm paraffin sections by staining for the T-cell marker CD3 (bar = 100 μm). (B) Day-4 to day-11 MCTSs were generated in the presence or absence of 60 mM oxamic acid (OA) and supernatants analyzed for lactate content. Horizontal bars represent the mean values. (C) Reduction of IFN-γ–positive CTLs relative to control cells without MCTSs against the lactate content of the MCTS coculture. (D) After 24 hours of coculture, day-5 MCTSs were disintegrated, and cells were stained intracellularly for IL-2 and IFN-γ. CTLs cultured in medium without MCTSs were used as control. Shown are representative data of 3 independent experiments.
Tumor spheroid–infiltrating CTLs exhibit a diminished ability for cytokine production, and oxamic acid is able to prevent this suppression. (A) MCTSs were generated in the presence or absence of oxamic acid from exponentially growing MelIm tumor cells. After 4 to 5 days, medium was replaced by a suspension containing 0.4 × 106/mL CTLs. Infiltrating CTLs were visualized in 5-μm paraffin sections by staining for the T-cell marker CD3 (bar = 100 μm). (B) Day-4 to day-11 MCTSs were generated in the presence or absence of 60 mM oxamic acid (OA) and supernatants analyzed for lactate content. Horizontal bars represent the mean values. (C) Reduction of IFN-γ–positive CTLs relative to control cells without MCTSs against the lactate content of the MCTS coculture. (D) After 24 hours of coculture, day-5 MCTSs were disintegrated, and cells were stained intracellularly for IL-2 and IFN-γ. CTLs cultured in medium without MCTSs were used as control. Shown are representative data of 3 independent experiments.
CTLs were cocultured for 24 hours with MCTSs and shown to strongly infiltrate all regions of the spheroids within 24 hours (Figure 6A). After CTL-MCTS coculture, spheroids were harvested, washed, and disintegrated by vigorous pipetting. The obtained single-cell suspensions were stained with mAbs recognizing various surface markers. As expected, we found that MCTS-infiltrating CTLs were not altered in their antigen expression profile by the tumor environment (data not shown). However, MCTS-infiltrating CTLs were suppressed in their IL-2 and IFN-γ production when compared with cells cultured without MCTSs (Figure 6D). While 62% and 91% of untreated CTLs produced IL-2 or IFN-gamma, respectively, 45% and 72% of CTLs showed cytokine production after 24-hour coculture with tumor spheroids. To verify that tumor-derived lactic acid was the reason for diminished cytokine production, we blocked lactic acid production by pretreating MCTSs with oxamic acid and observed a reversion of the MCTS effect (Figure 6D). Further analysis revealed a statistically significant correlation between lactate concentration in the MCTS cultures and inhibition of IFN-γ but not IL-2 production (Figure 6C and data not shown). These data clearly demonstrate that lactic acid in the tumor environment is a significant inhibitor of CTL function.
Lactic acid accumulation leads to a metabolic blockage in CTLs
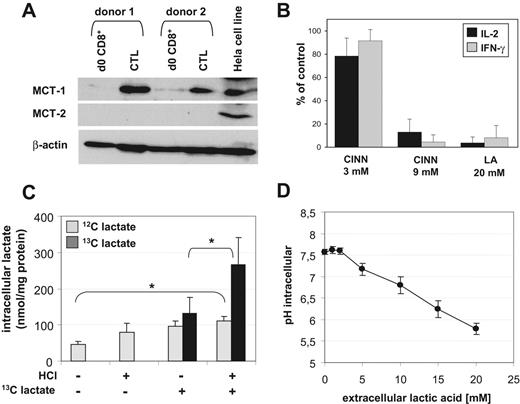
It is known that CTL effector functions are linked to a higher energetic demand and strongly depend on glucose availability. Thereby, a predominance of glycolysis over the respiratory chain metabolism has been reported by several groups.16,17 The increased glycolysis rate in CTLs is accompanied by an increased production and export of lactic acid. In exponentially growing CTL cultures, we determined lactate in the range of 5 to 15 mM in the supernatants (data not shown). The necessity for CTLs to discharge accumulating lactic acid from the cytoplasm indicates an important role of MCTs, which cotransport protons and lactate anions through the plasma membrane depending on the concentration gradient. We determined protein expression of MCT family members MCT-1 and MCT-2 in Melan-A–specific CTLs by Western blot and found a significant expression of MCT-1 but not MCT-2 in all CTL lines tested. Freshly isolated CD8+ T cells showed a weak MCT-1 expression (Figure 7A).
MCT-1 transport activity and lactic acid influx in CTLs. (A) Expression of MCT-1 and MCT-2 in CTL lines and unstimulated human CD8+ T cells (from the same donor, respectively) analyzed by Western blot. Hela cells were used as a positive control. (B) Blocking of MCT transport activity by treating CTLs for 5 hours with 3 or 9 mM CINN or 20 mM lactic acid. Intracellular cytokines IL-2 and IFN-γ were determined by FACS. Results are normalized to untreated CTLs. (C) Uptake of 13C-labeled lactate by CTLs. CTLs were incubated for 30 minutes with 20 mM external 13C-lactate in the presence or absence of HCl (*P < .05; n = 4). Endogenous lactate and the uptake of exogenous lactate and was determined in the cell lysates by mass spectrometry. (D) Decrease of the intracellular pH in CTLs 30 minutes after addition of lactic acid was determined flow cytometrically with SNARF-1. Shown are means ± SEM from 3 independent experiments with different donors.
MCT-1 transport activity and lactic acid influx in CTLs. (A) Expression of MCT-1 and MCT-2 in CTL lines and unstimulated human CD8+ T cells (from the same donor, respectively) analyzed by Western blot. Hela cells were used as a positive control. (B) Blocking of MCT transport activity by treating CTLs for 5 hours with 3 or 9 mM CINN or 20 mM lactic acid. Intracellular cytokines IL-2 and IFN-γ were determined by FACS. Results are normalized to untreated CTLs. (C) Uptake of 13C-labeled lactate by CTLs. CTLs were incubated for 30 minutes with 20 mM external 13C-lactate in the presence or absence of HCl (*P < .05; n = 4). Endogenous lactate and the uptake of exogenous lactate and was determined in the cell lysates by mass spectrometry. (D) Decrease of the intracellular pH in CTLs 30 minutes after addition of lactic acid was determined flow cytometrically with SNARF-1. Shown are means ± SEM from 3 independent experiments with different donors.
In order to investigate a functional involvement of MCT-1 in Melan-A–specific CTL suppression, we performed blocking studies with CINN, an MCT-1 inhibitor. A blockade of MCT during a 5-hour stimulation with PMA/ionomycin resulted in a dose-dependent inhibition of IL-2 and IFN-γ production in CTLs (Figure 7B), indicating that CTLs are strongly dependent not only on glucose availability but also on lactate removal.
A high extracellular level of lactic acid could block the lactic acid export, however an additional uptake of exogenous lactic acid in CTLs also seems likely. To address this issue, we analyzed lactate uptake into CTLs by mass spectrometry. CTLs were incubated for 30 minutes with 20 mM 13C-labeled lactate in the absence or presence of HCl. Control cells were cultured without 13C-labeled lactate (Figure 7C). CTLs contained a certain level of endogenous lactate that increased significantly after the addition of extracellular lactate in the presence of HCl, indicating a block in the export of endogenous lactate under these conditions. In addition, exogenous lactate was taken up by the CTL, and this process was increased about 2-fold by the addition of protons/HCl.
As MCT-1 acts as a cotransporter for H+ ions and lactate, we determined the intracellular pH value of CTLs after exposure to increasing amounts of lactic acid. As expected, all CTL lines showed a significant dose-dependent decrease in the intracellular pH value (from pH 7.6 to < pH 6.0, Figure 7D) after 30 minutes of incubation, which again strongly suggests a symport of H+/lactate anions by antigen-experienced CTLs.
Discussion
For many years, the phenomenon of paradoxical coexistence of tumor cells and tumor-specific CD8+ T cells has been of interest.27–29 Several mechanisms of tumor-induced immune defects in T cells have been proposed, including alterations in signal transduction,30 functional unresponsiveness,31 and an immunosuppressive environment32 leading to defective maturation and functional tolerance of tumor-specific T cells at the tumor site.1 We previously showed that lactic acid, a side product of tumor metabolism, alters the antigen-presenting capability of human monocyte-derived dendritic cells (DCs).33 In this study, we addressed the question whether lactic acid exerts an additional direct effect on T lymphocytes. The importance of this issue was highlighted by our data revealing a positive correlation between tumor burden and lactate serum concentrations in cancer patients.
An in vitro treatment of human Melan-A–specific CD8+ CTLs revealed a dual effect of lactic acid. While prolonged incubation (24 hours) induces cell death in a fraction (< 60%) of CTLs, short-term (3-6 hours) incubation rapidly suppresses various crucial T-cell functions. Upon exposure to external lactic acid, we observed a dose-dependent reduction in the proliferation of CTLs, as well as a dramatically reduced availability of IL-2 and IFN-γ on mRNA and protein level. Additionally, the cytolytic capacity, a key feature of Melan-A–specific CTLs, was hampered, probably due to a reduced amount of intracellular perforin and granzyme-B. This finding is in line with the observation that repeated injections of l-lactate suppress cytotoxic immune responses in vivo.34
Despite the fact that lactic acid causes an acidification of the medium, which has been shown before to inhibit natural killer cell activity and polymorphonuclear leukocyte chemotaxis and migration,35–37 we exclude that the inhibitory effect is mediated exclusively by protons: (1) Treatment with HCl was merely able to mediate about one half of the suppressive effect on proliferation and cytokine production compared with lactic acid. (2) In contrast to lactic acid, which clearly suppressed cytokine production on mRNA level, HCl seems to affect IL-2 and IFN-γ production posttranscriptionally. This is in line with data from Heming et al who observed a posttranscriptional modulation of TNF-α under acidic conditions in macrophages.38 Similar to our results, Redegeld et al found that pH differences do not affect Ca2+-dependent CTL-mediated cell lysis in vitro.39
One important point that needs to be considered is the question whether the lactic acid concentrations used for our in vitro experiments are reasonable to mimic the intratumoral micromilieu. Direct evidence exists from intratumoral analyses by Walenta et al that indeed revealed concentrations of up to 40 mM lactate inside the tumor tissue.10,12 Moreover, our own analyses from supernatants of exponentially growing tumor monolayer cultures showed a production of 5 to 20 mM lactic acid (E.G., unpublished results, 2006). To further address this issue, we aimed to verify the inhibitory effects of lactic acid in a 3-dimensional tumor cell model. MCTSs represent a complex 3-dimensional tissue culture model for avascular sites of a tumor or micrometastases with respect to growth kinetics, extracellular matrix, nutrient gradients, oxygen tension, and pH and mimic the in vivo situation better than monolayer cultures.40 After 24-hour coculture of Melan-A–specific CTLs with MCTSs, we could detect a huge amount of tumor-infiltrating CTLs by immunohistochemistry. As expected, these infiltrating T cells showed a suppressed ability for cytokine production. The limited ability for cytokine production is clearly caused by tumor-derived lactic acid, as oxamic acid, an LDH inhibitor, was able to circumvent CTL suppression. Together, these data suggest that infiltrating CTLs are disabled in crucial functions shortly after entering the tumor site. However, our data demonstrated that the suppressed CTL phenotype is not stable. A recovery period of 24 hours in medium without lactic acid restored cytokine production probably due to a resolution of the metabolic blockade. Together with the finding that the expression of important cell surface antigens was not influenced by external lactic acid, a therapeutic rescue of CTLs from lactic acid–mediated suppression seems conceivable.
The sensitivity of CTLs toward exogenous lactic acid can be attributed to the specific metabolism during T-cell activation. Activated T cells metabolize glucose predominantly via glycolysis16,17 in order to provide ATP for energy-consuming processes and to supply metabolic intermediates for the pentose-phosphate pathway. In order to maintain a high glycolysis rate, the removal of lactic acid is essential.41 This process involves MCTs, which cotransport bidirectionally lactate anions and H+ ions, depending on the respective concentration gradient.19 This is in line with our finding that sodium lactate (sodium salt of lactic acid) in the absence of protons had no suppressive effect on CTL functions, which is most likely linked to the H+/lactate cotransport activity of MCTs.
In human Ag-specific CD8+ CTLs, lactic acid transport seems to depend predominantly on MCT-1, which is strongly induced in these cells. As MCT-1 transport activity is mainly regulated by the concentration gradient over the cell membrane,19 exceeding concentrations of external lactic acid hamper continuous lactic acid efflux and thereby T-cell metabolism (see graphic, Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Accordingly, Murray et al found that a series of immunosuppressive compounds acts via blockade of MCT-1. This blockade resulted in a suppressed glycolytic flux, and thereby inhibited cellular metabolism in human T cells. In contrast to our findings, cytokine production was not impaired in their experiments.42 Lactate efflux via MCT-1 can also be disturbed by a blockade of MCT-1 by CINN. In our experiments, treatment of CTLs with CINN induced the same suppressed phenotype with low cytokine production as did external lactic acid. Moreover, lactate uptake into murine T cells has been demonstrated to be a pH- and activation-dependent process leading to an intracellular lactate accumulation.43 In line with these results, we could show an uptake of lactate and a decline in the intracellular pH value in CTLs during lactic acid treatment.
Finally, we conclude that lactic acid suppresses T-cell activation via blockade of lactate efflux and thereby disturbance of T-cell metabolism. Metabolic aspects of tumor immunology will enrich the debate on immune escape. Further studies may lead to new therapeutic approaches that target metabolic processes in cancer patients.
Authorship
Contribution: M.K., K.F., and E.G. designed the research and wrote the paper; S.W.K. collected and analyzed the patient data; R.A. and A.M. discussed the data; K.F., S.V., S.S., J.A., S.H., P.H., M.E., G.R., N.M., M.K., L.K.-S., K.R., and B.T. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Kreutz, University of Regensburg, Department of Hematology and Oncology, Franz-Josef Strauss Allee 11, 93042 Regensburg; e-mail: marina.kreutz@klinik.uni-regensburg.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by the Deutsche Forschungsgemeinschaft (Krm-1418/6-4).
The authors thank Rüdiger Eder, Alexandra Müller, Alice Peuker, Claudia Reichelt-Busch, and Jana Berger for excellent technical assistance and M. Rehli for critically reading the paper.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal