Abstract
A unique aspect of integrin receptor function is the transmission of bidirectional signals. In platelets αIIbβ3 integrins require “inside-out” signals to bind fibrinogen and form thrombi. Following ligand binding, αIIbβ3 integrins generate “outside-in” signals that contribute to thrombus stability. Because integrin cytoplasmic tails are short and lack enzymatic activity, bidirectional signals are believed to be mediated by interactions with intracellular proteins, but the molecular basis for integrin signal transduction remains poorly understood. In the present study we have used retroviral vectors to express αIIbβ3 integrins with mutant β3 tails in mouse platelets and test mechanisms of bidirectional signaling. Using this approach we identify mutations (eg, β3Y747A) that confer loss of signaling in both directions and others (eg, β3T762A) that confer a selective loss of outside-in signals. These results reveal the presence of discrete bidirectional signaling pathways controlled by integrin β subunits in platelets and describe a high-throughput means of further investigating these pathways in vivo.
Introduction
The binding of fibrinogen and von Willebrand factor by platelet αIIbβ3 integrins is required for the generation and stability of arterial thrombi.1-3 The ability of platelets to bind fibrinogen is tightly regulated by intracellular signals, termed inside-out signals, that induce allosteric changes in the αIIbβ3 integrin required for extracellular ligand binding.4 Once bound to fibrinogen, αIIbβ3 integrins are believed to generate additional intracellular signals, termed outside-in signals, that contribute to thrombus stability and wound healing.5 Remarkably, this complex and diverse signal transduction is accomplished by αIIbβ3 and other integrin receptors through short cytoplasmic tails that lack any intrinsic enzymatic activity. Integrin bidirectional signals are thought to be transmitted via protein-protein interactions involving these short cytoplasmic tails.6 Biochemical studies have identified a large number of proteins as potential binding partners of the αIIbβ3 integrin,7,8 and signaling studies have implicated a diverse array of pathways both upstream and downstream of integrins,6,9 but how the integrin cytoplasmic tail orchestrates the transmission of bidirectional signals is not well understood
Recent studies support a model in which intracellular protein interactions with the integrin β subunit tail play key roles for both inside-out and outside-in signal transduction.10-12 In contrast to integrin α subunit cytoplasmic tails, the cytoplasmic tails of integrin β subunits are highly conserved, suggesting the existence of defined motifs required for integrin function. The best characterized of these are the tandem NPxY motifs capable of binding phosphotyrosine binding (PTB) domains on partner proteins.8 Biochemical, structural, and in vivo studies indicate that integrin β subunit NPxY interaction with the intracellular protein talin is both necessary and sufficient for inside-out activation of platelet integrins.12-15 Interactions of these β integrin subunit motifs with other intracellular proteins (eg, the adaptor Shc and the nonreceptor tyrosine kinase Syk) are also postulated to mediate outside-in signals.16-18 The identity and function of other β integrin motifs and binding partners involved in platelet bidirectional signals, however, have been more difficult to define. The nonreceptor tyrosine kinase src has recently been found to bind the distal portion of the β3 integrin tail and proposed to participate in platelet outside-in signals,10 but these and other studies have relied primarily on biochemical interactions and measures of receptor function in surrogate cell types such as the Chinese hamster ovary (CHO) cell line. Whether these studies accurately define bidirectional signaling pathways in platelets and the identity of other essential motifs is not known.
A major limitation of studying integrin receptor signaling in platelets has been the difficulty of expressing transgenic proteins in these short-lived, anuclear cells and in their megakaryocyte precursors. Gene targeting to introduce mutations in genes encoding integrin receptor proteins has been successful15,17 but is a time-consuming and low-throughput approach. Megakaryocyte-specific promoters are generally weak, and transgenic animals must be crossed onto null backgrounds to assess mutant receptor function. To circumvent these obstacles and to address the mechanism of bidirectional αIIbβ3 integrin signals in platelets, we have used a complementation strategy in which lethally irradiated animals were reconstituted with β3-deficient hematopoietic tissue engineered to express wild-type and mutant β3 mRNAs using retroviral vectors. Expression of wild-type β3 integrins using this approach rescued both inside-out and outside-in signaling responses in β3-deficient platelets. In contrast, expression of mutant β3 integrins revealed some with a complete loss of bidirectional signals, some with partial loss of bidirectional signals, and one with selective loss of outside-in signals. These studies establish a high-throughput approach to testing integrin signaling in platelets and identify discrete mechanisms by which integrins transduce bidirectional signals.
Materials and methods
Animals
The generation of integrin β3-deficient animals has been previously described,3 and the animals used were on a BALB/c genetic background. Wild-type BALB/c recipient mice were purchased from Charles River Laboratories (Wilmington, MA).
Antibodies and reagents
The phycoerythrin (PE)–conjugated anti–mouse CD61 (integrin β3 chain) monoclonal antibody clone 2C9.G2 (subclone of Hmβ3-1) was purchased from BD PharMingen (catalog no. 553347) (San Diego, CA). Fibrinogen from human plasma conjugated to Alexa-Fluor 647 was purchased from Molecular Probes (catalog no. F35299) (Eugene, OR). Actin staining was performed using Alexa-Fluor 594–conjugated phalloidin purchased from Molecular Probes (catalog no. A12381).
Retrovirus construction
The cDNA of murine integrin β3 was cloned by polymerase chain reaction (PCR) from C57BL/6 spleen total RNA. Point mutations of the cytoplasmic domain of integrin β3 were generated using the Stratagene (La Jolla, CA) quick change mutagenesis kit. Primers used were as follows: W739A forward: 5′GAGCCAGAGCCAAGGCGGACACAGCAAACAACC3′, W739A reverse: 5′ GGTTGTTTGCTGTGTCCGCCTTGGCTCTGGCTC3′; Y747A forward: 5′GCAAACAACCCGCTGGCCAAAGAGGCCACCTCC3′, Y747A reverse: 5′GGAGGTGGCCTCTTTGGCCAGCGGGTTGTTTGC3′; Y759A forward: 5′CCTTCACCAATATCACCGCCCGGGGGACTTAAGAA3′, Y759A reverse: 5′TTCTTAAGTCCCCCGGGCGGTGATATTGGTGAAGG 3′; T762A forward: 5′ATATCACCTACCGGGGGGCATAAGAATTCGATCAATCACTA3′, T762A reverse: 5′TAGTGATTGATCCAATTCTTATGCCCCCCGGTAGGTGATAT 3′. Wild-type and mutant β3 cDNAs were subcloned into the MSCV MigR1 viral vector with an IRES-GFP inserted prior to the polyadenylation signal as previously described.19
Fetal liver cell transplantation
Fetal liver cells were harvested from itgβ3−/− embryos at E14.5 to E16.5, and mononuclear cells were purified with Lympholyte (Cedarlane Labs, Burlington, NC) gradient and cultured overnight in IMDM with a supplement of murine stem cell factor (MSCF), IL-3, and IL-6. The cells then were transduced twice with β3 retrovirus at a multiplicity of infection (MOI) equal to 5 virions per cell. A total of 1 × 106 cells were transplanted by retro-orbital injection (250 μL per mouse) into recipient animals (8 to 10 weeks old) conditioned with a lethal dose of 800 cGy total body irradiation. Platelet studies were performed 6 to 8 weeks after transplantation.
Blood collection
Mice were anesthetized with 16 μg/g body weight tribromoethanol, and whole blood (100 μL) was collected by retro-orbital bleeding with a heparinized capillary tube. Platelet-rich plasma (PRP) was obtained by diluting whole blood 1:3 in Tyrode buffer and performing centrifugation at 100g for 15 minutes.
Integrin β3 staining using flow cytometry
PRP was diluted to 2.5 × 107 platelets per milliliter with Tyrode buffer containing 1 mM MgCl2 and 1 mM CaCl2, and 2.5 × 106 platelets were incubated with PE-conjugated anti–mouse integrin β3 (CD61) (2 μL/mL) at room temperature for 30 minutes.
Fibrinogen binding assay
PRP was diluted to 2.5 × 107 platelets per milliliter with Tyrode buffer containing 1 mM MgCl2 and 1 mM CaCl2, and 2.5 × 106 platelets were incubated with 100 μg/mL Alexa-Fluor 647–conjugated fibrinogen for 12 minutes at room temperature. The reaction was stopped by fixation with 1% paraformaldehyde (PFA) for 5 minutes, and then platelets were washed twice with PBS by centrifugation at 1000g. β3 staining and fibrinogen binding were measured with a FACSort (BD Biosciences, San Jose, CA), and the data were analyzed with FlowJo 6.3.1 software (Tree Star, Ashland, OR).
Platelet-spreading assay
PRP was diluted to 2.5 × 107 platelets per milliliter with Tyrode buffer containing 1 mM MgCl2 and 1 mM CaCl2, and 5 × 106 platelets were incubated at 37°C for 45 minutes in multichamber slides coated with 100 μg/mL fibrinogen. The unbound platelets were gently washed off with PBS twice. The attached platelets were fixed with 2% PFA for 5 minutes and permeabilized with PBS containing 0.2% Triton X-100 for 5 minutes. Cytoskeletal actin was stained with Alexa-Fluor 594–conjugated phalloidin at room temperature for 1 hour. Platelet spreading was observed using a Nikon Eclipse E600 fluorescence microscope (Nikon, Melville, NY) equipped with a 100×/1.30 numerical aperture oil objective lens. Images were taken using a Nikon DXM1200 digital camera and Nikon ACT-1 software version 2. The images of GFP and actin staining were overlaid using Photoshop (Adobe, San Jose, CA) software, and only platelets with both GFP and actin staining were analyzed. Platelet surface area was measured in pixels using Image J software (National Institutes of Health, Bethesda, MD) particle analysis. Particles sizing from 225 to 4050 pixels, which represented those well-separated platelets, were measured. Results shown are the average spreading area of 100 to 1000 platelets from 7 animals for each genotype.
Statistics
Means and standard deviations are shown with the number of samples for each group indicated as “n.” P values shown were calculated using the 2-tailed Student t test.
Results
Retroviral expression of wild-type β3 restores inside-out and outside-in signaling responses in β3-deficient platelets
To address structure-function questions regarding bidirectional signaling by the β3 integrin subunit in platelets, we first developed a high-throughput means of expressing and testing wild-type and mutant β3 integrins in platelets. Because platelets are anuclear and synthesize little or no protein after release from the bone marrow, stable in vivo expression must be accomplished through megakaryocytes, the platelet precursor in the bone marrow. Megakaryocytes and platelets express very high levels of β3 integrins (approximately 50 000 to 80 000 per human platelet); thus, a useful experimental system must be robust as well as high-throughput. We and others have previously used retroviral vectors to express transgenes in platelets,20,21 suggesting that retroviral complementation of β3-deficient hematopoietic cells might allow in vivo structure-function analysis of platelet αIIbβ3 integrins.
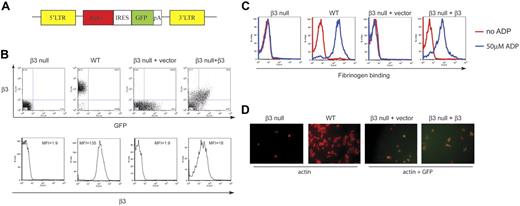
To test the ability of retrovirally expressed β3 integrin subunits to confer bidirectional signaling responses in platelets, we harvested fetal liver from E14.5 Itgβ3−/− embryos and exposed fetal liver cells to retrovirus engineered to express both the wild-type β3 integrin subunit and GFP (Figure 1A). Following ex vivo exposure to retrovirus, the fetal liver cells were injected into lethally irradiated wild-type mice and platelets harvested 6 to 8 weeks after transplantation. Analysis of platelets in the whole blood of reconstituted animals revealed that between 20% and 70% of platelets expressed both surface αIIbβ3 integrin and GFP (Figure 1B, top, and data not shown). Surface levels of αIIbβ3 integrins in GFP-positive platelets were approximately 2- to 3-fold lower than those in wild-type platelets (Figure 1B, bottom, and data not shown), and simultaneous measurement of GFP and β3 integrins in individual platelets revealed close correlation (Figure 1B, top). Virtually no platelets expressed β3 integrins in the absence of GFP, indicating that all αIIbβ3-expressing platelets were the product of retroviral β3 expression in β3-deficient donor cells and not derived from wild-type host hematopoietic cells that escaped irradiation (Figure 1B and data not shown).
Retroviral β3 expression restores bidirectional signaling in β3-deficient platelets. (A) Schematic representation of the retroviral vector used to express both integrin β3 and GFP in β3-deficient hematopoietic cells. LTR indicates long terminal repeat; itgβ3, coding region of the mouse β3 cDNA; IRES, internal ribosomal entry site; GFP, green fluorescent protein; pA, SV40 polyadenylation signal. (B) Retroviral expression of β3 integrins. β3-deficient fetal liver cells exposed to the retroviral vector shown in panel A or a control vector expressing only GFP were used to reconstitute lethally irradiated mice. Shown is the expression of β3 integrins in β3-deficient (β3 null) platelets, wild-type (WT) platelets, platelets from β3-deficient cells exposed to vector expressing GFP only (β3 null + vector), and platelets from β3-deficient cells exposed to vector expressing wild-type β3 and GFP (β3 null + β3). Dot plots show GFP vsersus β3 expression in individual platelets. The histograms show β3 expression in wild-type and β3 null platelets and in GFP-positive platelets from reconstituted animals. MFI indicates mean fluorescent intensity. (C) Retroviral expression of β3 integrins restores inside-out signaling in β3-deficient platelets. Fibrinogen binding was measured in the platelets described in panel B with (blue lines) and without (red lines) ADP stimulation and binding by soluble Alexa-Fluor 647–conjugated fibrinogen was measured. (D) Retroviral expression of β3 integrins restores outside-in signaling in β3-deficient platelets. The platelets described in panel B were exposed to immobilized fibrinogen and platelet spreading observed at 45 minutes. Platelets were visualized using Alexa-Fluor 594–conjugated phalloidin to detect cellular actin and GFP to detect platelets with retroviral expression of β3 integrins.
Retroviral β3 expression restores bidirectional signaling in β3-deficient platelets. (A) Schematic representation of the retroviral vector used to express both integrin β3 and GFP in β3-deficient hematopoietic cells. LTR indicates long terminal repeat; itgβ3, coding region of the mouse β3 cDNA; IRES, internal ribosomal entry site; GFP, green fluorescent protein; pA, SV40 polyadenylation signal. (B) Retroviral expression of β3 integrins. β3-deficient fetal liver cells exposed to the retroviral vector shown in panel A or a control vector expressing only GFP were used to reconstitute lethally irradiated mice. Shown is the expression of β3 integrins in β3-deficient (β3 null) platelets, wild-type (WT) platelets, platelets from β3-deficient cells exposed to vector expressing GFP only (β3 null + vector), and platelets from β3-deficient cells exposed to vector expressing wild-type β3 and GFP (β3 null + β3). Dot plots show GFP vsersus β3 expression in individual platelets. The histograms show β3 expression in wild-type and β3 null platelets and in GFP-positive platelets from reconstituted animals. MFI indicates mean fluorescent intensity. (C) Retroviral expression of β3 integrins restores inside-out signaling in β3-deficient platelets. Fibrinogen binding was measured in the platelets described in panel B with (blue lines) and without (red lines) ADP stimulation and binding by soluble Alexa-Fluor 647–conjugated fibrinogen was measured. (D) Retroviral expression of β3 integrins restores outside-in signaling in β3-deficient platelets. The platelets described in panel B were exposed to immobilized fibrinogen and platelet spreading observed at 45 minutes. Platelets were visualized using Alexa-Fluor 594–conjugated phalloidin to detect cellular actin and GFP to detect platelets with retroviral expression of β3 integrins.
The end point of inside-out signaling through αIIbβ3 integrins is the ability to bind soluble fibrinogen following stimulation by platelet agonists such as ADP. To test the ability of retrovirally expressed wild-type β3 integrins to restore inside-out signaling responses, we next measured ADP-induced fibrinogen binding by platelets from mice reconstituted with vector encoding GFP alone or vector encoding wild-type β3 plus GFP. Fibrinogen binding was measured using Alexa-Fluor 647–conjugated fibrinogen, a fluorescent molecule that can be detected in the presence of GFP using flow cytometry. More than 95% of GFP-positive platelets from animals reconstituted with wild-type β3-expressing vector, but none from animals reconstituted with GFP-expressing vector, showed robust fibrinogen binding (Figure 1C). Thus, expression of wild-type β3 integrins using a retroviral vector restores agonist-induced fibrinogen binding to β3-deficient platelets, indicating that retroviral complementation can be used to examine inside-out signaling responses mediated by β3 integrins in platelets.
In contrast to inside-out signaling, the measurement of outside-in signals through αIIbβ3 integrins in platelets is less well defined. Ideally, outside-in signals should be measurable independent of inside-out signals (ie, in unstimulated platelets in which inside-out activating signals do not obscure identification of subsequent outside-in signals). This ideal is constrained, however, by the stringent requirement of αIIbβ3 integrins for inside-out activation in order to bind ligand. To circumvent this limitation, outside-in signaling has been studied by allowing unstimulated platelets to settle on a fibrinogen-coated surface and the presence or absence of subsequent platelet spreading used as a measure of outside-in signaling.22-24 In this assay the limited number of αIIbβ3 integrin receptors capable of binding fibrinogen without inside-out activation are allowed to productively engage ligand over time and generate a signaling response that culminates in platelet spreading. Using this approach, the Syk/Slp-76/PLCγ2 signaling pathway has been identified as an outside-in pathway in platelets and these platelet findings confirmed by the loss of more easily quantified outside-in signaling responses by neutrophil integrins in the same animals.22,23,25 Platelet spreading on fibrinogen does not appear to be dependent on secondary signaling pathways, because inhibition of ADP signaling by the scavenger apyrase does not reduce the spreading of wild-type platelets (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). An important advantage of the platelet-spreading assay for a retroviral complementation strategy is that it is a single-cell assay in which the response of GFP-expressing platelets that coexpress β3 integrins can be selectively examined even if the percentage of reconstituted platelets is relatively low.
To test the ability of retrovirally expressed wild-type β3 integrins to restore outside-in signaling responses, we therefore examined the spreading of unstimulated platelets on a fibrinogen-coated surface. Expression of wild-type β3 resulted in a spreading response that more than doubled the platelet surface area compared with GFP-expressing platelets after 45 minutes (Figure 1D). These studies indicate that retroviral complementation of β3 restores outside-in signals as well as inside-out signals by platelet αIIbβ3 integrins.
Expression of β3 integrins with cytoplasmic tail mutations in β3-deficient platelets using retroviral vectors
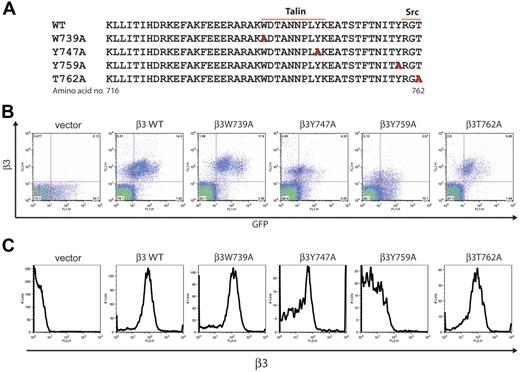
Studies in CHO cells and megakaryocytes suggest that binding of the protein talin to the β3 integrin cytoplasmic tail is both necessary and sufficient for inside-out integrin activation.12 Biochemical and structural studies indicate that talin-β3 binding is mediated by interaction between the talin PTB domain and β3 NPxY motifs, especially the region between W739 and K748 encompassing the N-terminal NPxY motif.14 In contrast, the more carboxy-terminal amino acids of the β3 tail have not been implicated in talin binding but have been found to bind molecules such as Syk and src that are proposed to mediate outside-in signals that require the C-terminal NPxY motif and T762, respectively.11,26 To test this model of N- and C-terminal coupling to inside-out and outside-in signaling pathways, respectively, in platelets, we generated retroviral vectors designed to express β3W739A, β3Y474A, β3Y759A, and β3T762A in β3-deficient platelets (Figure 2A).
Expression of β3 integrins with mutant cytoplasmic tails in β3-deficient mouse platelets. (A) The amino acid sequence of the cytoplasmic tail of the mouse β3 integrin subunit and the sites of alanine substitution for mutant subunits (red letters) are shown. The predicted sites of talin and src-family kinase binding are indicated by lines. (B) Retroviral expression of GFP and mutant β3 integrins in the platelets of reconstituted animals. Surface β3 integrin expression and GFP expression were measured in live platelets using flow cytometry. Platelets were harvested from animals reconstituted with β3-deficient fetal liver exposed to retroviral vectors encoding GFP only (vector), wild-type β3 (β3WT), and the β3 mutants indicated. The dot blots shown are representative of 7 animals of each type studied. Note the generally close correlation between GFP and β3 levels. (C) Expression of mutant β3 integrins on the platelet surface. Shown are histograms of surface β3 integrins in GFP-positive platelets from animals reconstituted with β3-deficient hematopoietic cells expressing the indicated mutant β3 subunits.
Expression of β3 integrins with mutant cytoplasmic tails in β3-deficient mouse platelets. (A) The amino acid sequence of the cytoplasmic tail of the mouse β3 integrin subunit and the sites of alanine substitution for mutant subunits (red letters) are shown. The predicted sites of talin and src-family kinase binding are indicated by lines. (B) Retroviral expression of GFP and mutant β3 integrins in the platelets of reconstituted animals. Surface β3 integrin expression and GFP expression were measured in live platelets using flow cytometry. Platelets were harvested from animals reconstituted with β3-deficient fetal liver exposed to retroviral vectors encoding GFP only (vector), wild-type β3 (β3WT), and the β3 mutants indicated. The dot blots shown are representative of 7 animals of each type studied. Note the generally close correlation between GFP and β3 levels. (C) Expression of mutant β3 integrins on the platelet surface. Shown are histograms of surface β3 integrins in GFP-positive platelets from animals reconstituted with β3-deficient hematopoietic cells expressing the indicated mutant β3 subunits.
Following transduction of β3-deficient fetal liver and transplantation into lethally irradiated hosts, platelet surface expression of the β3 mutant receptors was measured using flow cytometry. Surface levels of all mutant β3 integrin receptors were similar to wild type with the exception of Y759A, a mutant subunit that repeatedly conferred lower levels of surface β3 despite similar vector titers (Figure 2B-C and data not shown). Thus, most mutant β3 subunits associate normally with the integrin alpha subunit and are expressed as a complete integrin receptor.
Inside-out αIIbβ3 integrin signals require the N-terminal β3 NPxY motif
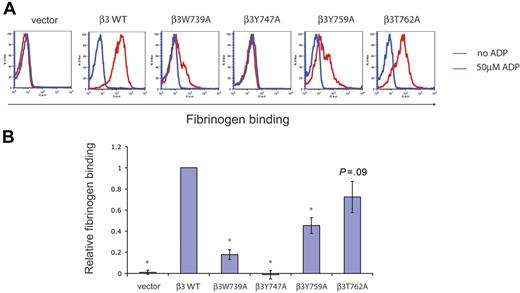
To determine the ability of αIIbβ3 receptors with mutant C tails to transmit inside-out signals, we next measured fibrinogen binding by reconstituted platelets following ADP stimulation. Fibrinogen binding by GFP-positive platelets reconstituted with wild-type and mutant β3 receptors with and without ADP stimulation is shown by a representative fluorescence-activated cell sorter (FACS) plot (Figure 3A) and a graph comparing the mean fibrinogen binding of each mutant with that of wild-type β3 (Figure 3B). A complete defect in inside-out signaling was observed in β3Y747A platelets and partial defects observed in β3W739A and β3Y759A platelets (Figure 3A). In contrast, β3T762A platelets exhibited inside-out signaling that was not significantly reduced compared with wild type (Figures 3). These results demonstrate that the N-terminal NPxY motif of the β3 integrin cytoplasmic tail is required for inside-out signaling by αIIbβ3. In contrast, the adjacent residues W739 and the C-terminal NPxY motif are contributory but nonessential and the most carboxy-terminal region noncontributory for inside-out signal transduction.
Inside-out αIIbβ3 signals require the N-terminal β3 NPxY motif. (A) ADP-stimulated fibrinogen binding by wild-type and mutant β3 integrins in platelets. β3-deficient platelets expressing wild-type (WT) and mutant β3 integrins were exposed to 50 μM ADP (red lines) or buffer (blue lines) and the binding of soluble fibrinogen used to measure inside-out signal transduction. Note the complete loss of inside-out integrin activation in β3Y747A platelets. The experiment shown is representative of more than 15 experiments in 7 individual animals performed for each mutant. (B) Relative inside-out signal transduction by β3 mutant receptors. To obtain a relative measure of inside-out signaling, the mean fluorescent intensity of bound fibrinogen was normalized to the mean fluorescent intensity of surface β3 expression. P values for values obtained in platelets reconstituted with wild-type versus individual mutant β3 integrins were calculated. Asterisk indicates P < .05; n = 7 animals studied for each group; error bars indicate mean ± standard deviation.
Inside-out αIIbβ3 signals require the N-terminal β3 NPxY motif. (A) ADP-stimulated fibrinogen binding by wild-type and mutant β3 integrins in platelets. β3-deficient platelets expressing wild-type (WT) and mutant β3 integrins were exposed to 50 μM ADP (red lines) or buffer (blue lines) and the binding of soluble fibrinogen used to measure inside-out signal transduction. Note the complete loss of inside-out integrin activation in β3Y747A platelets. The experiment shown is representative of more than 15 experiments in 7 individual animals performed for each mutant. (B) Relative inside-out signal transduction by β3 mutant receptors. To obtain a relative measure of inside-out signaling, the mean fluorescent intensity of bound fibrinogen was normalized to the mean fluorescent intensity of surface β3 expression. P values for values obtained in platelets reconstituted with wild-type versus individual mutant β3 integrins were calculated. Asterisk indicates P < .05; n = 7 animals studied for each group; error bars indicate mean ± standard deviation.
Outside-in αIIbβ3 integrin signals require C-terminal β3 motifs
Analysis of β3 mutant receptor inside-out signaling in platelets suggested that inside-out and outside-in signals might be spatially divided such that the former were mediated primarily by interactions at the N-terminal region of the receptor tail and the latter by interactions at the receptor C terminus. To address this possibility, we next tested outside-in signaling in the β3 mutants described using platelet spreading on fibrinogen as described in Figure 1D. β3Y747A platelets that were completely deficient in inside-out signaling demonstrated a significant but not absolute defect in outside-in–mediated platelet spreading (Figure 4). Similar to their inside-out responses, β3W739A and β3Y759A platelets exhibited partial defects in platelet spreading, although the outside-in defect observed with β3Y759A platelets was relatively greater than the inside-out defect observed (Figures 3 and 5). Remarkably, β3T762A platelets that exhibited normal fibrinogen binding exhibited a nearly complete loss of platelet spreading (Figure 4A). These findings support the existence of overlapping but discrete structural requirements for inside-out and outside-in signaling pathways in platelet β3 integrins.
Outside-in αIIbβ3 signals require C-terminal β3 motifs. (A) Platelet spreading on immobilized fibrinogen mediated by wild-type and mutant β3 integrins. Outside-in signaling was measured using platelet spreading after 45 minutes of incubation on a fibrinogen-coated surface. Adherent platelets were visualized using actin staining with Alexa-Fluor 594 phalloidin (top) and green fluorescence to detect β3-expressing platelets (middle). The overlay of actin and GFP staining is shown on the bottom row. (B) Relative outside-in signaling by platelets expressing wild-type and mutant β3 integrins. The area occupied by adherent platelets was measured using the Image J program. P values are shown for comparison of values obtained in platelets expressing wild-type and each mutant β3 integrin type. Asterisk indicates P < .01; n = 100 to 1000 individual platelets from 7 animals analyzed for each type of platelet; error bars indicate mean ± standard deviation.
Outside-in αIIbβ3 signals require C-terminal β3 motifs. (A) Platelet spreading on immobilized fibrinogen mediated by wild-type and mutant β3 integrins. Outside-in signaling was measured using platelet spreading after 45 minutes of incubation on a fibrinogen-coated surface. Adherent platelets were visualized using actin staining with Alexa-Fluor 594 phalloidin (top) and green fluorescence to detect β3-expressing platelets (middle). The overlay of actin and GFP staining is shown on the bottom row. (B) Relative outside-in signaling by platelets expressing wild-type and mutant β3 integrins. The area occupied by adherent platelets was measured using the Image J program. P values are shown for comparison of values obtained in platelets expressing wild-type and each mutant β3 integrin type. Asterisk indicates P < .01; n = 100 to 1000 individual platelets from 7 animals analyzed for each type of platelet; error bars indicate mean ± standard deviation.
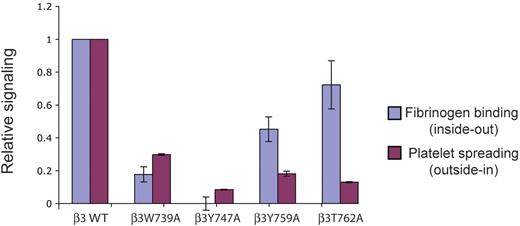
Relative defects in bidirectional signaling conferred by β3 cytoplasmic tail mutations. A bar graph is used to show the relative change in soluble fibrinogen binding (a measure of inside-out signaling) versus that in spreading on immobilized fibrinogen (a measure of outside-in signaling) in platelets expressing each mutant β3 C-tail; n = 7 for each group; error bars indicate mean ± standard deviation.
Relative defects in bidirectional signaling conferred by β3 cytoplasmic tail mutations. A bar graph is used to show the relative change in soluble fibrinogen binding (a measure of inside-out signaling) versus that in spreading on immobilized fibrinogen (a measure of outside-in signaling) in platelets expressing each mutant β3 C-tail; n = 7 for each group; error bars indicate mean ± standard deviation.
Discussion
The molecular basis of bidirectional integrin signaling has been difficult to establish because it appears to be mediated by complex and dynamic associations between short integrin cytoplasmic tails and a large pool of cytoplasmic target proteins. Biochemical studies have provided important clues regarding the identity of candidate interacting proteins, but how small cytoplasmic domains of the receptor transmit complex signals in 2 directions over brief periods of time remains unknown. In the present study we have performed in vivo structure-function analysis to test findings previously reached using biochemical, structural, and in vitro approaches in platelets. Our results provide strong support for the hypothesis that talin binding is directly responsible for inside-out activation of β3 integrins and direct evidence that αIIbβ3 outside-in signaling pathways are spatially distinct from those of inside-out pathways in platelets.
To perform structure-function analysis of the αIIbβ3 integrin in platelets, we used a complementation strategy in which retroviral vectors drove expression of wild-type and mutant β3 integrins in β3-deficient hematopoietic cells. A similar approach has previously employed lentivirus with an αIIb promoter element to drive platelet-specific expression of β3 in deficient megakaryocytes and platelets as a model for gene therapy.20 Although our approach expressed levels of β3 integrins similar to those observed with the αIIb lentivirus, in both cases expression varied both between animals and between platelets due to variable transduction efficiency in hematopoietic stem cells. This limitation can be overcome for structure-function analysis, however, by the use of an IRES-GFP to identify individual cells that express the transgene and by the use of single-cell assays to measure bidirectional signals in GFP-positive cells. Thus, retroviral complementation is an efficient and fairly high-throughput means of testing mutant integrin receptor function in platelets. In addition, because retroviral vector transduction efficiency is variable and individual animals may have large or small populations of platelets that remain deficient, assays that test the function of the entire platelet population (eg, biochemical assays, platelet aggregation, or in vivo thrombosis studies) will vary on the basis of transduction efficiency. Ideally, structural studies and biochemical studies performed using cell lines and purified proteins can be coupled to retroviral expression in platelets to test structure-function models of receptor function.
Using this approach we have begun to test proposed mechanisms by which αIIb3 integrins transmit inside-out signals required to bind fibrinogen and outside-in signals believed to play a role in later thrombus stability. A key inside-out signal has been proposed to be talin binding to the β3 integrin cytoplasmic tail. Structural studies indicate that the FERM domain of talin uses a variant PTB domain to bind β3 in a region between amino acids 739 and 750,14 a region that encompasses the N-terminal β3 NPxY motif. Biochemical analysis of the binding of this talin domain to β3 tail peptides reveals a complete loss of binding to the β3Y747A mutant and a severe but incomplete loss of binding to the β3W739A mutant.12 In the present study we have tested these predictions in platelets. Indeed, we find that β3Y747A integrins are entirely deficient in fibrinogen binding after ADP stimulation while β3W739A integrins are severely but not completely defective, a remarkably close concordance to the biochemical interaction with talin. In contrast, β3 mutations affecting more carboxy-terminal regions of the receptor tail (eg, Y759A and T762A) have less impact on inside-out signaling. Thus, structural analysis, biochemical studies, and in vivo function support talin binding to the β3 integrin subunit as a critical step for inside-out signal transduction. Interestingly, we recently reported a similar loss of inside-out activation of β1 integrins in platelets in association with mutations of the 2 β1 NPxY motifs using gene targeting,15 suggesting that this mechanism of inside-out activation extends to all integrins. Our observation that β3Y759A platelets exhibit reduced fibrinogen binding may indicate contribution by proteins other than talin in the transmission of inside-out signals, but the low surface expression of the β3Y759A receptor limits interpretation of this experiment.
In contrast to inside-out signaling, the molecular basis of αIIbβ3 integrin outside-in signaling is less investigated. In part, this stems from the difficulty in generating and measuring outside-in integrin signals in the absence of the inside-out signals required for the integrin to bind ligand. This limitation has been partially circumvented by examining the spreading of unstimulated platelets on fibrinogen, a response that is believed to be mediated by outside-in integrin signals. This response is deficient in platelets that lack Syk, Slp-76, and PLCγ2 that exhibit normal inside-out responses to G protein–coupled receptor agonists,22,23,25 and biochemical studies suggest that both Syk and the src-family kinases (SFKs) required to activate Syk bind the β3 integrin tail. These studies predict that Syk binds the region encompassing the 2 tyrosine residues of the β3 tail18,26 and that SFKs bind the carboxy-terminal RGT sequence in β3.10 In fibroblasts and CHO cells mutant integrins lacking this region of the β3 tail do not mediate spreading,10,11 but responses in these cells required coexpression of SFKs and whether such responses are predictive of platelet outside-in signals has not been tested. Our finding that platelet-spreading responses are almost completely deficient in β3T762A mutant platelets suggests that this mechanism is operative in platelets. The observed reduction in outside-in responses in β3Y759A mutant platelets despite preserved inside-out signals is also notable, but interpretation of this result must be made cautiously given the low receptor expression level.
Most importantly, our findings provide direct evidence that inside-out and outside-in signaling through β3 integrins in platelets are mediated by discrete pathways involving distinct protein interactions with the β3 tail, a hypothesis previously put forward on the basis of studies in cell lines.11 β3T762A platelets demonstrate profound outside-in defects despite preserved inside-out responses, a phenotype similar to that reported for β3Y747F, Y759F double-mutant mouse platelets.17 Similarly, although β3W739A platelets exhibit defects in both signaling pathways, the inside-out signaling defect appears greater than the outside-in signaling defect (Figure 5). Thus, defects in one directional pathway do not necessarily confer defects in the other.
How integrin β subunit cytoplasmic tails orchestrate bidirectional signaling remains unknown. The recent observation that filamin binding to β integrin cytoplasmic tails is competitive with that of talin27 suggests a competitive model in which multiple proteins jockey for position on the β integrin tail, but studies of protein-protein interactions in live cells will be necessary to determine which biochemical interactions take place and how they are orchestrated by cells. The findings presented here should facilitate the testing of existing and new molecular hypotheses in hematopoietic cells in vivo and accelerate investigation of the molecular basis of integrin signaling.
Authorship
Contribution: Z.Z. designed and performed the experiments described and wrote the paper; H.C. designed and performed the experiments described; A.A.S. designed and performed platelet adhesion assays; R.O.H. contributed to the design of the experiments and wrote the paper; and M.L.K. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark L. Kahn, BRBII/III Room 952, 421 Curie Blvd, University of Pennsylvania, Philadelphia, PA 19104; e-mail: markkahn@mail.med.upenn.edu.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr Joel Bennett for critical reading of this manuscript and for thoughtful advice throughout the course of these studies.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal