Abstract
The phenotype and function of murine dendritic cells (DCs) are primarily studied using bone-marrow–derived DCs (BM-DCs), but may be hampered by the heterogenous phenotype of BM-DCs due to their differential state of maturation. Here we characterize a newly established murine DC line (SP37A3) of myeloid origin. During maintainance in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and M-CSF, SP37A3 cells resemble immature DCs characterized by low expression of major histocompatibility complex (MHC) II and costimulatory molecules and low T-cell stimulatory capacity. Upon stimulation, SP37A3 cells acquire a mature phenotype and activate naive T cells as potently as BM-DCs. Similar to BM-DCs, SP37A3 cells activated in the presence of dexamethasone-induced regulatory T cells, which were anergic upon restimulation and suppressed proliferation of naive T cells. This tolerogenic state was reflected by lower expression levels of costimulatory molecules and proinflammatory cytokines compared with mature cells, as well as up-regulated expression of FcγRIIB and interleukin-1RA (IL-1RA). SP37A3 cells were responsive to dexamethasone even when applied at later time points during activation, suggesting functional plasticity. Thus, DC line SP37A3 represents a suitable model to study functions of immature and mature as well as tolerogenic myeloid DCs, circumventing restrictions associated with the use of primary DCs and BM-DCs.
Introduction
Immature dendritic cells (DCs), specialized in the uptake of antigen,1 reside as sentinels in almost every tissue. Under steady-state conditions, a small fraction of DCs acquire a semimature state and migrate to the draining secondary lymphoid organs. There, these semimature DCs exert tolerogenic functions by inducing apoptosis, anergy, or even a regulatory state in interacting autoreactive T cells that escaped elimination during negative thymic selection. In addition to natural regulatory T cells (Treg's), which originate in the thymus, induced Treg's, activated by tolerogenic DCs, are essential for maintainance of peripheral tolerance2 and ensure tolerance to harmless environmental antigens.3 In contrast, during infection, DCs engulf and process pathogenic material. Exposure to pathogen-derived molecules like lipopolysaccharide (LPS) or proinflammatory cytokines produced by cells in the microenvironment induces the full maturation of DCs, characterized by strong up-regulation of expression of costimulatory molecules and the production of proinflammatory cytokines.4 Mature DCs constitute the most potent antigen-presenting cells, which are capable of stimulating naive antigen-specific T cells and thereby inducing a primary immune reaction.
Several DC subsets with distinct phenotypes and functions have been identified.5 In mice, CD11c has been acknowledged as a useful marker for DCs. DC subsets of myeloid origin (mDCs) mature in response to bacterial products and proinflammatory cytokines, and fully mature mDCs are inducers of strong immune responses. However, following treatment with anti-inflammatory cytokines (IL-10, transforming growth factor-β [TGF-β]) or pharmacologic agents like dexamethasone (DEX) and vitamin D3, mDCs differentiate into a tolerogenic state.6,7 Plasmacytoid DCs (pDCs) express markers like GR-1 and, in mice, B220, although a distinct lymphoid origin of pDCs is still a matter of debate.8,9 pDCs have been ascribed a profound tolerogenic potential both under homeostatic conditions and even upon activation, mainly induced by DNA and RNA viruses.10
DC functions are mainly studied using bone-marrow–derived DCs (BM-DCs) which are as potent as primary DCs isolated from spleen.11 BM-DCs exhibit myeloid characteristics upon generation from progenitors in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas a major fraction displays a pDC-like phenotype when progenitors are cultivated with Flt3-L.12 However, the generation of BM-DCs is time consuming, cultures may contain contaminating cell types, and BM-DCs display a heterogenous phenotype concerning the expression of major histocompatibility complex (MHC) II and costimulatory molecules, and therefore T-cell stimulatory capacity.13 To circumvent these problems, several cell lines with DC-like characteristics have been derived either from long-term cultures of murine skin Langerhans cells14,15 or spleen cell suspensions,16 or by immortalizing primary DCs with retroviral vectors encoding oncogenes.17,18 These DC lines have proven a valuable tool for studying aspects of DC function and the regulatory mechanisms involved. However, the functional properties of these DC cell lines do not always reflect the plasticity and potency of primary DCs.
In this study, we have extensively characterized a newly established murine DC line (SP37A3) of myeloid origin. SP37A3 cells remain immature in phenotype and function during propagation, but acquire features of mature DCs upon stimulation. Notably, upon activation in the presence of glucocorticoid, SP37A3 cells show a tolerogenic, Treg-inducing function.
Materials and methods
Mice
Female C57BL/6, BALB/c, FVB/N, and OT-II mice were bred and maintained in the Central Animal Facilities of the University of Mainz under specific pathogen–free conditions on a standard diet. The “Principles of Laboratory Animal Care” (National Institutes of Health publication no. 85-23, revised 1985) were followed.
Generation and culture of SP37A3 cells
The DC line SP37A3 was derived by growth factor–dependent long-term culture of C57BL/6 spleen cells according to the method of Winzler et al16 with modifications. After removal of erythrocytes by lysis (155 mM NH4Cl, 10 mM KHCO3, and 100 μM EDTA-disodium [pH 7.4]), spleen cells (0.5 × 106/mL) were cultured in SP37A3 medium (RPMI 1640 [Invitrogen Life Technologies, Karlsruhe, Germany] with 10% fetal calf serum [FCS] [low endotoxin; PAA, Cölbe, Germany], 2 mM L-glutamine [Biochrom AG, Berlin, Germany], 100 U/mL penicillin, and 100 μg/mL streptomycin [Gibco, Paisley, United Kingdom]), and supplemented with 10 ng/mL recombinant murine (rm) GM-CSF and 50 ng/mL recombinant human (rh) M-CSF (R&D Systems, Wiesbaden, Germany). After 1 week, loosely adherent cells and cells in suspension containing DC precursors were recovered by washing and passaged at a density of 2 × 104 cells/cm2 in fresh medium. Cells were subcultured twice at weekly intervals at the same cell density using PBS/0.1% EDTA for detachment of the cells. Cultures were fed by addition of both growth factors every 2 to 3 days. After this growth period, the cells ceased to proliferate. Passaging of the cells nevertheless had to be continued in order to remove newly developing adherent macrophages in the culture. Growth factors were replenished every 3 to 4 days. During this growth stagnation period no morphologic changes of the cells were observed. After 8 weeks cells resumed proliferation. Cell stocks were cryopreserved at that stage. Immature SP37A3 cells could be subcultured every week up to passage 38 without any morphologic and functional (maturation potential after stimulation with inflammatory cytokines) changes.
To induce maturation, SP37A3 cells were washed twice and cultured for 3 days in 6-well tissue-culture plates (Greiner, Frickenhausen, Germany) at 106 cells/4 mL SP37A3 medium, supplemented with 10 ng/mL rmGM-CSF, 20 ng/mL rmIL-1β, and 20 ng/mL rm tumor necrosis factor-α (TNF-α; Immuno Tools, Friesoythe, Germany). Where indicated, DEX (Sigma-Aldrich, Deisenhofen, Germany) was added during this 3-day culture period (alternative activation).
Generation of BM-DCs
BM-DCs were generated as described19 with minor modifications20 using BM-DC medium (IMDM [PAA] with 10% FCS, L-glutamine, penicillin, and streptomycin) supplemented with 20 ng/mL rmGM-CSF. Starting on day 3, DEX was added to parallel cultures. On day 10, nonadherent immature BM-DCs were harvested and further differentiated into mature BM-DCs on 100-mm2 Cellstar tissue-culture dishes (Greiner) by stimulation in the presence of 1 μg/mL LPS for 48 hours.
Flow cytometry
Cells (5 × 105) harvested on day 3 after subculture were washed in staining buffer (PBS/2% FCS). To block Fc receptor–mediated staining, cells were incubated with mouse IgG (Dianova, Hamburg, Germany) for 15 minutes on ice. Afterward, cells were incubated with the primary antibody (20 minutes, on ice). Fluorescein isothiocyanate–conjugated antibodies (Abs) recognized CD3 (clone 145-2C11), CD11b (M1/70.15), CD11c (HL3), CD14 (rmC5-3), CD16/32 (2.4G2), CD19 (1D3), CD45R/B220 (RA3-6B2), Gr-1 (RB6-8C5), MHC class II I-A/I-E (2G9) (all from BD Pharmingen, San Diego, CA), CD4 (GK1.5), CD8a (53-6.7) (Miltenyi Biotec, Bergisch Gladbach, Germany), CD49b (DX5), CD205 (NLDC 145) (Dianova), MHC class I H-2Db (CTDb; Serotec, Oxford, United Kingdom), and F4/80 (C1:A3-1; American Type Culture Collection [ATCC], Rockville, MD). R-phycoerythrin–labeled Ab against CD25 (PC61 5.3) was obtained from Caltag (Burlingame, CA), and R-phycoerythrin–labeled Ab against CD83 from eBioscience (San Diego, CA). Biotinylated Ab specific for CD120a (HM1010) and CD120b (HM1012) were obtained from HyCult (Uden, the Netherlands), and were detected using R-phycoerythrin–conjugated streptavidin (BD Pharmingen). Unlabeled Ab recognized CD45 (30-F11), CD80 (1G10) (BD Pharmingen), CD86 (GL1), CD54 (KAT-1) (Dianova), and CD40 (FGK45.5F; a kind gift from Dr A. Rolink, Pharmazentrum Basel, Switzerland). All Abs were of rat origin, except Ab to CD3 and CD11c (hamster origin) and Ab to CD120b and CD120b (mouse origin). After washing, cells were stained with FITC-labeled goat anti–rat IgG (R35-95; BD Pharmingen), where appropriate (20 minutes, on ice). For detection of the intracellular actin-bundling protein fascin, cells were first permeabilized in absolute methanol for 5 minutes before treatment with rat anti–mouse fascin (55-k2; DakoCytomation, Hamburg, Germany), and stained with FITC-labeled goat anti–rat IgG (Southern Biotechnology, Birmingham, AL). Appropriate isotype controls were used. Flow cytometric analysis was performed using a FACScan flow cytometer (BD Biosciences) equipped with CellQuest Software.
In vivo cytotoxicity assay
Mature SP37A3 cells (1 × 106/250 μL) were transferred intravenously into BALB/c mice. Later (7 days), alloantigen-specific cytotoxic activity was assessed in vivo. Syngeneic BALB/c (H-2d) spleen cells and allogeneic spleen cells from C57BL/6 (H-2b) or FVB/N (H-2q) mice were labeled for 10 minutes at 37°C with low (0.2 μM) and high (1.7 μM) concentrations, respectively, of carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Leiden, the Netherlands). After labeling, 1 × 107 cells of each population were combined and injected intravenously in a final volume of 250 μL PBS per recipient. As control, naive mice were adoptively transferred with CFSElow and CFSEhigh cells. After 12 hours, mice were killed, and single-cell suspensions of spleen and axial/inguinal lymph nodes were prepared and analyzed by flow cytometry. CFSE+ cells were gated, and the percentage of CFSEhigh allogeneic target cells was determined.
T-cell proliferation assays
Allogeneic T-cell proliferation was assayed in a mixed lymphocyte reaction (MLR). BALB/c splenocytes depleted of erythrocytes were passed over nylon wool columns to enrich T cells. T cells (3 × 105) were cocultured for the indicated time on 96-well tissue-culture plates (Corning, Cambridge, MA) with graded numbers of irradiated (30 Gy) C57BL/6 BM-DCs or SP37A3 cells in 200 μL of BM-DC or SP37A3 medium. Cell proliferation was assessed by the uptake of [3H] thymidine (0.5 μCi/well [0.0185 MBq/well]) for the last 16 hours of culture. Cells were harvested onto glass fiber filters, and retained radioactivity was measured in a liquid scintillation counter (1205 Betaplate; LKB Wallac, Turcu, Finland).
Syngeneic T-cell proliferation was assessed following antigen-specific activation of ovalbumin (OVA)–TCR-transgenic OT-II T cells. T cells were enriched from splenocytes of OT-II mice21 by immunomagnetic sorting with CD90 MicroBeads (Miltenyi Biotec) as recommended by the manufacturer. T cells (5 × 104) were cocultured with graded numbers of irradiated DCs, previously pulsed with OVA323-339 peptide (10 μg/2 × 106 cells; Peptide Protein Research, Wickham, United Kingdom) for 3 hours, in 200 μL of BM-DC or SP37A3 medium. Cell proliferation was assessed as described above.
Alternatively, OT-II T-cells (5 × 104), labeled for 10 minutes at 37°C with 1.5 μM CFSE, were cocultured with OVA323-339 peptide-pulsed DCs (104) as described. CFSE staining intensity of T cells was assessed by flow cytometry.
T-cell restimulation and suppression assays
SP37A3 cells (106/well) were cocultured on 6-well tissue culture plates in a volume of 4 mL with nylon wool–enriched BALB/c T cells (6 × 106/well). After 7 days, T cells were harvested (prestimulated T cells). C57BL/6 splenocytes were depleted of erythrocytes and were γ-irradiated (30 Gy). Splenocytes (3 × 105) were cocultured with graded numbers of prestimulated T cells in SP37A3 medium for 6 days. To test for suppressive potential, graded numbers of prestimulated T cells were added to cocultures of splenocytes and 3 × 105 freshly isolated BALB/c T cells. T-cell proliferation was assessed as described above under “T-cell proliferation assays.”
Cytokine assays
Cytokines were quantified using a sandwich enzyme-linked immunosorbent assay (ELISA) as previously described.22 ELISA capture Abs for murine IFN-γ (clone R4-6A2) and IL-4 (11B11) were purchased from BD Pharmingen; Ab for murine IL-10 (JES052A5) was obtained from R&D Systems. Biotinylated detection Abs for IFN-γ (XMG1.2) and IL-4 (BVD6-24G2) were obtained from BD Pharmingen, and Ab to IL-10 (BAF417) was obtained from R&D Systems. Abs were used as recommended by the manufacturer. Recombinant murine cytokines IFN- γ and IL-4 used for ELISA standards were purchased from BD Pharmingen, and rmIL-10 was purchased from R&D Systems.
Real-time RT-PCR analysis
Total RNA was isolated from at least 5 × 105 cells per isolation by using the RNeasy MiniPrep kit and performing on-column DNase treatment (both from Qiagen, Hilden, Germany) according to the protocol recommended by the manufacturer. Eluted RNA (10 μL) was reverse-transcribed applying a 1:1 mix of Oligo-dT and random hexamer primers by using Moloney murine leukemia virus reverse transcriptase (M-MuLV RT; Fermentas, St Leon-Rot, Germany) as recommended. Primer sequences were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi),23 and primer pairs bridging intron-exon boundaries were chosen. Primers were purchased from MWG Biotech (Ebersberg, Germany). Primer sequences were as follows: B7-H2, 5′-AGCCTCAAGAACCCCAGATT-3′ (sense), 5′-GAACCCGCTAGAAACATGGA-3′ (antisense); B7-H3, 5′-AGTCTGGCACAGCTCAACCT-3′, 5′-CAAAGTCCTGGATGCTCACA-3′; 4-1BBL, 5′-CAACAGGGCTCTCCTGTGTT-3′, 5′-TGGCCTGTGTTTGTGAATGT-3′; CD40, 5′-CCTGGCTTTGGAGTTATGGA-3′, 5′-CCGGGACTTTAAACCACAGA-3′; CD80, 5′-CCATGTCCAAGGCTCATTCT-3′, 5′-TTCCCAGCAATGACAGACAG-3′; CD83, 5′-GCCTCCAGCTCCTGTTTCTA-3′, 5′-TTGGATCGTCAGGGAATAGG-3′; CD86, 5′-CAGTTACTGTGGCCCTCCTC-3′, 5′-ACTCTGCATTTGGTTTTGCT-3′; fascin, 5′-AACCCCTTGCCTTTCAAACT-3′, 5′-CATGGAAAGAAGGGGACAGA-3′; FcγRIIB: 5′-CCAAAGGCTGTGGTCAAACT-3′, 5′-TGCTCCATTTGACACCGATA-3′; ICAM-1, 5′-TTCACACTGAATGCCAGCTC-3′, 5′-GTCTGCTGAGACCCCTCTTG-3′; IL-1β, 5′-GCCCATCCTCTGTGACTCAT-3′, 5′-AGGCCACAGGTATTTTGTCG-3′; IL-1RA, 5′-CCAGCTCATTGCTGGGTACT-3′, 5′-TTCTCAGAGCGGATGAAGGT-3′; IL-1R2, 5′-GCATCATTGGGGTCAAGACT-3′, 5′-GATCCTCCCTTGTGACTGGA-3′; IL-6, 5′-CCGGAGAGGAGACTTCACAG-3′, 5′-CAGAATTGCCATTGCACAAC-3′; IL-10, 5′-CCAAGCCTTATCGGAAATGA-3′, 5′-TTTTCACAGGGGAGAAATCG-3′; IL-12b, 5′-CATCTGCTGCTCCACAAGAA-3′, 5′-CGCCATTCCACATGTCACTGC-3′; OX40L, 5′-GCTAAGGCTGGTGGTCTCTG-3′, 5′-ACCGAATTGTTCTGCACCTC-3′; PD-L1, 5′-AGTCTCCTCGCCTGCAGATA-3′, 5′-ACCACTAACGCAAGCAGGTC-3′; PD-L2, 5′-TGTGCTGCCTTTTCTGTGTC-3′, 5′-ATTCTCTGCGGTCAAAATCG-3′; Pir-b, 5′-GAAGATCCCCAGGGAGAGAC-3′, 5′-CTGGGAGAGAGGAGATGCAG-3′; TGF-β1, 5′-TTGCTTCAGCTCCACAGAGA-3′, 5′-TGGTTGTAGAGGGCAAGGAC-3′; and ubiquitin C, 5′-GTCTGCTGTGTGAGGACTGC-3′, 5′-CAGGGTGGACTCTTTCTGGA-3′. Reaction mixtures for real-time polymerase chain reaction (PCR) had a final volume of 25 μL and included 200 ng cDNA, 70 nM of each primer, and 12.5 μL of SYBRGreen mastermix (ABgene, Hamburg, Germany). PCR conditions were 15 minutes at 95 °C, and 50 cycles at 95°C for 15 seconds and 60°C for 1 minute. Each sample was run in duplicate. In pilot experiments we tested several housekeeping genes for their potential to be used as internal control. Since ubiquitin C showed the lowest level of variability under different experimental conditions, it was chosen for internal normalization. Specificity of product amplification was confirmed by automated melting curve analysis. Relative quantification of gene expression was performed according to the comparative threshold cycle method.24
Statistical analysis
Data were analyzed for statistically significant differences by applying the Student t test.
Results
SP37A3 cells display a DC-like phenotype
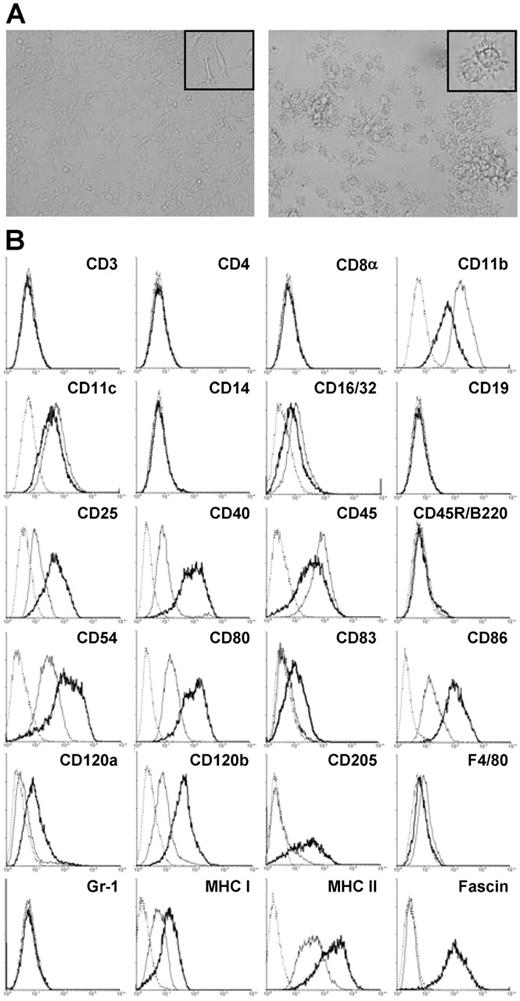
Several growth factor–dependent cell lines were obtained by long-term culture of spleen cells from several mouse strains in the presence of GM-CSF and M-CSF, of which cell line SP37A3 of C57BL/6 origin was characterized in more detail. Immature SP37A3 cells grew adherent and displayed filopodial extensions interacting with those of neighboring cells (Figure 1A; left panel). Phenotypic characterization by flow cytometry revealed that SP37A3 cells constitute DCs of myeloid origin: they expressed the typical murine DC marker CD11c25 and lacked markers of pDCs (CD45R/B220, GR-1) (Figure 1B). The cells did not express CD83. In accordance with the sentinel function of immature DCs,26 SP37A3 cells expressed receptors enabling antigen uptake and processing (CD11b, CD16/32, and CD205). Indicative of T-cell stimulatory capacity, they expressed MHC I (H-2K) and MHC II (H-2I-A/I-E) as well as costimulatory molecules (CD40, CD80, and CD86) and the accessory molecule CD54 involved in DC–T-cell interaction. Responsiveness to proinflammatory cytokines was indicated by the expression of both TNF-α receptors (CD120a, CD120b). In accordance with previous reports demonstrating expression of the low-affinity IL-2 receptor by DCs,27 SP37A3 cells expressed CD25. They lacked markers characteristic of lympoid cell types, including T cells (CD3, CD4, and CD8α) and B cells (CD19, CD45R/B220) as well as characteristic markers of the monocytic/macrophage lineage (CD14, F4/80).
Morphology and phenotype of SP37A3 cells. SP37A3 cells were kept at an immature proliferative state by addition of GM-CSF and M-CSF. To induce maturation, cells were cultivated for 3 days with GM-CSF and proinflammatory cytokines IL-1β and TNF-α. (A) Images of immature (left panel) and mature (right panel) SP37A3 cells were taken at room temperature with cells kept in the respective culture medium (see above) at ×20 magnification (UPlanFI 20×/0.50, 40×/0.75; Olympus Optical, Hamburg, Germany) using an Olympus IX70 inverted microscope (Olympus Optical) equipped with a DXC-950P digital camera (Sony Electronics, NJ) and AnalySIS 3.00 software (Olympus Soft Imaging Solutions, Münster, Germany). Representative sections are shown at ×40 magnification (UPlanFI 40×/0.75; Olympus Optical) in the inserts. Colors, brightness, and contrast of images were adjusted using PhotoShop 5.0 (Adobe Systems, Saggart, Ireland). (B) Cells were stained with mAbs specific for the indicated cell-surface markers. Histograms represent specific mAb binding to immature (thin line) and mature (thick line) SP37A3 cells, as well as binding of isotype-matched control mAb (dotted line). Graphs are representative of at least 2 independent experiments.
Morphology and phenotype of SP37A3 cells. SP37A3 cells were kept at an immature proliferative state by addition of GM-CSF and M-CSF. To induce maturation, cells were cultivated for 3 days with GM-CSF and proinflammatory cytokines IL-1β and TNF-α. (A) Images of immature (left panel) and mature (right panel) SP37A3 cells were taken at room temperature with cells kept in the respective culture medium (see above) at ×20 magnification (UPlanFI 20×/0.50, 40×/0.75; Olympus Optical, Hamburg, Germany) using an Olympus IX70 inverted microscope (Olympus Optical) equipped with a DXC-950P digital camera (Sony Electronics, NJ) and AnalySIS 3.00 software (Olympus Soft Imaging Solutions, Münster, Germany). Representative sections are shown at ×40 magnification (UPlanFI 40×/0.75; Olympus Optical) in the inserts. Colors, brightness, and contrast of images were adjusted using PhotoShop 5.0 (Adobe Systems, Saggart, Ireland). (B) Cells were stained with mAbs specific for the indicated cell-surface markers. Histograms represent specific mAb binding to immature (thin line) and mature (thick line) SP37A3 cells, as well as binding of isotype-matched control mAb (dotted line). Graphs are representative of at least 2 independent experiments.
Upon stimulation with IL-1β and TNF-α for 3 days in the presence of GM-CSF, SP37A3 cells stopped growing, detached, and acquired features characteristic of mature DCs, like formation of numerous dendritic protrusions and cell aggregation (Figure 1A; right panel). In accordance with the maturation process of DCs,25,28 stimulated SP37A3 cells up-regulated CD83 and down-regulated the expression of molecules involved in antigen uptake (CD11b, CD16/32; Figure 1B). As previously shown, CD205 is regulated inversely in maturing DCs,29 and was up-regulated in matured SP37A3 cells as well. In addition, the expression of receptors for TNF-α promoting maturation of DCs was up-regulated (CD120a, CD120b). Expression of CD25 was up-regulated as well, indicating responsiveness of DCs to IL-2 derived from activated T cells.27 Elevated levels of surface MHC I and MHC II, costimulatory molecules (CD40, CD80, and CD86), and CD54 suggested increased T-cell stimulatory capacity. In addition, the actin-bundling protein fascin, previously shown by us to be essential for formation of dendritic processes30,31 and serving as a general marker of DC maturation, was strongly up-regulated in mature cells.
Mature SP37A3 cells exert potent T-cell activation
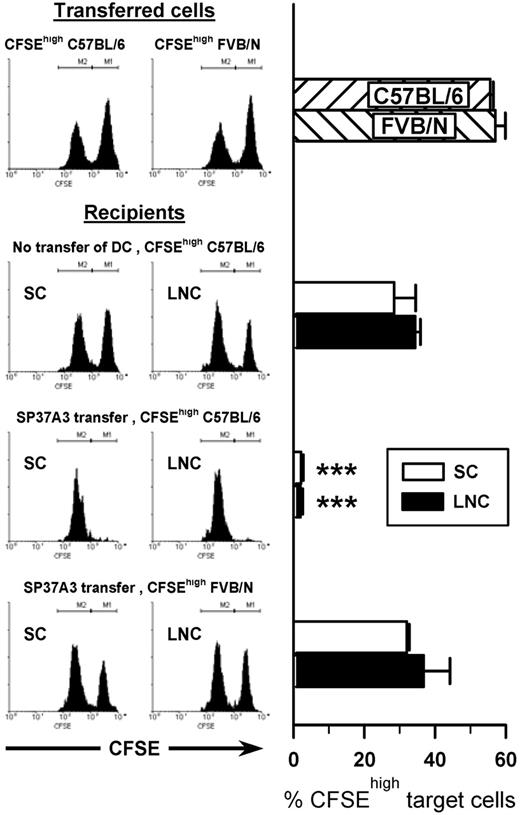
To confirm the DC-like phenotype of SP37A3 cells on a functional level, T-cell stimulatory potential was assessed by their capacity to induce in vivo cytotoxic activity. After injection of mature SP37A3 cells into BALB/c mice (7 days), a 1:1 mixture of differentially CFSE-labeled BALB/c and C57BL/6 splenocytes was given intravenously. SP37A3 cells induced strong in vivo cytotoxic activity that was antigen-specific, because third-party FVB/N splenocytes were not efficiently killed (Figure 2).
SP37A3 cells stimulate alloantigen-specific cytotoxic T cells in vivo. Mature SP37A3 cells (1 × 106) were transferred intravenously into BALB/c mice. After 7 days, equal numbers (1 × 107) of syngeneic CFSElow-labeled BALB/c spleen cells and allogeneic CFSEhigh-labeled spleen cells from C57BL/6 and FVB/N mice, respectively, were mixed and injected intravenously into recipient mice or naive mice as control. In vivo cytotoxicity against the allogeneic target cells was assessed after 12 hours. Spleen cells (SCs) and lymph node cells (LNCs) were collected, and the proportion of CFSEhigh target cells as a percentage of total CFSE+ cells was determined by flow cytometry. Histograms show representative data from individual mice; bars represent mean ± SEM of 6 mice per group, obtained in 2 independent experiments. ***P < .001 compared with naive mice receiving CFSEhigh C57BL/6 target cells.
SP37A3 cells stimulate alloantigen-specific cytotoxic T cells in vivo. Mature SP37A3 cells (1 × 106) were transferred intravenously into BALB/c mice. After 7 days, equal numbers (1 × 107) of syngeneic CFSElow-labeled BALB/c spleen cells and allogeneic CFSEhigh-labeled spleen cells from C57BL/6 and FVB/N mice, respectively, were mixed and injected intravenously into recipient mice or naive mice as control. In vivo cytotoxicity against the allogeneic target cells was assessed after 12 hours. Spleen cells (SCs) and lymph node cells (LNCs) were collected, and the proportion of CFSEhigh target cells as a percentage of total CFSE+ cells was determined by flow cytometry. Histograms show representative data from individual mice; bars represent mean ± SEM of 6 mice per group, obtained in 2 independent experiments. ***P < .001 compared with naive mice receiving CFSEhigh C57BL/6 target cells.
In a further step, the T-cell stimulatory capacity of SP37A3 cells was compared with that of BM-DCs in an MLR. In their mature state, SP37A3 cells were similarly potent as BM-DCs in activating naive allogeneic T cells (Figure 3A-B). Unlike immature BM-DCs, which were poor T-cell stimulators, unstimulated SP37A3 cells displayed some allogeneic T-cell–activating potential at higher cell numbers. This might be due to single mature cells arising spontaneously during culture of SP37A3 cells.
The allogeneic T-cell stimulatory capacity of SP37A3 cells depends on their state of differentiation. Titrated numbers of irradiated DCs were cocultured with 3 × 105 nylon wool–enriched T cells of BALB/c mice for 4 days in triplicate cultures. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. (A) BM-DCs were used either at an immature state, or were matured by addition of LPS (1 μg/mL for 48 hours). In parallel cultures, DEX was applied at 10−6 M and 10−5 M starting on day 3 of culture, and stimulation with LPS was performed as described. (B) Immature and mature SP37A3 cells were generated as described (Figure 1). In parallel cultures, DEX was coapplied during induction of maturation. Data represent mean ± SEM of triplicate cultures and are representative of 4 (A) and 5 (B) independent experiments. *P < .05 compared with immature and DEX-cotreated DCs (10−5 M, 10−6 M); +P < .05 compared with DEX-cotreated DCs (10−5 M, 10−6 M). For the sake of clarity, indication of distinct levels of significance was omitted. (C) A micrograph of SP37A3 cells stimulated in the presence of 10−5 M DEX, as described in panel B. The image was captured as described in Figure 1. (D) The relative allogeneic T-cell stimulatory capacity of differentially conditioned SP37A3 cells was determined. Experiments were performed as described for panel B. Results obtained with 1.7 × 104 immature, mature, and DEX-treated SP37A3 cells are shown. The respective T-cell proliferation induced by mature SP37A3 cells was set to 1 (horizontal dashed line). Data represent mean ± SEM of 5 independent experiments. *P < .05; **P < .01; ***P < .001.
The allogeneic T-cell stimulatory capacity of SP37A3 cells depends on their state of differentiation. Titrated numbers of irradiated DCs were cocultured with 3 × 105 nylon wool–enriched T cells of BALB/c mice for 4 days in triplicate cultures. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. (A) BM-DCs were used either at an immature state, or were matured by addition of LPS (1 μg/mL for 48 hours). In parallel cultures, DEX was applied at 10−6 M and 10−5 M starting on day 3 of culture, and stimulation with LPS was performed as described. (B) Immature and mature SP37A3 cells were generated as described (Figure 1). In parallel cultures, DEX was coapplied during induction of maturation. Data represent mean ± SEM of triplicate cultures and are representative of 4 (A) and 5 (B) independent experiments. *P < .05 compared with immature and DEX-cotreated DCs (10−5 M, 10−6 M); +P < .05 compared with DEX-cotreated DCs (10−5 M, 10−6 M). For the sake of clarity, indication of distinct levels of significance was omitted. (C) A micrograph of SP37A3 cells stimulated in the presence of 10−5 M DEX, as described in panel B. The image was captured as described in Figure 1. (D) The relative allogeneic T-cell stimulatory capacity of differentially conditioned SP37A3 cells was determined. Experiments were performed as described for panel B. Results obtained with 1.7 × 104 immature, mature, and DEX-treated SP37A3 cells are shown. The respective T-cell proliferation induced by mature SP37A3 cells was set to 1 (horizontal dashed line). Data represent mean ± SEM of 5 independent experiments. *P < .05; **P < .01; ***P < .001.
Similar to these results, mature SP37A3 cells like BM-DCs, when pulsed with OVA323-339 peptide, were potent stimulators of naive syngeneic OVA-TCR–transgenic OT-II T cells as determined by incorporation of tritiated thymidine (Figure 4A) and by measurement of CFSE staining intensity (Figure 4B). Both immature SP37A3 cells and BM-DCs pulsed with OVA323-339 peptide induced significantly less OT-II T-cell proliferation.
The protein antigen-specific T-cell stimulatory capacity of SP37A3 cells correlates with their state of maturation. OVA-specific T-cell stimulatory capacity of differentially conditioned BM-DCs (left panels) and SP37A3 cells (right panels) was assessed by determining proliferation of OT-II T cells. DCs were differentiated as described (Figures 1,3) and were pulsed for 3 hours with 10 μg/mL OVA323-339 peptide or medium and were irradiated. (A) Titrated numbers of DCs were cocultured with 5 × 104 OT-II T cells for 3 days in triplicate cultures. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. Data represent mean ± SEM of triplicate cultures and are representative of 3 independent experiments. (B) DCs (3 × 103) were cocultured with CFSE-labeled T cells (5 × 104) for 3 days, and T-cell proliferation was determined as relative CFSE staining intensity by flow cytometry. Graphs are representative of 3 independent experiments. *P < .05 compared with immature DCs and DCs matured in the presence of DEX (10−5 M); +P < .05 compared with alternatively activated DCs (10−5 M).
The protein antigen-specific T-cell stimulatory capacity of SP37A3 cells correlates with their state of maturation. OVA-specific T-cell stimulatory capacity of differentially conditioned BM-DCs (left panels) and SP37A3 cells (right panels) was assessed by determining proliferation of OT-II T cells. DCs were differentiated as described (Figures 1,3) and were pulsed for 3 hours with 10 μg/mL OVA323-339 peptide or medium and were irradiated. (A) Titrated numbers of DCs were cocultured with 5 × 104 OT-II T cells for 3 days in triplicate cultures. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. Data represent mean ± SEM of triplicate cultures and are representative of 3 independent experiments. (B) DCs (3 × 103) were cocultured with CFSE-labeled T cells (5 × 104) for 3 days, and T-cell proliferation was determined as relative CFSE staining intensity by flow cytometry. Graphs are representative of 3 independent experiments. *P < .05 compared with immature DCs and DCs matured in the presence of DEX (10−5 M); +P < .05 compared with alternatively activated DCs (10−5 M).
Similar to BM-DCs, SP37A3 cells mediate hypoproliferation of T cells when matured in the presence of DEX
Given these properties, SP37A3 cells may serve as a model to study the functional characteristics of DCs in a tolerogenic state. Treatment of DCs with the synthetic glucocorticoid DEX was demonstrated to modulate DC maturation and function.32,33 SP37A3 cells and BM-DCs treated with DEX during stimulation displayed an overall similar morphology to that of cells matured in the absence of DEX, although the formation of dendritic protrusions was markedly reduced in SP37A3 cells (Figure 3C) and BM-DCs (data not shown).
Treatment of SP37A3 cells with DEX during stimulation, similar to BM-DCs, significantly impaired their allogeneic T-cell stimulatory capacity. BM-DCs matured in the presence of DEX at 10−6 and 10−5 M were as poor allogeneic T-cell stimulators as in their immature state (Figure 3A). Likewise, the T-cell stimulatory capacity of DEX-treated SP37A3 cells was decreased in a dose-dependent manner (Figure 3B,D). In addition, coapplication of DEX during stimulation of SP37A3 cells similar to that of BM-DCs prevented the acquisition of strong stimulatory potency for OT-II T cells (Figure 4). Due to their impaired T-cell stimulatory capacity, SP37A3 cells cotreated with DEX during stimulation with IL-1β and TNF-α were considered “alternatively activated.”
Alternatively activated SP37A3 cells induce regulatory T cells
Since distinctly activated SP37A3 cells showed significantly divergent T-cell stimulatory capacities, we assessed the cytokine profiles of T cells cocultured with differentially treated SP37A3 cells for 7 days. Stimulation of allogeneic BALB/c T cells with mature SP37A3 cells resulted in strong expression of the T helper 1 (Th1) marker cytokine IFN-γ (Figure 5A). Allogeneic T cells primed by immature or alternatively activated SP37A3 cells produced significantly less IFN-γ, with T cells cocultured with alternatively activated SP37A3 cells showing the lowest IFN-γ expression level. T cells stimulated by mature DCs produced the highest amount of the Th2 marker cytokine IL-4, while T cells cocultured with immature or alternatively activated SP37A3 cells secreted less IL-4, which was statistically significant in the case of immature SP37A3 cells. Notably, T cells stimulated by mature or alternatively activated SP37A3 cells displayed comparable secretion levels of the Th2/Treg cytokine IL-10 that were significantly higher compared with T cells cocultured with immature SP37A3 cells.
Alternatively activated SP37A3 cells exhibit tolerogenic potential. (A) SP37A3 cells were differentiated as described (Figures 1,3). Irradiated cells (2.5 × 105) were cocultured for 7 days with 106 nylon wool–enriched allogeneic BALB/c T cells in 1 mL culture medium. Culture supernatants were harvested and assayed by ELISA for contents of IFN-γ, IL-4, and IL-10. Data represent mean ± SEM of at least 3 experiments. *P < .05. (B-D) Differentially conditioned (Figures 1,3) irradiated SP37A3 cells (106) were cocultured with 6 × 106 nylon wool–enriched T cells of BALB/c mice in 4 mL volume in wells of 6-well cluster plates for 7 days, and T cells were harvested afterward. (B) T cells (105) primed by immature, mature, or alternatively activated SP37A3 cells were restimulated with irradiated C57BL/6 spleen cells (105) in 0.2 mL culture medium for 4 days. (C-D) Graded numbers of T cells primed by immature, mature, or alternatively activated SP37A3 cells (pTCs) were cocultured with 3 × 105 naive BALB/c T cells (nTCs) and 3 × 105 C57BL/6 spleen cells in 0.2 mL volume for 6 days in triplicate cultures. To account for the doubled number of T cells used in suppression assays (pTC + nTC), T-cell proliferation of 6 × 105 naive T cells (2 × nTC) was determined. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. Data represent mean ± SEM of triplicate cultures and are representative of 2 (B) and 3 (C) independent experiments. (D) Assessment of suppressive activity of T cells primed by alternatively activated SP37A3 cells (pTC; 3 × 105). The respective proliferation of naive T cells (nTC; 3 × 105) induced by C57BL/6 spleen cells was arbitrarily set to 1. Data repesent mean ± SEM of 3 independent experiments. *P < .05; **P < .001; ***P < .001 compared with 3 × 105 nTCs (C) or between indicated groups (B,D).
Alternatively activated SP37A3 cells exhibit tolerogenic potential. (A) SP37A3 cells were differentiated as described (Figures 1,3). Irradiated cells (2.5 × 105) were cocultured for 7 days with 106 nylon wool–enriched allogeneic BALB/c T cells in 1 mL culture medium. Culture supernatants were harvested and assayed by ELISA for contents of IFN-γ, IL-4, and IL-10. Data represent mean ± SEM of at least 3 experiments. *P < .05. (B-D) Differentially conditioned (Figures 1,3) irradiated SP37A3 cells (106) were cocultured with 6 × 106 nylon wool–enriched T cells of BALB/c mice in 4 mL volume in wells of 6-well cluster plates for 7 days, and T cells were harvested afterward. (B) T cells (105) primed by immature, mature, or alternatively activated SP37A3 cells were restimulated with irradiated C57BL/6 spleen cells (105) in 0.2 mL culture medium for 4 days. (C-D) Graded numbers of T cells primed by immature, mature, or alternatively activated SP37A3 cells (pTCs) were cocultured with 3 × 105 naive BALB/c T cells (nTCs) and 3 × 105 C57BL/6 spleen cells in 0.2 mL volume for 6 days in triplicate cultures. To account for the doubled number of T cells used in suppression assays (pTC + nTC), T-cell proliferation of 6 × 105 naive T cells (2 × nTC) was determined. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. Data represent mean ± SEM of triplicate cultures and are representative of 2 (B) and 3 (C) independent experiments. (D) Assessment of suppressive activity of T cells primed by alternatively activated SP37A3 cells (pTC; 3 × 105). The respective proliferation of naive T cells (nTC; 3 × 105) induced by C57BL/6 spleen cells was arbitrarily set to 1. Data repesent mean ± SEM of 3 independent experiments. *P < .05; **P < .001; ***P < .001 compared with 3 × 105 nTCs (C) or between indicated groups (B,D).
To test whether hypoproliferation of alloreactive T cells cocultured with alternatively activated DCs was due to the induction of T-cell anergy, T cells were harvested at the end of a 7-day primary MLR and restimulated with allogeneic C57BL/6 spleen cells. While T cells prestimulated with mature SP37A3 cells proliferated well, T cells primed by immature and alternatively activated SP37A3 cells were refractory to restimulation (Figure 5B). Since anergy is a key characteristic of Treg's,2 we tested T cells anergized by prestimulation with alternatively activated SP37A3 cells for potential suppressor activity. In coculture experiments, anergized T cells significantly inhibited proliferation of naive T cells in a dose-dependent manner (Figure 5C-D), and therefore resemble induced Treg's.
Alternative activation of SP37A3 cells impairs up-regulation of maturation-induced molecules required for T-cell activation
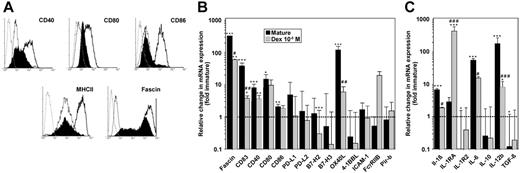
The tolerogenic function of DCs results from the impaired delivery of costimulatory signals to antigen-specific T cells. Therefore, we analyzed surface expression of several important costimulatory molecules involved in DC–T-cell interactions. Compared with mature SP37A3 cells, alternatively activated DCs showed reduced expression of CD40, CD80, and CD86 (Figure 6A). Moreover, maturation-induced up-regulation of MHC II and the DC activation marker fascin was largely impaired in alternatively activated SP37A3 cells.
Alternatively activated SP37A3 cells display a semimature phenotype. SP37A3 cells were differentiated as described (Figures 1,3). (A) Histograms represent expression of the markers indicated by immature (thin line), mature (thick line), and DEX-cotreated SP37A3 cells (filled histograms), and isotype-matched controls (dotted line). Graphs are representative of 3 independent experiments. (B-C) The relative mRNA expression level of genes encoding intracellular and transmembrane (B) and secreted (C) products was assessed by quantitative real-time PCR. The housekeeping gene ubiquitin C was used for internal normalization. Relative gene expression levels were determined by applying the comparative threshold cycle method. Data indicate fold changes in mRNA expression by mature and alternatively activated SP37A3 cells, respectively, versus immature cells (dashed line). Data represent the mean ± SEM of 3 to 6 experiments performed in duplicate. *Statistically significant differences in mRNA expression in mature or DEX-cotreated versus immature SP37A3 cells; statistically significant differences in mRNA expression in alternatively activated versus mature SP37A3 cells (*P < .05; #P < .05; **P < .01; ##P < .01; ***P < .001; ###P < .001).
Alternatively activated SP37A3 cells display a semimature phenotype. SP37A3 cells were differentiated as described (Figures 1,3). (A) Histograms represent expression of the markers indicated by immature (thin line), mature (thick line), and DEX-cotreated SP37A3 cells (filled histograms), and isotype-matched controls (dotted line). Graphs are representative of 3 independent experiments. (B-C) The relative mRNA expression level of genes encoding intracellular and transmembrane (B) and secreted (C) products was assessed by quantitative real-time PCR. The housekeeping gene ubiquitin C was used for internal normalization. Relative gene expression levels were determined by applying the comparative threshold cycle method. Data indicate fold changes in mRNA expression by mature and alternatively activated SP37A3 cells, respectively, versus immature cells (dashed line). Data represent the mean ± SEM of 3 to 6 experiments performed in duplicate. *Statistically significant differences in mRNA expression in mature or DEX-cotreated versus immature SP37A3 cells; statistically significant differences in mRNA expression in alternatively activated versus mature SP37A3 cells (*P < .05; #P < .05; **P < .01; ##P < .01; ***P < .001; ###P < .001).
Although SP37A3 cells stimulated in the presence of DEX displayed higher surface-expression levels of costimulatory molecules than in their immature state, they exerted a tolerogenic function. In order to characterize the underlying molecular events in more detail, we assessed the transcriptional profiles of immature, mature, and DEX-treated SP37A3 cells (Figure 6B-C). Corresponding to protein levels, mRNA expression of fascin and CD83 was strongly up-regulated in mature SP37A3 cells, but showed significantly less increase in DEX-treated SP37A3 cells. In broad accordance with their respective surface expressions, mature SP37A3 cells displayed significant increases in the mRNA expression of CD40, CD80, and CD86. Interestingly, while alternatively activated SP37A3 cells showed lower cell-surface expression of the respective proteins than mature SP37A3 cells, they displayed similar mRNA expression levels. Expression of other molecules of the B7 family (PD-L1, PD-L2, B7-H2, and B7-H3), the costimulatory molecule 4-1BBL, the adhesion molecule ICAM-1, and Pir-b was not affected by maturation or alternative activation, with the exception of B7-H2, which was significantly down-regulated in alternatively activated SP37A3 cells. On the other hand, the costimulatory molecule OX40L was drastically up-regulated in mature SP37A3 cells only. In contrast, FcγRIIB displayed selective up-regulation in DEX-treated SP37A3 cells.
Concerning the maturation-dependent regulation of T-cell–polarizing cytokines, mRNA levels of IL-6 and IL-12b (p40) were significantly up-regulated in mature SP37A3 cells, but significantly less so in alternatively activated SP37A3 cells (Figure 6C). Conversely, we noted a significant increase of mRNA expression of the anti-inflammatory cytokine IL-1RA in alternatively activated SP37A3 cells. Interestingly, transcript levels of IL-1R2 and TGF-β1 were significantly reduced in mature SP37A3 cells only. Transcript levels of IL-1β and IL-10 were not significantly modulated.
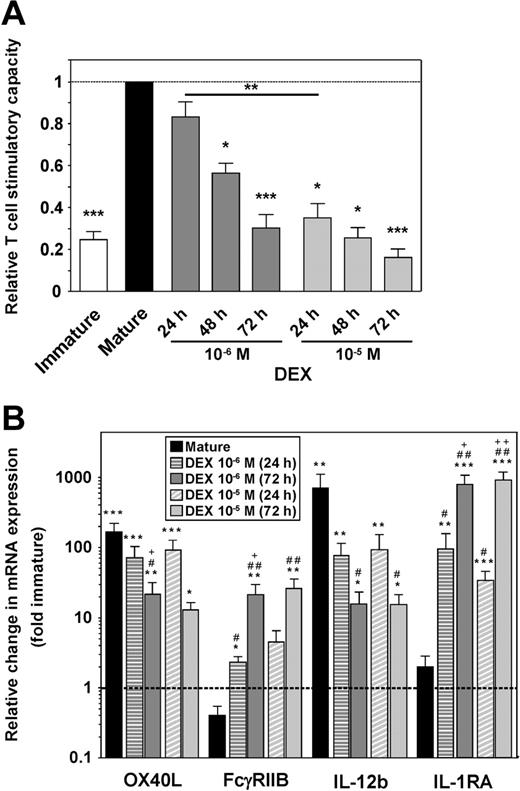
SP37A3 cells display functional plasticity in response to DEX during stimulation in a dose- and time-dependent manner
To assess the functional plasticity of SP37A3 cells in the course of differentiation, DEX was added at 2 different concentrations (10−6 M and 10−5 M) either at the onset of stimulation with IL-1β and TNF-α or at later time points. In MLRs, the T-cell stimulatory capacity of SP37A3 cells correlated inversely with the dose of DEX applied and the duration of DEX cotreatment (Figure 7A). In accordance with the functional impairment of alternatively activated SP37A3 cells, the level of maturation-induced up-regulation of OX40L mRNA was significantly impaired after 72 hours of cotreatment with 10−6 M DEX, and of IL-12b mRNA with either dose of DEX (Figure 7B). Moreover, OX40L mRNA expression inversely correlated with the duration of application of 10−6 M DEX. Compared with immature SP37A3 cells, the expression levels of FcγRIIB and IL-1RA were significantly increased in alternatively activated but not mature SP37A3 cells, and correlated with the duration of DEX treatment for 10−6 M concentration in case of FcγRIIB, and for both concentrations in case of IL-1RA.
SP37A3 cells retain functional plasticity during maturation. SP37A3 cells were differentiated as described (Figures 1,3), with DEX added at 2 different concentrations (10−6 M, 10−5 M) for the last 24 or 48 hours of the 3-day activation period with TNF-α and IL-1β, or for the entire 72 hours. Conditioned SP37A3 cells were subjected to allogeneic MLR (Figure 3) (A) and quantitative real-time PCR (Figure 6B-C) (B). (A) Proliferation of BALB/c T cells induced by mature SP37A3 cells was set to 1 (dashed line). Data represent mean ± SEM of 4 experiments performed in triplicate. Asterisks indicate statistically significant (*P < .05; **P < .001; ***P < .001) differences compared with proliferation induced by mature SP37A3 cells or between the indicated groups. (B) Data indicate fold changes in mRNA expression by mature and alternatively activated SP37A3 cells, versus immature cells (dashed line). Data represent mean ± SEM of 3 experiments performed in duplicate. *Statistically significant differences in mRNA expression by mature or alternatively activated versus immature SP37A3 cells; #statistically significant differences in mRNA expression by DEX-cotreated versus mature SP37A3 cells; +statistically significant differences in mRNA expression by SP37A3 cells cotreated with the same dose of DEX for 72 versus 24 hours (*P < .05; #P < .05; +P < .05; **P < .01; ##P < .01; ++P < .01; ***P < .001).
SP37A3 cells retain functional plasticity during maturation. SP37A3 cells were differentiated as described (Figures 1,3), with DEX added at 2 different concentrations (10−6 M, 10−5 M) for the last 24 or 48 hours of the 3-day activation period with TNF-α and IL-1β, or for the entire 72 hours. Conditioned SP37A3 cells were subjected to allogeneic MLR (Figure 3) (A) and quantitative real-time PCR (Figure 6B-C) (B). (A) Proliferation of BALB/c T cells induced by mature SP37A3 cells was set to 1 (dashed line). Data represent mean ± SEM of 4 experiments performed in triplicate. Asterisks indicate statistically significant (*P < .05; **P < .001; ***P < .001) differences compared with proliferation induced by mature SP37A3 cells or between the indicated groups. (B) Data indicate fold changes in mRNA expression by mature and alternatively activated SP37A3 cells, versus immature cells (dashed line). Data represent mean ± SEM of 3 experiments performed in duplicate. *Statistically significant differences in mRNA expression by mature or alternatively activated versus immature SP37A3 cells; #statistically significant differences in mRNA expression by DEX-cotreated versus mature SP37A3 cells; +statistically significant differences in mRNA expression by SP37A3 cells cotreated with the same dose of DEX for 72 versus 24 hours (*P < .05; #P < .05; +P < .05; **P < .01; ##P < .01; ++P < .01; ***P < .001).
Discussion
Both myeloid and plasmacytoid DCs contribute to the maintainance of peripheral T-cell tolerance against self-antigens as well as harmless environmental antigens (reviewed by Smits et al34 ). Since autoimmune diseases and allergies arise from the induction of immune responses directed against such antigens, recent work has focused on the generation of tolerogenic DCs that present the relevant antigen to specific T cells in order to reestablish tolerance to these antigens.7 Similarly, aiming at suppressing transplant rejection, tolerogenic donor and host DCs have been used to induce tolerance in transplant recipients.35
Various methods of inducing the differentiation of DCs into a semimature tolerogenic phenotype in vitro have been established, including the use of anti-inflammatory cytokines (IL-10, TGF-β) or pharmacologic inhibitors, all acting at least in part by inhibiting NF-κB.6,7 However, all of the compounds used to generate tolerogenic DCs exert pleiotropic effects, and include unwanted side effects like reduced viability, impaired migratory behavior, and reduced antigen presentation due to lowered expression of MHC molecules.36,37 In order to minimize these effects, current work focuses on the identification and selective modulation of key molecules which actively mediate the overall tolerogenic phenotype of the conditioned DC. The identification of effector molecules for tolerizing DCs by transcriptional and translational profiling prompted the use of homogenous DC populations that react uniformly to the tolerizing treatment. Both isolated primary DCs and progenitor-derived DCs consist of different subpopulations, thus the requirement to sort these prior to analysis.11
Therefore, a DC cell line with plasticity in differentiation/maturation would be of great advantage. In this study we have extensively characterized the newly established murine myeloid DC cell line SP37A3. SP37A3 cells resembled immature DCs during propagation, but readily matured when stimulated with proinflammatory cytokines. In accordance with the expression pattern of surface receptors and proinflammatory cytokines, immature SP37A3 cells stimulated naive T cells only to a low extent, while mature cells were equally potent as LPS-stimulated BM-DCs in activating naive T cells. Therefore, the SP37A3 cell line constitutes a suitable model to study the functions of myeloid DCs at either stage of differentiation. In more detail, we tested the possibility to induce a tolerogenic state in SP37A3 cells.
We chose the anti-inflammatory and immunosuppressive drug DEX as tolerizing agent because glucocorticoids are generated endogenously as a negative feedback regulatory mechanism upon inflammation by cells of the adrenal cortex.38 In clinical practice, glucocorticoids are frequently prescribed in the treatment of autoimmune diseases as well as severe allergic diseases due to their anti-inflammatory effects exerted in a variety of cell types.39 Glucocorticoids diffuse through the cellular plasma membrane and bind to the glucocorticoid receptor (GR). Upon binding of its ligand, the activated GR dissociates from its multimeric retention complex and translocates into the nucleus, where it binds to glucocorticoid response elements, and thereby induces or represses expression of genes.40 In addition, activated GR interferes with inflammatory gene expression due to direct protein-protein interaction with members of the NF-κB and AP-1 transcription factor families. Moreover, activated GR inhibits the respective upstream signaling cascades on the level of IKK and JNK activation.
In accordance with the essential role of NF-κB and AP-1 for the maturation of DCs,41,42 impairment of maturation and T-cell stimulatory capacity of both human and murine DCs treated with glucocorticoids has been shown in several studies.32,33 In agreement with these findings, DCs tolerized upon alternative activation by cotreatment with DEX during stimulation displayed reduced up-regulation of MHC II, costimulatory molecules (CD40, CD80, CD86, and OX40L), and proinflammatory cytokines (IL-6, IL-12b) on mRNA and/or protein levels compared with mature cells. Likewise, fascin expression was decreased, which corresponded with the markedly reduced formation of dendrites. Although expression levels for several of these molecules were considerably increased compared with immature SP37A3 cells, alternatively activated SP37A3 cells were as weak T-cell stimulators as immature SP37A3 cells, both in a soluble protein antigen-specific context (OVA) as well as in an allogeneic setting. Impaired T-cell stimulatory capacity was due to a tolerogenic function, since alloreactive T cells were refractory to restimulation. This result corresponds with a previous report demonstrating induction of anergy in human T cells after coculture with antigen-pulsed DCs matured in the presence of DEX.43 Furthermore, we demonstrated that these anergized T cells exerted suppressive activity because they inhibited proliferation of naive T cells upon allogeneic stimulation. This finding is in agreement with a study showing induction of Treg's as a consequence of stimulation with allogeneic DEX-treated immature BM-DCs in rats.44 However, in contrast to the latter study, which used DCs treated with DEX without applying a maturation stimulus, we used DCs induced to mature by IL-1β and TNF-α while treated with DEX. We showed that Treg's induced by stimulation with these alternatively activated DCs produced considerable amounts of IL-10 but no IFN-γ. Consistently, in murine CD4+ T cells prestimulated with either mature BM-DCs or BM-DCs treated with DEX during maturation, Matyszak et al45 observed IL-10 secretion levels that were comparable to those of T cells primed with mature DCs, but significantly decreased amounts of IFN-γ.
Several regulatory DC subsets are capable of inducing IL-10–producing Treg's. Both in humans and mice, plasmacytoid DCs have been shown to induce IL-10–producing CD4+46 and CD8+47 Treg's. Furthermore, human immature myeloid DCs have been shown to induce CD4+48 and CD8+49,50 Treg's, the latter mediating suppression by IL-10. In mice, Wakkach et al51 identified a DC subpopulation with plasmacytoid-like morphology and a rather immature phenotype that secreted high amounts of IL-10 upon activation, and was capable of inducing IL-10–producing CD4+ Treg's. Other subsets of tolerogenic DCs may induce Treg's with different characteristics. For example, in humans, treatment of monocyte-derived DCs with IL-10 resulted in the induction of CD4+ and CD8+ Treg's that inhibited proliferation of other T cells in a contact-dependent manner.52 In general, tolerogenic DCs of immature or semi-ature states are characterized by low to moderate expression levels of costimulatory molecules and cytokines,1,34 while tolerogenic steady-state DCs with a mature phenotype that were isolated from mucosal sites with a tolerizing micromilieu produced high levels of IL-10. However, the actual effector molecules of tolerogenic DCs are still largely unknown.
In several studies, analysis of the gene-expression profile of human DCs, tolerized by cultivation with IL-10, revealed suppression of proinflammatory genes53 and up-regulation of receptors with coinhibitory potential.54 Selective modulation of such key molecules in DCs may be sufficient to render them tolerogenic and induce T-cell tolerance. Since alternatively activated SP37A3 cells, despite moderate up-regulation of some costimulatory molecules, were as weak T-cell stimulators as immature SP37A3 cells, their tolerogenic phenotype may be attributed at least in part to DEX-induced overexpression of molecules with coinhibitory function. In this regard, we noted up-regulated transcript expression of the inhibitory receptor FcγRIIB. As revealed by studies in knock-out mice, expression of FcγRIIB is essential for the maintainance of tolerogenic function of DCs under homeostatic conditions due to binding of local IgG in mucosa-draining lymph nodes.55 In addition, we detected enhanced mRNA expression of the anti-inflammatory cytokine IL-1RA in alternatively activated SP37A3 cells. IL-1RA counteracts the activity of IL-1 due to competitive binding to the IL-1 receptor. Overexpression of IL-1RA may promote tolerance by interfering with IL-1–induced maturation of DCs56,57 as well as T-cell stimulation and polarization.58,59 Consistently, in clinical trials, application of IL-1RA proved efficient in the treatment of rheumatoid arthritis,60 and has been shown to prevent allergic reactions61 and to prolong allograft survival.62 Comprehensive transcriptional profiling may be used to identify other molecules up-regulated in SP37A3 cells upon DEX-treatment during stimulation. Overexpression of such candidate factors in DCs may reveal their ability to induce a tolerogenic phenotype in these cells.
In conclusion, the murine cell line SP37A3 constitutes a suitable model to study DC functions at immature, mature, and inducible tolerogenic states. To our knowledge, this is the first study demonstrating induction of Treg's in vitro by using conditioned DC-line cells. Since SP37A3 cells readily proliferate in their immature state, large-scale preparation of conditioned DCs and therefore stimulation of great numbers of T cells is possible in a short time. This may overcome limitations arising when studying characteristics of induced Treg's where hypoproliferation is an obstacle.
Authorship
Contribution: M.B. designed and performed research, analyzed data, and wrote the paper; A.K., N.W., and N.-A.D. performed research; F.J. and A.S. established and partially characterized the SP37A3 cell line; R.R. designed and performed research; J.K. wrote the paper; S.S. designed research, analyzed data, and wrote the paper; and A.B.R.-K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: F.J. and A.S., who established the DC line SP37A3, are employees of Merck KGaA.
Correspondence: A. B. Reske-Kunz, Clinical Research Unit Allergology, Department of Dermatology/VFG, Johannes Gutenberg-University, Obere Zahlbacher Str 63, D-55131 Mainz, Germany; e-mail: a.reske-kunz@uni-mainz.de
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 548, and the Stiftung Rheinland-Pfalz für Innovation (15212-386261/761).

![Figure 3. The allogeneic T-cell stimulatory capacity of SP37A3 cells depends on their state of differentiation. Titrated numbers of irradiated DCs were cocultured with 3 × 105 nylon wool–enriched T cells of BALB/c mice for 4 days in triplicate cultures. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. (A) BM-DCs were used either at an immature state, or were matured by addition of LPS (1 μg/mL for 48 hours). In parallel cultures, DEX was applied at 10−6 M and 10−5 M starting on day 3 of culture, and stimulation with LPS was performed as described. (B) Immature and mature SP37A3 cells were generated as described (Figure 1). In parallel cultures, DEX was coapplied during induction of maturation. Data represent mean ± SEM of triplicate cultures and are representative of 4 (A) and 5 (B) independent experiments. *P < .05 compared with immature and DEX-cotreated DCs (10−5 M, 10−6 M); +P < .05 compared with DEX-cotreated DCs (10−5 M, 10−6 M). For the sake of clarity, indication of distinct levels of significance was omitted. (C) A micrograph of SP37A3 cells stimulated in the presence of 10−5 M DEX, as described in panel B. The image was captured as described in Figure 1. (D) The relative allogeneic T-cell stimulatory capacity of differentially conditioned SP37A3 cells was determined. Experiments were performed as described for panel B. Results obtained with 1.7 × 104 immature, mature, and DEX-treated SP37A3 cells are shown. The respective T-cell proliferation induced by mature SP37A3 cells was set to 1 (horizontal dashed line). Data represent mean ± SEM of 5 independent experiments. *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-07-035576/4/m_zh80090700400003.jpeg?Expires=1769091578&Signature=mRI7vApD3tCGptyTiKyE6pljczcjnzIHgB3Gu8cZXZvmEnA50apZBHmU427pkLyCflMb4-ruxtR3ItAg2bFDg5ET~BYKz0vti1ugFjkQWMtkcD75BQKp9iACuGJ0ia3ga74CMtMJxViBstafA2UX399C1empLQVyUi46i78tSeFMI1pFcwFaev1cn~hZ~1e8VX8YhX3Qf1ZQOn8uwoglarlj0ClCr3n9TkRAojhK5HU1JuXf2Pyu7lRdrsYLZg25vgnmUOAXGktnmMgombMRBocfzLkD-gLVI4jhvlxdDfYV1rAi9xEDE8GAOJr30~8zABG~X9XwtnEiVpMkfJgQxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. The protein antigen-specific T-cell stimulatory capacity of SP37A3 cells correlates with their state of maturation. OVA-specific T-cell stimulatory capacity of differentially conditioned BM-DCs (left panels) and SP37A3 cells (right panels) was assessed by determining proliferation of OT-II T cells. DCs were differentiated as described (Figures 1,3) and were pulsed for 3 hours with 10 μg/mL OVA323-339 peptide or medium and were irradiated. (A) Titrated numbers of DCs were cocultured with 5 × 104 OT-II T cells for 3 days in triplicate cultures. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. Data represent mean ± SEM of triplicate cultures and are representative of 3 independent experiments. (B) DCs (3 × 103) were cocultured with CFSE-labeled T cells (5 × 104) for 3 days, and T-cell proliferation was determined as relative CFSE staining intensity by flow cytometry. Graphs are representative of 3 independent experiments. *P < .05 compared with immature DCs and DCs matured in the presence of DEX (10−5 M); +P < .05 compared with alternatively activated DCs (10−5 M).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-07-035576/4/m_zh80090700400004.jpeg?Expires=1769091578&Signature=Y9lY8VcJyXpRjqr8kr41V1bUucph9Oo2c6Rr8T0-H1S2ZVDotkfLF6s-YmhdGhybQ21vkGKHRK4fJAeFCgKuaT5CwoXJ4HAQbSbnV6ObRPCTeFdjqOM-qNkImSkhPMvn5JJJwmSacKYhwCOfXl055DnvmE404LELTSuSyXbcM4H~EEc85TrfSw-~NFETdybcctWMtnv-L-v-oZLBuwIfZVTx1vnL~5WzVpv93JDuBeO~p9pqK5vr~~x-kivZq1FxlbSCIy0gVCrOLLBDT-zPHcnjg1yrctua5P0cfZ59qnYvVgF0wfcutyn7HtUmroJZeAKwqHtx5Pa-ZIMlfWqXbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Alternatively activated SP37A3 cells exhibit tolerogenic potential. (A) SP37A3 cells were differentiated as described (Figures 1,3). Irradiated cells (2.5 × 105) were cocultured for 7 days with 106 nylon wool–enriched allogeneic BALB/c T cells in 1 mL culture medium. Culture supernatants were harvested and assayed by ELISA for contents of IFN-γ, IL-4, and IL-10. Data represent mean ± SEM of at least 3 experiments. *P < .05. (B-D) Differentially conditioned (Figures 1,3) irradiated SP37A3 cells (106) were cocultured with 6 × 106 nylon wool–enriched T cells of BALB/c mice in 4 mL volume in wells of 6-well cluster plates for 7 days, and T cells were harvested afterward. (B) T cells (105) primed by immature, mature, or alternatively activated SP37A3 cells were restimulated with irradiated C57BL/6 spleen cells (105) in 0.2 mL culture medium for 4 days. (C-D) Graded numbers of T cells primed by immature, mature, or alternatively activated SP37A3 cells (pTCs) were cocultured with 3 × 105 naive BALB/c T cells (nTCs) and 3 × 105 C57BL/6 spleen cells in 0.2 mL volume for 6 days in triplicate cultures. To account for the doubled number of T cells used in suppression assays (pTC + nTC), T-cell proliferation of 6 × 105 naive T cells (2 × nTC) was determined. T-cell proliferation was assessed as uptake of [3H] thymidine for the final 16 hours of culture. Data represent mean ± SEM of triplicate cultures and are representative of 2 (B) and 3 (C) independent experiments. (D) Assessment of suppressive activity of T cells primed by alternatively activated SP37A3 cells (pTC; 3 × 105). The respective proliferation of naive T cells (nTC; 3 × 105) induced by C57BL/6 spleen cells was arbitrarily set to 1. Data repesent mean ± SEM of 3 independent experiments. *P < .05; **P < .001; ***P < .001 compared with 3 × 105 nTCs (C) or between indicated groups (B,D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-07-035576/4/m_zh80090700400005.jpeg?Expires=1769091578&Signature=PH6eWbU85VG~~RLM-7OQhmz4YEFFHBzoBeuK3y8UDTG7DndGOY55h5pyZc1olafzB5AI1sLPVZnpnuvst45E4E7fHNrcAq0PM3IZwGCGAEHatfql8TmaQJKej2~Vg8VP-GA0KnjaJA93s5gp4tQokPr1W0e-MfMS6BWDehv7~ccGnQVCmr7O6-tSSk6SL4XV5-oEfMPrqeph4cT6cgNoIBbCP3D9nNU7UU4c4zt9U-tnBvotrPbX4TR7WqNPhQ75egG6WQwgj6xXQOfOWm6gdnSVK2GMJcSpzz18G8T-j7zu6EaK7GadqIohJlVVR4hEAUJUC68vG-3iNYxccPNAZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal