Abstract
The systemic administration of keratinocyte growth factor (KGF) enhances T-cell lymphopoiesis in normal mice and mice that received a bone marrow transplant. KGF exerts protection to thymic stromal cells from cytoablative conditioning and graft-versus-host disease–induced injury. However, little is known regarding KGF's molecular and cellular mechanisms of action on thymic stromal cells. Here, we report that KGF induces in vivo a transient expansion of both mature and immature thymic epithelial cells (TECs) and promotes the differentiation of the latter type of cells. The increased TEC numbers return within 2 weeks to normal values and the microenvironment displays a normal architectural organization. Stromal changes initiate an expansion of immature thymocytes and permit regular T-cell development at an increased rate and for an extended period of time. KGF signaling in TECs activates both the p53 and NF-κB pathways and results in the transcription of several target genes necessary for TEC function and T-cell development, including bone morphogenetic protein 2 (BMP2), BMP4, Wnt5b, and Wnt10b. Signaling via the canonical BMP pathway is critical for the KGF effects. Taken together, these data provide new insights into the mechanism(s) of action of exogenous KGF on TEC function and thymopoiesis.
Introduction
Decreased T-cell cellularity and a skewed TCR repertoire are hallmarks of an immune deficiency commonly observed in old age, as a consequence of general infectious diseases and aggressive lymphocyte-depleting therapies for diverse malignancies.1–4 The regeneration of a phenotypically and functionally normal T-cell compartment is curtailed for an extended period of time in patients receiving a hematopoietic stem cell transplant (HSCT).5–7 This lack in T-cell reconstitution is associated with opportunistic infections, the reactivation of latent viral and parasitic infections, chronic inflammation, and autoimmunity.3,4
Following cytoablative therapy, the recovery of the T-cell compartment relies on 2 independent pathways, that is, the expansion of peripheral T cells and, alternatively, the de novo production of T cells in the thymus.1,2,7–10 The latter assures the generation of a population of naive T cells expressing a diverse repertoire of TCR specificities.5,7,8,10,11 The extent of thymus-dependent T-cell reconstitution correlates directly with thymic size following immune ablation and hematopoietic stem cell (HSC)–derived reconstitution7,12 but is inversely related to age and transplant-related toxicities such as graft-versus-host disease (GVHD).10,13–17 The generation of new T cells of donor origin depends on the migration of hematopoietic precursors to the thymus. Normal thymic T-cell development is in turn contingent on the regular maintenance of the stromal microenvironment. However, age-related thymic involution18 and injury from radiation,19 GVHD,20 chemotherapy,12,21 or infection3,4,12,18–23 preclude normal thymopoiesis to occur as they directly affect thymic epithelial cells (TECs). There has been considerable interest in identifying strategies to prevent TEC injury. Recently, robust T-cell lymphopoiesis has been maintained in myeloablated HSCT recipients by pretransplantation administration of different factors such as IL-7,24,25 androgen antagonists,26 and fibroblast growth factor 7 (Fgf7; aka, keratinocyte growth factor [KGF]).20,27–29 KGF belongs to the family of the structurally related Fgfs and is a potent epithelial cell mitogen.27,30 KGF is expressed under physiological conditions within the thymus both by mesenchymal cells and by T cells at specific developmental stages. To exert its biologic activity, KGF activates the IIIb variant of the FgfR2 receptor (FgfR2IIIb), which is expressed within the thymus exclusively on TECs.31 Experiments using mice deficient for FgfR2IIIb or the removal of mesenchyme from normal embryos revealed the importance of Fgf signaling during early thymus organogenesis.32 The postnatal thymic epithelial compartment may continue to require growth-regulating signals including possibly endogenous KGF, whose thymic expression is sustained throughout life.28
Although of considerable therapeutic potential, little is known regarding KGF's mode of action on adult thymopoiesis and the thymic microenvironment. Here, we report on the cellular and molecular response of adult TECs to a systemic treatment with recombinant human KGF and how the ensuing changes enhance thymopoiesis.
Materials and methods
Animals
Female C57BL/6 and B6.SJL-PtprcaPep3b/BoyJ (B6.CD45.1; CD45.1+) mice were purchased from Charles River (Lyon, France) and the Jackson Laboratories (Bar Harbor, ME), respectively. Mice were 6 weeks of age at the time of KGF administration. Animals were kept under specific pathogen-free conditions and in accordance with federal regulations. [Smad4lox/lox: Foxn1-cre]F2 mice were generated by crossing B6.129Smad4lox/lox mice (a gift from C. Deng, Bethesda, MD) to B6;D2-Tg(Foxn1-cre)8Ghr transgenic mice that express the Cre-recombinase in TECs (L.T.J. and G.A.H., manuscript in preparation).
In vivo and in vitro KGF treatment
Mice were injected intraperitoneally for 3 days (days 0, 1, and 2) with Hanks balanced salt solution (HBSS) or recombinant human KGF (palifermin, generously provided by Amgen, Thousand Oaks, CA) solubilized in HBSS at a dose of 5 mg/kg per day. For in vitro studies, thymic stromal cell preparations taken from E15.5 fetal thymic lobes were cultured for the indicated times in media supplemented with KGF (100 ng/mL) or HBSS (vol/vol).
Flow cytometry
For flow cytometric analyses and cell purifications, fluorochrome-conjugated or unconjugated moAbs against TCRβ (clone H57-592), CD8 (53-6.7), CD4 (RM4-5), CD3 (145-2C11), CD44 (IM7), CD25 (PC61), CD45 (30-F11), CD45.1 (A20), CD45.2 (104), I-Ab (AF6-120.1), CD117 (2B8), and CD127 (A7R34) were used (BD Biosciences, San Diego, CA; eBioscience, San Diego, CA; Caltag Laboratories, Burlingame, CA). To reveal biotinylated moAbs, streptavidin-conjugated Cy5, PerCP, CyChrome, phycoerythrin, and APC (Zymed Laboratories, San Francisco, CA; and Caltag Laboratories) were used. Three-color analyses were performed using a FACSCalibur (Becton Dickinson, Mountain View, CA). The different medullary (m) and cortical (c) TEC subpopulations were identified in stromal cell preparations by flow cytometry by use of the lectin UEA-1 and antibodies specific for the surface molecule MTS24 (a TEC precursor marker33,34 ) and the cytokeratin 5 (K5) and K18.20,35–37 To this end, thymic stromal cells were stained with a moAb to K18 (Ks18.04; Progene, Heidelberg, Germany), polyclonal antibody to K5 (Covance, Princeton, NJ), biotinylated UEA1 lectin (Vector Laboratories, Burlingame, CA), and the MTS24 moAb (a gift from R. Boyd, Melbourne, Australia). Large granular cells (FSChighSSChigh) present in the freshly isolated thymic stromal cell preparations were gated for analysis. Early thymic progenitors (ETPs) were identified and purified (≥ 98%) by cell sorting as Lin− (that is, TCRβ−, CD3−, CD4−, CD8−) CD44+CD25−CD117high lymphoid cells.
Thymic stromal cell preparations and culture
To generate thymic stromal cell preparations, thymic cell suspensions from adult C57BL/6 mice (6 weeks old) were digested with collagenase D and DNAse I for 3 cycles, as described.33,38 For quantification, CD45−I-Ab int+high stromal cells present in each cell fraction were counted by flow cytometry. CD45−I-Ab int+high stromal cells were sorted to purity (> 99%) using a FACSVantage cell sorter (Becton Dickinson). Thymic stromal cells from fetal (E14.5 or E15.5) C57BL/6 mice were isolated and depleted from lymphoid cells with 2-deoxyguanosine, as described.39 Purified adult TECs or fetal thymic stromal cells were starved for 24 hours in IMDM/1% FCS, and subsequently stimulated with KGF. Stromal cell proliferation was assessed by 3H-thymidine incorporation. E15.5 fetal thymic stromal cells were cultured in the presence or absence of the selective IκB kinase inhibitor PS1145 (10 mM; a gift from Millennium, Cambridge, MA), the farnesyltransferase inhibitor L-778123 (1 mM; Merck Research Laboratories, Rahway, NJ), and the p53 inhibitor PFT102 (10 mM; provided by A. V. Gudkov, Cleveland, OH).
Assessment of TEC proliferation in vivo
Adult C57BL/6 mice (6 weeks old) were injected intraperitoneally with 5′-bromo-2′-deoxyuridine (BrdU; 1 mg in 0.2 mL PBS; Sigma, Buchs, Switzerland) 48 and 24 hours before being killed and analyzed for BrdU incorporation, as described.40 DNA-synthesizing cells were represented as frequencies of BrdU+ cells (in %) among total FSChighSSChighCD45−I-Ab+ cells present in the thymic stromal cell preparations.
Detection of recent thymic emigrants
Anesthetized adult mice treated with either KGF or HBSS were injected in one thymic lobe with 10 μL FITC (125 μg/mL diluted in PBS; Sigma). Splenic FITC+CD4+ and CD8+ lymphocytes were detected by flow cytometry 16 hours after intrathymic injection.
Transfer of ETPs
ETPs were purified from adult B6.CD45.1 mice on day 6 after initiation of KGF or HBSS treatment and transferred intrathymically (1000 ETPs/lobe) into C57BL/6 (CD45.2+) recipients that had received either HBSS or KGF 15 days earlier.
Time-lapse visualization of T-cell precursor attraction
Thymocyte-depleted fetal thymic stromal cell preparations (E14.5) that had been exposed for 24 hours to either KGF (100 ng/mL) or HBSS were cocultured with T-cell precursors. Cell culture, visualization, reaggregate preparations, and data acquisition were carried out as published.41
Immunohistofluorescence confocal microscopy
For analysis of mTECs and cTECs by immunohistology, frozen thymic sections (6 μm) were fixed with 4% paraformaldehyde/PBS and stained with a panel of reagents that was also used for flow cytometry (above; Gill et al33 ; and Bennett et al34 ) and the cytokeratin 5 (K5) and K18.20,35–37 In addition, moAbs against MTS10 (BD Biosciences) and polyclonal anti-FgfR2IIIb Ab (R&D Systems, Minneapolis, MN) were used. Images were captured on a Zeiss LSM 510 confocal microscope with a 20×/0.5 NA objective (Carl-Zeiss AG, Feldbach, Switzerland). Overlays of blue (Cy5), red (Alexa555), and green (Alexa488) stainings were colored by computer-assisted management of data using the Zeiss LSM 510 software version 3.2.
Quantitative PCR
cDNA was generated from total RNA and amplified using primers designed for real-time polymerase chain reaction (PCR). Primer sequences for IL-7, GAPDH, Wnt5b, Wnt10b, BMP2, BMP4, CCL21, and CCL25 are available from the authors. RNA transcription levels in TECs from KGF-treated mice or stromal culture levels were normalized to GAPDH and compared with their expression in HBSS-treated control mice or thymic stromal control cultures.
Statistical analysis
Groups were compared by one-way ANOVA and Bonferroni/Dunn posthoc test. The overall statistical significance level was set to 5%. For 2-group comparisons, the Mann-Whitney test was applied. StatView from SAS Institute (Cary, NC) was used for statistical analysis.
Results
KGF increases thymocyte numbers by differentially stimulating cell proliferation
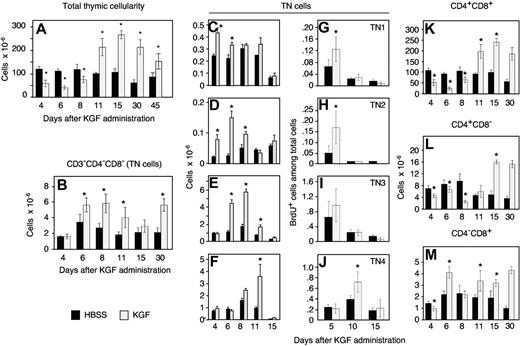
We first determined in adult C57BL/6 mice the consequences of KGF signaling for thymopoiesis. Following treatment for 3 consecutive days (days 0, 1, and 2) with KGF, the total thymic cellularity was transiently diminished (days 4-8) but recovered to higher than normal values on and beyond day 11 (Figure 1A). Supranormal numbers of thymocytes were sustained for no fewer than 2 months thereafter (data not shown). The increase in total thymocyte cellularity noted beyond day 11 was initially caused by an expansion due to cell proliferation of the most immature, CD3/CD4/CD8 triple-negative (TN), thymocytes (Figure 1B-J). These cells sequentially gave rise to cells with a more mature phenotype. On day 4, the absolute number of all TN thymocytes was comparable for both treatment groups, although TN1 and TN2 cells were already increased due to a higher proliferation rate. Over the next few days, TN3 and TN4 followed suit. Thus, KGF affected the most immature thymocytes, resulting in a developmental wave of new T cells that followed the correct sequence of TN maturational stages. KGF treatment also resulted in a transient decrease of mature CD4+CD8− and CD4−CD8+ thymocytes (Figure 1L-M). Absolute numbers of mature thymocytes stably rose above levels found in HBSS-treated mice, however, once a higher number of immature CD4+CD8+ thymocytes had been achieved (ie, at day 11 and thereafter).
KGF increases thymocyte numbers via induction of TN cell division. Adult naive C57BL/6 mice were treated with KGF (5 mg/kg, ⊡) or HBSS (▪) on 3 consecutive days (days 0, 1, and 2), and thymi were analyzed by flow cytometry at the indicated days. (A) Total thymocyte numbers (cells × 10−6). (B-F) TN cell numbers per thymus (× 10−6). TN indicates CD3−4−8−; TN1, CD44+CD25−; TN2, CD44+CD25+; TN3, CD44−CD25+; and TN4, CD44−CD25− cells. (G-J) BrdU incorporation into TN cells as a measure of cell division. The data represent the fraction (in %) of cells among total thymocytes that have incorporated BrdU. (K-M) Total numbers per thymus of CD4+CD8+, CD4+CD8−, and CD4−CD8+ cells. Three independent experiments were performed, and one representative experiment is shown. Mean ± SD; *P < .05 versus HBSS controls, with 5 mice per group and time point.
KGF increases thymocyte numbers via induction of TN cell division. Adult naive C57BL/6 mice were treated with KGF (5 mg/kg, ⊡) or HBSS (▪) on 3 consecutive days (days 0, 1, and 2), and thymi were analyzed by flow cytometry at the indicated days. (A) Total thymocyte numbers (cells × 10−6). (B-F) TN cell numbers per thymus (× 10−6). TN indicates CD3−4−8−; TN1, CD44+CD25−; TN2, CD44+CD25+; TN3, CD44−CD25+; and TN4, CD44−CD25− cells. (G-J) BrdU incorporation into TN cells as a measure of cell division. The data represent the fraction (in %) of cells among total thymocytes that have incorporated BrdU. (K-M) Total numbers per thymus of CD4+CD8+, CD4+CD8−, and CD4−CD8+ cells. Three independent experiments were performed, and one representative experiment is shown. Mean ± SD; *P < .05 versus HBSS controls, with 5 mice per group and time point.
An increased thymic size in KGF-treated mice correlates with enhanced thymic T-cell export
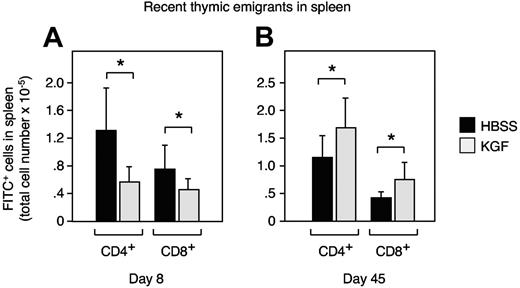
Next, we quantified thymic export in response to KGF treatment by detection of intrathymically FITC-labeled T cells that have recently emigrated into the spleen. In agreement with the reduction of mature CD4+CD8− thymocytes at 8 days after initiation of KGF treatment (Figure 1L), we found in KGF-treated mice a significant decrease in the export of CD4+ cells to the periphery (Figure 2A). Thymic export of CD4−CD8+ cells was similarly affected. Six weeks later (day 45), thymic export of CD4+ and CD8+ cells to the spleen was significantly increased in the KGF-treated mice (Figure 2B), corresponding to the overall increase in mature thymocytes. These results demonstrated during steady-state conditions of thymopoiesis a direct correlation between thymic cellularity, the absolute increase in mature thymocytes, and the number of recent thymic emigrants.
Thymocyte numbers correlate with T-cell exit into the periphery. Adult C57BL/6 mice were first treated either with KGF (⊡) or HBSS (▪) as in Figure 1 and then intrathymically injected with FITC (10 μL) 7 or 44 days later. After 24 hours of in vivo labeling, the export of thymus-derived cells into the periphery was assessed by enumeration of FITC-positive cells (× 10−6) on day 8 (A) and day 45 (B). Mean ± SD; *P < .05 versus HBSS controls, with 6 mice per group and time point.
Thymocyte numbers correlate with T-cell exit into the periphery. Adult C57BL/6 mice were first treated either with KGF (⊡) or HBSS (▪) as in Figure 1 and then intrathymically injected with FITC (10 μL) 7 or 44 days later. After 24 hours of in vivo labeling, the export of thymus-derived cells into the periphery was assessed by enumeration of FITC-positive cells (× 10−6) on day 8 (A) and day 45 (B). Mean ± SD; *P < .05 versus HBSS controls, with 6 mice per group and time point.
KGF affects numbers and thymic entry of early T-cell precursors
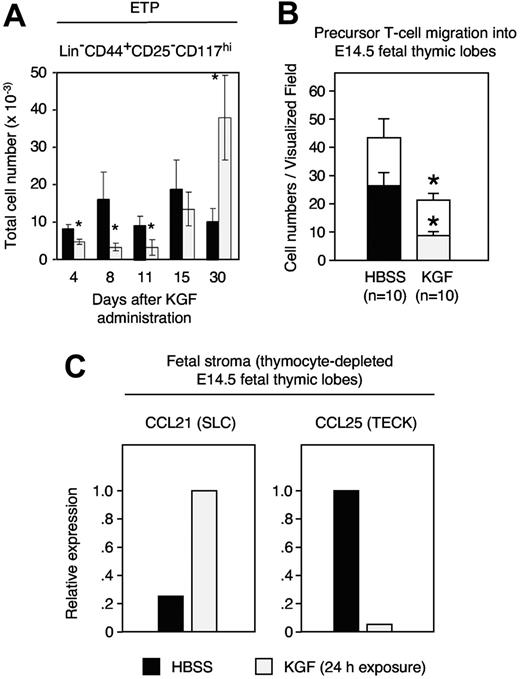
As the absolute number of TN1 cells had increased by 4 days after KGF treatment (Figure 1C), we investigated whether this effect was due to expansion of the most immature intrathymic cell precursors (early thymic progenitors [ETPs]). These cells reside within the population of TN1 cells and display a Lin−CD44+CD25−CD117+ phenotype.42,43 Although the frequency of ETPs was expanded to supranormal values on day 30, their number was decreased early (ie, 4 to 11 days) after initiation of KGF treatment (Figure 3A). To investigate the cause for this transient reduction, we sought to quantify the attraction and subsequent entry of hematopoietic precursors to the thymus. This was most informatively achieved by time-lapse video microscopy of fetal thymic lobes as previously demonstrated by us.41 Following the exposure of alymphoid E14.5 thymic lobes to KGF, both the number of precursors attracted to and the number of precursors then entering into the lobes were significantly reduced (Figure 3B). Several chemokines, including CCL21 and CCL25, have been implicated for the colonization of hematopoietic precursor cells as they are expressed both in the combined fetal thymus/parathyroid anlage and in the postnatal thymus.41,44–46 The exposure of fetal thymic lobes to KGF caused an up-regulation of CCL21 but a reduction in CCL25 transcripts (Figure 3C). Thus, the decrease in CCL25 correlated with the decreased seeding of T-lymphoid precursors to the thymus.
KGF inhibits the capacity of the thymic microenvironment to attract T-cell precursors. (A) Adult C57BL/6 mice were treated either with KGF (⊡) or HBSS (▪) as in Figure 1, and the numbers of ETPs (Lin−CD44+CD25−CD117hi) were determined at the indicated days. (B) Time-lapse video microscopy recorded E14.5 fetal thymocytes that migrated toward (open bars) and then entered (filled bars) an alymphoid E14.5 fetal thymic lobe that had been pre-exposed for 24 hours to KGF (100 ng/mL, ⊡) or HBSS-supplemented medium (▪) before the start of the migration experiments. The graph depicts the numbers of cells that migrated in a directed fashion and that reached the thymic lobe. Statistical significance was evaluated individually for open bars (□) and filled bars. (C) Changes in chemokine expression following exposure to exogenous KGF. Chemokine mRNA levels were determined by quantitative reverse-transcription PCR qRT. The y-axis shows the relative transcript levels (the group with maximal chemokine expression was set as 100%, which is displayed as value of 1.0). Mean ± SD; *P < .05 versus HBSS controls in panel A, with 5 mice per group and time point. Mean ± SEM; *P < .01 versus HBSS controls in panel B where data are representative of 10 independent in vitro experiments.
KGF inhibits the capacity of the thymic microenvironment to attract T-cell precursors. (A) Adult C57BL/6 mice were treated either with KGF (⊡) or HBSS (▪) as in Figure 1, and the numbers of ETPs (Lin−CD44+CD25−CD117hi) were determined at the indicated days. (B) Time-lapse video microscopy recorded E14.5 fetal thymocytes that migrated toward (open bars) and then entered (filled bars) an alymphoid E14.5 fetal thymic lobe that had been pre-exposed for 24 hours to KGF (100 ng/mL, ⊡) or HBSS-supplemented medium (▪) before the start of the migration experiments. The graph depicts the numbers of cells that migrated in a directed fashion and that reached the thymic lobe. Statistical significance was evaluated individually for open bars (□) and filled bars. (C) Changes in chemokine expression following exposure to exogenous KGF. Chemokine mRNA levels were determined by quantitative reverse-transcription PCR qRT. The y-axis shows the relative transcript levels (the group with maximal chemokine expression was set as 100%, which is displayed as value of 1.0). Mean ± SD; *P < .05 versus HBSS controls in panel A, with 5 mice per group and time point. Mean ± SEM; *P < .01 versus HBSS controls in panel B where data are representative of 10 independent in vitro experiments.
The effect of KGF on early T-cell precursors is not T-cell autonomous
The effect of KGF on early thymocyte proliferation may have been driven by the acquisition of a cell-autonomous capacity of TN cells to proliferate better or, alternatively, by an improved thymic stromal microenvironment via the creation of additional developmental niches. We therefore measured the extent of donor-derived thymopoiesis in recipients of intrathymically injected, congenic ETPs. We purified ETPs from B6.CD45.1 donors and intrathymically transferred them to KGF-treated C57BL/6 mice. In a second group, C57BL/6 recipients received ETPs isolated from KGF-treated B6.CD45.1 mice. One week after injection, these mice were compared with a control group where neither donors nor hosts had been treated with KGF. The thymic cellularity was enhanced only under conditions where the C57BL/6 recipient thymic stromal environment had been exposed to KGF (Figure 4A dashed bar). Here, the absolute numbers of intrathymic CD45.1+ cells (ie, ETP progeny) were found to be the highest among the 3 transplantation groups (Figure 4B). In contrast, ETPs isolated from mice treated with KGF did not exhibit an increased growth rate upon intrathymic transfer into HBSS-injected recipients (gray bars). Thus, the treatment with KGF resulted in an improved stromal microenvironment able to increase thymopoiesis, but the stroma did not impart on ETPs a cell-autonomous capacity for enhanced reconstitution.
Non–cell-autonomous effect of KGF on early thymic progenitors. ETPs (Lin−CD44+CD25−CD117hi cells) were purified on day 6 after initiation of treatment of B6.CD45.1 donors (CD45.1+) that had received KGF (5 mg/kg per day, ⊡) or HBSS (▪, ▨) on days 0, 1, and 2. Afterward, ETPs (1 × 103) from either group were transferred separately to a single thymic lobe of adult C57BL/6 recipients (CD45.2+) that had received 15 days earlier either HBSS (▪, ⊡) or KGF (5 mg/kg per day, ▨) on 3 consecutive days. The panels depict recipient thymic total cellularity (A) and CD45.1+ congenic ETP progeny (B) that were determined 7 days after intrathymic (i.t.) injection. Mean ± SD; *P < .001 versus HBSS controls (black bars), with 5 mice per group and time point.
Non–cell-autonomous effect of KGF on early thymic progenitors. ETPs (Lin−CD44+CD25−CD117hi cells) were purified on day 6 after initiation of treatment of B6.CD45.1 donors (CD45.1+) that had received KGF (5 mg/kg per day, ⊡) or HBSS (▪, ▨) on days 0, 1, and 2. Afterward, ETPs (1 × 103) from either group were transferred separately to a single thymic lobe of adult C57BL/6 recipients (CD45.2+) that had received 15 days earlier either HBSS (▪, ⊡) or KGF (5 mg/kg per day, ▨) on 3 consecutive days. The panels depict recipient thymic total cellularity (A) and CD45.1+ congenic ETP progeny (B) that were determined 7 days after intrathymic (i.t.) injection. Mean ± SD; *P < .001 versus HBSS controls (black bars), with 5 mice per group and time point.
Exogenous KGF is mitogenic for all TEC subpopulations in adult mice
Our data indicated that KGF triggered a temporary deficiency to attract hematopoietic precursor cells but eventually may have created new developmental niches for T lymphopoiesis or, alternatively, enhanced the ability of TECs to support thymopoiesis. To examine whether KGF differentially acted on the known TEC subpopulations, we first determined in adult C57BL/6 mice the FgfR2IIIb expression among major cortical (K18+K5−MTS 10−UEA1−), minor cortical (K18+K5+), major medullary (K5+MTS10+), and minor medullary (K18+UEA1+) TECs as well as MTS24+ TEC precursors. Using immunohistofluorescence confocal microscopy, we found that a minority of the K18+ cTECs and K5+ mTECs expressed FgfR2IIIb (Figure 5A-B), whereas almost all UEA1+ and the large majority of MTS24+ epithelial cells were positive for FgfR2IIIb (Figure 5C-D). Thymocytes did not express FgfR2IIIb (data not shown, and Rossi et al20 ). To test the in vivo responses of the diverse TEC subpopulations to KGF, adult mice were treated for 3 consecutive days (days 0, 1, and 2) with KGF or HBSS. On day 3, thymic tissue was collected and stromal cell preparations were subjected to flow cytometric analysis. Following treatment with KGF, the relative frequencies of the different TEC subsets increased each by at least 2-fold when compared with HBSS-treated mice (Figure 5E-H). However, the architectural organization of all tested TEC populations appeared normal on day 15 by immunohistofluorescence analysis (Figure 5I-N). To determine TEC proliferation in vivo, adult mice were treated with KGF and then injected with BrdU. The frequency of TECs (CD45−I-Ab+) incorporating BrdU was increased by more than 10-fold in mice injected 3 days earlier with KGF when compared with animals treated with HBSS (Figure 5O). This proliferative response was transient as the frequency of BrdU+ TECs on day 6 was enhanced by only 3-fold in response to KGF. The increase in BrdU incorporation, as observed 3 days after initiation of KGF treatment, translated at day 6 into enhanced TEC numbers. While HBSS-treated 6-week-old normal C57BL/6 mice held 3.6 ± 0.7 × 105 CD45−I-Ab+ TECs per thymus, the cellularity for both immature (MTS24+) and mature (MTS24−) TECs rose to 5.4 ± 0.7 × 105 cells upon KGF administration (Figure 5P). The number of TECs returned to normal values by day 15 after initiation of KGF treatment in parallel with a shift in the ratio of MTS24+ to MTS24− TECs (Figure 5P). Taken together, these data demonstrated a transitory effect of pharmacological doses of KGF on TEC proliferation in vivo leading to an early expansion of both mature and immature TECs, and on the transition of epithelial precursors to more mature TECs.
KGF enhances TEC numbers via induction of cell division in adult mice. (A-D) Adult TECs express the receptor for KGF. Thymic sections from 6-week-old, naive C57BL/6 mice were analyzed by confocal immunohistofluorescence for the expression of FgfR2IIIb on K18+ epithelial cells (A), K5+ cells (B), MTS24+ cells (C), and on TECs binding UEA1 (D). The colocalization denotes distinct TEC subpopulations expressing the KGF receptor (arrows). A total of 3 experiments were performed, providing comparable results. (E-H) TECs expand in response to KGF in vivo. Adult C57BL/6 mice were treated with KGF (5 mg/kg, ⊡) or HBSS (▪) on 3 consecutive days (days 0, 1, and 2). Frequencies of the 4 TEC subpopulations were assessed on day 3 by flow cytometry. Relative frequencies are given (x-fold changes KGF vs HBSS treatment, whereby frequencies [in %] for HBSS-treated mice were set as 1.0 and shown as dashed lines). Mean ± SD. *P < .01 versus HBSS. (I-N) KGF administration to adult mice does not alter the normal architecture and epithelial composition of the thymus. Confocal microscopy analysis was performed 15 days after treatment of adult C57BL/6 mice with HBSS (I-K) or KGF (L-N) for 4 TEC subpopulations, as indicated. mTEC indicates medullary TEC; cTEC, cortical TEC. (O) Adult C57BL/6 mice were treated as described in panels E-H and then injected with BrdU 48 and 24 hours before killing. BrdU+ cells among FSChighSSChighCD45−I-Ab+ cells present in freshly isolated thymic stromal cells (days 3 and 6) were analyzed (x-fold change KGF vs HBSS, whereby frequencies [in %] in HBSS-treated mice were set as 1.0 [dashed lines]). Mean ± SD; *P < .05 versus HBSS controls, with 6 mice per group and time point. A total of 3 experiments were performed, providing comparable results. (P) Absolute TEC numbers on days 6 and 15 following treatment of mice with either KGF (⊡) or HBSS (▪). Total CD45−I-Ab int+high stromal cells were counted by flow cytometry. Relative frequencies of total TECs are given, whereby the values for HBSS-treated mice were set as 1.0. The fractions of MTS24+ and MTS24− cells (separated by horizontal lines) among total TECs and their absolute cell numbers are also indicated. Mean ± SD; *P < .01, total TEC numbers and #P < .02, total MTS24+ cells, respectively, in KGF-treated mice versus those in HBSS controls. A total of 12 individual mice were analyzed.
KGF enhances TEC numbers via induction of cell division in adult mice. (A-D) Adult TECs express the receptor for KGF. Thymic sections from 6-week-old, naive C57BL/6 mice were analyzed by confocal immunohistofluorescence for the expression of FgfR2IIIb on K18+ epithelial cells (A), K5+ cells (B), MTS24+ cells (C), and on TECs binding UEA1 (D). The colocalization denotes distinct TEC subpopulations expressing the KGF receptor (arrows). A total of 3 experiments were performed, providing comparable results. (E-H) TECs expand in response to KGF in vivo. Adult C57BL/6 mice were treated with KGF (5 mg/kg, ⊡) or HBSS (▪) on 3 consecutive days (days 0, 1, and 2). Frequencies of the 4 TEC subpopulations were assessed on day 3 by flow cytometry. Relative frequencies are given (x-fold changes KGF vs HBSS treatment, whereby frequencies [in %] for HBSS-treated mice were set as 1.0 and shown as dashed lines). Mean ± SD. *P < .01 versus HBSS. (I-N) KGF administration to adult mice does not alter the normal architecture and epithelial composition of the thymus. Confocal microscopy analysis was performed 15 days after treatment of adult C57BL/6 mice with HBSS (I-K) or KGF (L-N) for 4 TEC subpopulations, as indicated. mTEC indicates medullary TEC; cTEC, cortical TEC. (O) Adult C57BL/6 mice were treated as described in panels E-H and then injected with BrdU 48 and 24 hours before killing. BrdU+ cells among FSChighSSChighCD45−I-Ab+ cells present in freshly isolated thymic stromal cells (days 3 and 6) were analyzed (x-fold change KGF vs HBSS, whereby frequencies [in %] in HBSS-treated mice were set as 1.0 [dashed lines]). Mean ± SD; *P < .05 versus HBSS controls, with 6 mice per group and time point. A total of 3 experiments were performed, providing comparable results. (P) Absolute TEC numbers on days 6 and 15 following treatment of mice with either KGF (⊡) or HBSS (▪). Total CD45−I-Ab int+high stromal cells were counted by flow cytometry. Relative frequencies of total TECs are given, whereby the values for HBSS-treated mice were set as 1.0. The fractions of MTS24+ and MTS24− cells (separated by horizontal lines) among total TECs and their absolute cell numbers are also indicated. Mean ± SD; *P < .01, total TEC numbers and #P < .02, total MTS24+ cells, respectively, in KGF-treated mice versus those in HBSS controls. A total of 12 individual mice were analyzed.
Bone morphogenetic proteins play a role in adult TECs in their response to KGF
Early TEC development as well as the survival, proliferation, differentiation, and migration of thymocytes are under the precise control of different molecules produced by TECs, including Wnt glycoproteins and the bone morphogenetic proteins BMP2 and BMP4.47–51 We therefore tested whether KGF affected the expression of these factors in TECs from adult mice. CD45−I-Ab+ TECs were isolated on day 7 from adult C57BL/6 mice treated with either KGF or HBSS. As shown in Figure 6A, transcripts for Wnt5b, Wnt10b, BMP2, and BMP4 were up-regulated in vivo by 2- to 5-fold above normal in response to KGF. To determine a definitive role for BMPmediated signaling in the TEC response to KGF, we next investigated mice that lacked Smad4 specifically in TECs. Cytoplasmic Smad4 acts as an indispensable partner in the canonical BMP signaling pathway leading to target gene transcription.52 When compared with B6.129Smad4lox/lox controls, [Smad4lox/lox: Foxn1-cre]F2 mice displayed a smaller thymus whose cellular composition and architectural organization were proportionally normal (L.T.J. and G.A.H., manuscript in preparation). However, the latter failed to increase their thymic cellularity in response to KGF treatment (Figure 6B). This deficit was independent of the age of the mouse (data not shown). Hence, the KGF-mediated increase in thymic cellularity was dependent on signals in TECs that engaged Smad4, thus indicating a definitive role of BMPs in the response of thymic epithelium to KGF.
Wnts and BMPs are target genes of KGF. (A) Analysis of gene transcription by qRT-PCR in adult TECs. Six-week-old C57BL/6 mice were treated with KGF or HBSS as described in Figure 1 and sorted CD45−I-Ab+ TECs were analyzed on day 7 for mRNA expression of Wnt5b, Wnt10b, BMP2, and BMP4. Expression levels in KGF-treated mice (⊡) were compared with those in control mice treated with HBSS (x-fold change KGF vs HBSS, whereby the expression levels in the latter were set as 1.0; dotted line). (B) The increase in thymic cellularity in response to KGF depends on signals mediated by Smad4. Adult wild-type B6.129Smad4lox/lox mice (− Cre) and [Smad4lox/lox: Foxn1-cre]F2 mice (+ Cre) were treated with KGF (⊡) or HBSS (▪). Six and 15 weeks after the initiation of treatment, total thymocyte cellularity was determined. Absolute thymocyte numbers (× 10−6) are shown. Mean ± SD versus HBSS controls. For data shown in panel A, 3 experiments were performed whereby material from 15 mice was pooled for each experiment.
Wnts and BMPs are target genes of KGF. (A) Analysis of gene transcription by qRT-PCR in adult TECs. Six-week-old C57BL/6 mice were treated with KGF or HBSS as described in Figure 1 and sorted CD45−I-Ab+ TECs were analyzed on day 7 for mRNA expression of Wnt5b, Wnt10b, BMP2, and BMP4. Expression levels in KGF-treated mice (⊡) were compared with those in control mice treated with HBSS (x-fold change KGF vs HBSS, whereby the expression levels in the latter were set as 1.0; dotted line). (B) The increase in thymic cellularity in response to KGF depends on signals mediated by Smad4. Adult wild-type B6.129Smad4lox/lox mice (− Cre) and [Smad4lox/lox: Foxn1-cre]F2 mice (+ Cre) were treated with KGF (⊡) or HBSS (▪). Six and 15 weeks after the initiation of treatment, total thymocyte cellularity was determined. Absolute thymocyte numbers (× 10−6) are shown. Mean ± SD versus HBSS controls. For data shown in panel A, 3 experiments were performed whereby material from 15 mice was pooled for each experiment.
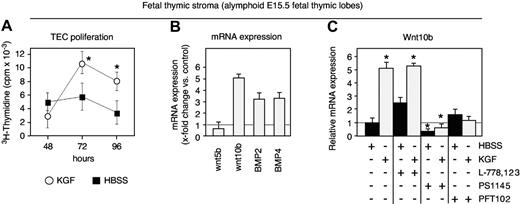
As freshly isolated TECs are not suited to manipulations in vitro due to their rapid loss of TEC-specific features, we used E15.5 fetal thymic epithelial cultures to investigate in more detail 2 important issues related to our findings in adult mice: (1) the regulation in the expression of growth and differentiation factors; and (2) the signal transduction pathways in TECs activated by KGF. As demonstrated in Figure 7A, the in vitro exposure to KGF (100 ng/mL) robustly increased TEC proliferation. Similar to the results obtained with adult TECs, the exposure of fetal TECs to KGF resulted in an up-regulation of BMP2, BMP4, and Wnt10b (Figure 7B). The signaling pathways previously shown to mediate KGF activity have included Ras/MAP kinases, phosphatidylinositol 3′kinase (PI3K), and p53.53–57 We therefore assessed the effect of KGF on TECs by independently blocking these different signaling pathways (Figure 7C). As Wnt molecules provide signals critical for normal TEC development,49 Wnt10b mRNA expression was used as a quantifiable readout for our experiments. The blocking of p53 with the inhibitor PFT102,58 and that of NF-κB (a downstream target of PI3K) with the IκB kinase inhibitor PS1145,59 suppressed the up-regulation of Wntl0b, suggesting that this effect was highly dependent on signaling via both NF-κB and p53. In contrast, using the farnesyltransferase inhibitor L-778123,60 the activation of the Ras pathway was not found to be obligatory to mediate the observed KGF effect in fetal TECs.
KGF signaling engages NF-κB and p53 in TECs. (A) Proliferation of cells present in thymocyte-depleted E15.5 fetal thymic stromal cell preparations was assessed in culture by 3H-thymidine incorporation (cpm). The x-axis indicates the time (in hours) after exposure to exogenous KGF (100 ng/mL, ○) or HBSS (▪); n = 6 experiments. Mean ± SD; *P < .05. (B) Transcripts for Wnt5b, Wnt10b, BMP2, and BMP4 were quantified by qRT-PCR after 24 hours of culture. Expression levels in KGF-treated mice (⊡) were compared with those in control mice treated with HBSS (x-fold change KGF vs HBSS, whereby the expression levels in the latter were set as 1.0; dotted line). A total of 3 experiments were performed, whereby material from 15 mice was pooled for each experiment. (C) Transcripts for Wnt10b were quantified by qRT-PCR in alymphoid E15.5 fetal thymic lobes exposed in culture for 24 hours to either KGF (⊡) or HBSS (▪) in the presence or absence of the following inhibitors: the selective IκB kinase (IKK) inhibitor PS1145, the farnesyltransferase (FTase) inhibitor L-778123, which blocks the Ras pathway, or PFT102, a specific small-molecular inhibitor of p53. Transcription levels were compared with control cultures exposed to HBSS but not supplemented with any of the inhibitors (dotted line = 1.0). Three independent experiments were performed; mean ± SD. *P < .05 versus HBSS control, with 6 lobes examined per time point (A), 3 lobes per group and experiment (B, total of 3 experiments), and 4 experiments (C).
KGF signaling engages NF-κB and p53 in TECs. (A) Proliferation of cells present in thymocyte-depleted E15.5 fetal thymic stromal cell preparations was assessed in culture by 3H-thymidine incorporation (cpm). The x-axis indicates the time (in hours) after exposure to exogenous KGF (100 ng/mL, ○) or HBSS (▪); n = 6 experiments. Mean ± SD; *P < .05. (B) Transcripts for Wnt5b, Wnt10b, BMP2, and BMP4 were quantified by qRT-PCR after 24 hours of culture. Expression levels in KGF-treated mice (⊡) were compared with those in control mice treated with HBSS (x-fold change KGF vs HBSS, whereby the expression levels in the latter were set as 1.0; dotted line). A total of 3 experiments were performed, whereby material from 15 mice was pooled for each experiment. (C) Transcripts for Wnt10b were quantified by qRT-PCR in alymphoid E15.5 fetal thymic lobes exposed in culture for 24 hours to either KGF (⊡) or HBSS (▪) in the presence or absence of the following inhibitors: the selective IκB kinase (IKK) inhibitor PS1145, the farnesyltransferase (FTase) inhibitor L-778123, which blocks the Ras pathway, or PFT102, a specific small-molecular inhibitor of p53. Transcription levels were compared with control cultures exposed to HBSS but not supplemented with any of the inhibitors (dotted line = 1.0). Three independent experiments were performed; mean ± SD. *P < .05 versus HBSS control, with 6 lobes examined per time point (A), 3 lobes per group and experiment (B, total of 3 experiments), and 4 experiments (C).
Discussion
Administration of KGF has been among the therapeutic strategies to promote thymic function.20,28 Here, we have detailed the molecular and cellular mechanisms by which KGF stimulates thymic T lymphopoiesis in adult mice. We show that the KGF-specific receptor, FgfR2IIIb, is expressed by both mature cortical and medullary as well as immature TECs (Figure 5). Upon exposure to exogenous KGF, these stromal cells proliferate and express several growth and differentiation factors, including different members of the family of Wnt and BMP molecules (Figures 5–6). In consequence, a robust and sustained increase in thymopoiesis occurs, which is initiated as a single wave affecting first the most immature T-cell precursors (Figure 1). This effect of enhanced thymopoiesis is uniquely dependent on the exposure of the stromal microenvironment to KGF as the transfer of ETPs from KGF-treated donor mice to naive recipients fails to impart an enhancement in thymopoiesis (Figure 4). The increased thymic cellularity leads to an enhanced export of mature T cells to the periphery (Figure 2). Thus, exogenous KGF displays a strong pharmacological activity in the thymus of naive adult mice that assures a vigorous and enduring increase in thymic T-cell development.
Here, we report the normal TEC cellularity of 6-week-old C57BL/6 mice (3.6 × 105 cells; see also Gray et al61 ) can transiently be expanded by exogenous KGF (Figure 5). In parallel with a higher frequency of mature (that is, MTS24−) TECs, the total number of TECs returned at day 15 after initiation of KGF treatment to normal values. These kinetics are consistent with the noted physiological turnover of TECs in adult mice.61,62 Due to an increased proliferation rate following KGF stimulation, the resultant TEC expansion has likely an initial but nonetheless decisive impact on lymphoid cellularity after day 11. It is conceivable that enhanced TEC numbers create a “larger” microenvironment able to accommodate increased amounts of early-stage thymocytes. Due to the time required for the transition from TN1 to mature thymocytes,63–65 an appreciable increase in total thymocyte cellularity secondary to quantitative TEC changes may be noted only after such a maturational progression has occurred (that is, after about 13 days). With respect to our results at and beyond day 15, we would argue for a qualitative rather than a quantitative change in the ability of TECs to support thymopoiesis. Such an interpretation is in keeping with Wnt data shown in Figure 6A, the reported role of Wnts in thymopoiesis,66–68 and the changes in the ratio of MTS24+ to MTS24− TECs.
The stromal response to KGF allowed for an early and sequential increase in TN thymocytes (Figure 1), suggesting that the transient expansion of the thymic epithelial scaffold rendered the microenvironment more proficient to support early thymopoiesis. This interpretation is consistent with previous work demonstrating that thymic size is limited by the availability of stromal niches for TN thymocytes.69 Cells at and beyond the CD4+CD8+ stage were, in contrast, decreased during this initial phase following KGF treatment. This finding implied a transient but substantial change in the potential of the microenvironment to sustain the survival and/or the differentiation of more mature thymocytes. A likely explanation for this finding may be the observation that proliferating TECs lose their capacity to support CD4+CD8+ thymocytes since this deficit correlated with TEC proliferation in response to KGF. Independent of the immediate changes in thymocyte cellularity following KGF treatment, a robust and long-lasting increase in the cellularity of all thymocyte subpopulations was eventually achieved. The transient increase of CD4−CD8+ cells at day 6 was caused by immature single-positive thymocytes, while the delayed appearance of CD4+CD8− cells could be secondary to the decreased MHC class II expression of KGF-treated cortical TECs as previously reported.70 Our results are in agreement with a recent report28 and with the observations that the number of CD4+CD8+ thymocytes is proportional to the number of TN cells present.71 Thus, the increase in thymic size together with a restoration of the different thymocyte subpopulations in a proportionally correct fashion reflect the establishment of a regular thymic homeostasis 4 weeks after treatment with KGF.
As the thymus does not contain hematopoietic stem cells with an unlimited self-renewal capacity, T-cell progenitors need to be recruited from the blood to maintain thymopoiesis. Several mechanisms may account for the decrease in ETPs following the treatment with KGF, including the possibility that KGF affects the number of T-cell precursors homing to the thymus. The necessary steps in the homing process are initiated in vessels at the corticomedullary junction and involve not only the expression of different adhesion molecules on endothelial cells but also the presence of different chemokines41,44,46,72 Recent data suggest that CCL25 controls the seeding of hematopoietic precursors to both fetal nonvascularized as well as adult thymic tissue.46,73 The exposure of the thymic microenvironment to KGF not only decreased the attraction of T-cell precursors to the thymus but also reduced their entry. This fact was correlated with a decrease in CCL25 expression by the fetal thymic microenvironment exposed to KGF. A causal link between these 2 findings is possible because age-matched embryos deficient for CCL25 display a considerable decrease in thymocyte numbers,44 and the use of neutralizing antibodies against CCL25 in adult mice decreases the thymic accumulation of T-cell precursors.46 Thus, the down-regulation of CCL25 expression by TECs exposed to KGF may, at least partly, account for the observed paucity of ETPs present early after treatment.
In addition to a phase during which the thymus may have been partially inaccessible for blood-borne T-cell precursors, changes in the kinetics of early thymocyte development could further affect the thymic entry of precursors and/or their developmental expansion in KGF-treated mice. It appears that thymic size is controlled by the number of TN thymocytes, which in turn depends on the availability of developmental niches.69 In KGF-treated mice, TN1 and TN2 cells were indeed significantly increased as early as day 4 after the initiation of KGF treatment and displayed a notably higher proliferation rate when compared with the same cell populations in control mice. Yet, all TN subpopulations and their proliferative responses returned to normal values within 2 weeks after initiation of KGF treatment. At this point, the number of CD4+CD8+ thymocytes had increased above the values noted in control animals. However, the kinetics of the changes in the number of TN thymocytes in response to KGF (Figure 1) was in keeping with the time reported that TN cells require to transit to CD4+CD8+ thymocytes,63–65 and hence excludes a decrease in the TN transition time as an explanation for the observed increase in cellularity. Our findings, however, suggest that the early and sequential increase in the different TN subpopulations was caused by a single developmental wave initiated by ETPs or their immediate progeny. Beyond day 15 after KGF treatment, increased and proportionally normal thymopoiesis was present for an extended period of time.
The KGF-mediated stimulation of fetal and adult TECs enhanced BMP2 and BMP4 transcripts. Induction of BMP4 expression is consistent with comparable findings in tracheal epithelium cultures exposed to either KGF or Fgf10, which binds also to FgfR2IIIb. It is likely but not formally demonstrated by our experiments that enhancement of BMP2/4 mediated the increase in thymic cellularity as Smad4−/− mice failed to expand their thymocyte numbers in response to KGF. Normal thymic cellularity is indeed dependent on the correct BMP dose, as mice either transgenic for the BMP antagonist, noggin, or deficient for the BMP4 inhibitor, twisted gastrulation, display a reduced thymic size secondary to microenvironmental changes.50,74 Even though our results demonstrated an increase in BMP2/BMP4 transcripts as a consequence of KGF signaling, a previous report had placed the effect of BMP4 on TECs upstream of KGF/Fgf10.51 This discrepancy is likely explained by differences in the experimental design, as the latter study investigated the effects of BMP4 on thymocyte development, while our analysis examined the immediate transcriptional changes in TECs upon KGF signaling. However, it is also conceivable that KGF/Fgf10 are part of a positive feedback loop regulating BMP4 signaling in TECs.
KGF treatment of TECs also enhanced Wnt expression, albeit at different magnitudes when comparing fetal and adult cells (Figures 6–7). This divergence most likely reflects differences in maturation and thus function. Wnt molecules exert a profound effect on TECs (S.Z., M.P.K., and G.A.H., manuscript in preparation; and Balciunaite et al49 ). We used Wnt10b as a readout in primary TECs and have established a link between KGF signaling and the activation of the PI3K/Akt/NF-κB and the p53 pathways. Stimulation of the PI3K/Akt/NF-κB pathway constitutes a typical feature of KGF signaling in some epithelial cells, but the activation of the p53 pathway has not yet been linked to KGF signaling.56,57 A functional link between the PI3K/Akt/NF-κB and the p53 pathways may occur indirectly since Akt can modulate p53 activity via a positive feedback loop, thus coordinating p53 activity with other signaling events including the Wnt pathway.75,76 Activation of the Ras/MAP kinase pathway was not required for the KGF-mediated up-regulation of Wnt10b. This finding was unexpected as Fgf signaling typically leads to the phosphorylation of the docking protein FRS2α and the recruitment of multiple Grb2/Sos complexes, which eventually results in the activation of the Ras/MAP kinase pathway.53,54 KGF may elicit differential effects in TECs, whereby only some necessitate the activation of the Ras/MAP kinase pathway.
Taken together, the exposure of TECs to KGF has revealed 3 specific aspects. First, KGF enhances in fetal and adult TECs the expression of growth and differentiation factors previously demonstrated to affect TEC biology. In consequence, a transiently enlarged but regularly structured thymic microenvironment is generated able to impart enhanced T-cell production. Second, the exposure of the thymic microenvironment to KGF decreased the expression of the chemokine CCL25 in TECs and thus may have affected the homing of T-cell precursors. This effect was, however, temporary and a proportionally normal representation of the different thymocyte subpopulations was re-established within 4 weeks after the start of treatment. Third, KGF caused changes in the stroma's ability to support separate phases in thymocyte maturation. While these changes allowed for an early and sequentially correct expansion of the TN thymocytes, the immediate consequences of the KGF treatment precluded in parallel a simultaneous increase in CD4+CD8+ and mature single-positive thymocytes. Transient alterations in the number, function, and/or composition of niches specific for the development and selection of more mature thymocytes could account for this finding. Thus, the treatment of naive mice with KGF may serve as an informative model to assess the molecular regulation of distinct microenvironmental niches necessary for mouse T-cell development.
KGF-induced thymopoiesis resulted in enhanced export of mature thymic T cells to the periphery. As there is no evidence that the thymus senses peripheral lymphopenia and gauges its T-cell export accordingly, and as changes in thymic output will contribute to the replenishment of the peripheral T-cell compartment, any increase in thymopoiesis may therefore be beneficial for individuals with lymphopenia. The enhanced thymic export of naive T cells will also secure a diverse T-cell repertoire, as it will concurrently offset the homeostatic expansion of a limited number of mature T cells. Hence, the use of KGF in clinical medicine may produce an efficient restoration of the T-cell compartment and thus the competence for an effective adaptive immune response.
Authorship
Contribution: S.W.R., W.K., and G.A.H. designed and performed work, analyzed the data, and wrote the paper; T.U., S.K., Y.T., L.T.J., M.P.K., and S.Z. designed and performed work; B.R.B. designed work and wrote the paper; A.V.G. provided materials. W.K. and G.A.H. share senior authorship.
Conflict-of-interest disclosure: The authors declare no competing financial interests. Human recombinant KGF (palifermin) was a generous gift of Amgen Inc (Thousand Oaks, CA). A.V.G. provided the p53 inhibitor.
Correspondence: Georg A. Holländer or Werner Krenger, Laboratory of Pediatric Immunology, Center for Biomedicine, University of Basel, Mattenstrasse 28, 4058 Basel, Switzerland; e-mail: georg-a.hollaender@unibas.ch or werner.krenger@unibas.ch
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grants 3100-68310.02 to G.A.H., and NFP46 to G.A.H. and W.K.), the European Community 6th Framework Program Euro-Thymaide Integrated Project (G.A.H.), the Basel Cancer League (W.K., M.P.K.), NIH grants R01-A1057477 (G.A.H) and R01-HL073794 (B.R.B), and the Dana Foundation.


![Figure 5. KGF enhances TEC numbers via induction of cell division in adult mice. (A-D) Adult TECs express the receptor for KGF. Thymic sections from 6-week-old, naive C57BL/6 mice were analyzed by confocal immunohistofluorescence for the expression of FgfR2IIIb on K18+ epithelial cells (A), K5+ cells (B), MTS24+ cells (C), and on TECs binding UEA1 (D). The colocalization denotes distinct TEC subpopulations expressing the KGF receptor (arrows). A total of 3 experiments were performed, providing comparable results. (E-H) TECs expand in response to KGF in vivo. Adult C57BL/6 mice were treated with KGF (5 mg/kg, ⊡) or HBSS (▪) on 3 consecutive days (days 0, 1, and 2). Frequencies of the 4 TEC subpopulations were assessed on day 3 by flow cytometry. Relative frequencies are given (x-fold changes KGF vs HBSS treatment, whereby frequencies [in %] for HBSS-treated mice were set as 1.0 and shown as dashed lines). Mean ± SD. *P < .01 versus HBSS. (I-N) KGF administration to adult mice does not alter the normal architecture and epithelial composition of the thymus. Confocal microscopy analysis was performed 15 days after treatment of adult C57BL/6 mice with HBSS (I-K) or KGF (L-N) for 4 TEC subpopulations, as indicated. mTEC indicates medullary TEC; cTEC, cortical TEC. (O) Adult C57BL/6 mice were treated as described in panels E-H and then injected with BrdU 48 and 24 hours before killing. BrdU+ cells among FSChighSSChighCD45−I-Ab+ cells present in freshly isolated thymic stromal cells (days 3 and 6) were analyzed (x-fold change KGF vs HBSS, whereby frequencies [in %] in HBSS-treated mice were set as 1.0 [dashed lines]). Mean ± SD; *P < .05 versus HBSS controls, with 6 mice per group and time point. A total of 3 experiments were performed, providing comparable results. (P) Absolute TEC numbers on days 6 and 15 following treatment of mice with either KGF (⊡) or HBSS (▪). Total CD45−I-Ab int+high stromal cells were counted by flow cytometry. Relative frequencies of total TECs are given, whereby the values for HBSS-treated mice were set as 1.0. The fractions of MTS24+ and MTS24− cells (separated by horizontal lines) among total TECs and their absolute cell numbers are also indicated. Mean ± SD; *P < .01, total TEC numbers and #P < .02, total MTS24+ cells, respectively, in KGF-treated mice versus those in HBSS controls. A total of 12 individual mice were analyzed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-10-049767/4/m_zh80090700090005.jpeg?Expires=1769218700&Signature=i-wv8GeLdyWM-I9XNZOgfQLsO2OythS-dBMMt6p8UX9SEYWXJkJWIvn-GH871aNDn98zlQqrVjHz6zLLt8RJ8XX3uscrU96RBMmB0VXAW88S7VSPyK1oMHzyWLjg5zcsLHhL1nC-4GaTcIVgjgp~nJoocKT~9nLWGVFu1IsJwJP~HWZ~2wXcuFtev0irLjjD6hyCK~8o6kDanUP7eT2ptl-hpQO9k4hACvU0dosKzNoWgL7Gpt0mhMlqV1TY~5-SB3hP2QFLBw7r4iRyRpTl3lGPbTzXY626NOC~9o7lnS-zZRYHPXsmNtt5FrbsoQnl8FqhnyVI1yrtAq-Pe5PoFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Wnts and BMPs are target genes of KGF. (A) Analysis of gene transcription by qRT-PCR in adult TECs. Six-week-old C57BL/6 mice were treated with KGF or HBSS as described in Figure 1 and sorted CD45−I-Ab+ TECs were analyzed on day 7 for mRNA expression of Wnt5b, Wnt10b, BMP2, and BMP4. Expression levels in KGF-treated mice (⊡) were compared with those in control mice treated with HBSS (x-fold change KGF vs HBSS, whereby the expression levels in the latter were set as 1.0; dotted line). (B) The increase in thymic cellularity in response to KGF depends on signals mediated by Smad4. Adult wild-type B6.129Smad4lox/lox mice (− Cre) and [Smad4lox/lox: Foxn1-cre]F2 mice (+ Cre) were treated with KGF (⊡) or HBSS (▪). Six and 15 weeks after the initiation of treatment, total thymocyte cellularity was determined. Absolute thymocyte numbers (× 10−6) are shown. Mean ± SD versus HBSS controls. For data shown in panel A, 3 experiments were performed whereby material from 15 mice was pooled for each experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-10-049767/4/m_zh80090700090006.jpeg?Expires=1769218700&Signature=dr~yaAuHt6cEd90JtuT0WT~6kQl1cTR5p86CLtWRSYMXdRM2qwoGHmkIdblqnIvouPKkpldYz2MFgRvn95pyWh~idsjtCGz3EiXc0Cvxmt-sITe7pAXpbQ9NkEQFd8Uo5Ii506dJcvhKwEcbQXfdg3Bje~7UAA~wjVPl6NBcLqvoCAFB9TxweJ0A8jmcc7PW6JUAQA1mVUgYczgoFirZF1jANKBpMCCIYIvc121RSAQ~DU07zOIvVrK2-zQaEgXFNdhDJRCtYIbUqokbzpTMp7HN1GheCInnTSQPAchOfgbmJsG87YOUfIvhcTPEdNoo5UkNS7ij9VUqOca1W59YLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal