Abstract
Suppression of thromboxane (Tx) A2 biosynthesis retards atherogenesis. In this setting, the coincidental presence of nonconventional ligands for the TxA2 receptor (TP), such as isoprostanes, could still induce a proatherogenic vascular phenotype. However, no data are available on the effect of combining suppression of TxA2 formation with blockade of TP in atherogenesis. To this end, we tested the effect of a selective COX-1 inhibitor, SC560, a TP antagonist, BM-573, or a combination of both in low-density lipoprotein receptor-deficient mice on a high-fat diet. None of the treatments affected body weight or plasma cholesterol or triglycerides levels. Although SC-560 suppressed TxA2 biosynthesis, BM-573 reduced its levels by 35%; in contrast, the 2 drugs, alone or in combination, did not significantly affect prostacyclin levels. At the end of the study, SC560 and BM-573 reduced atherogenesis; however, a further significant decrease was observed in mice receiving both drugs. This effect was associated with a further significant reduction of vascular inflammation, a decrease in macrophages, and an increase in the content of collagen and smooth muscle cells of the atherosclerotic lesions. These results show for the first time that the addition of a TP antagonist increases the antiatherogenic effect of COX-1–dependent TxA2 suppression.
Introduction
Atherosclerosis is a complex and chronic inflammatory disease of the arterial wall influenced by diverse biochemical factors, including cytokines, chemokines, and growth factors.1 One group of these mediators is represented by the prostanoids, a large family of bioactive lipids generating from arachidonic acid by the enzyme cyclooxygenase (COX).2 The biosynthesis of prostanoids, specifically thromboxane A2 (TxA2) and prostacyclin (PGI2), is grossly altered in human as well as in experimental atherosclerosis.3,4 The increase in PGI2 reflects in part the induction of both COX isoforms, COX-1 and COX-2, in the arterial wall.5 However, most of the TxA2 in this clinical setting derives from platelet COX-1 activation.6 Both compounds may influence atherogenesis in different ways by modulating vascular inflammatory responses, cell growth, apoptosis, migration, and proliferation, processes that have all been implicated in atherosclerosis.7 Cardioprotection from aspirin is widely attributed to its inhibitory effect on platelet COX-1–dependent TxA2 formation.8 Previously, we have provided evidence for the importance of COX-1–dependent TxA2 formation in atherogenesis. Thus, targeted pharmacological inhibition of COX-1 by indomethacin as well as low-dose aspirin, but not COX-2 by nimesulide, retards atherogenesis in low-density lipoprotein receptor–deficient (LDLR KO) mice.9-11 Consistent with our observation, selective inhibition of COX-1 enzyme activity and genetic deletion of COX-1 gene expression both reduce atherogenesis in another mouse model, the apolipoprotein E-KO (apoE-KO).12,13 However, challenging this view, a recent report showed that COX-1 deficiency in bone marrow-derived cells worsens early atherosclerosis.14
In general, although inhibition of COX-1 activity suppresses TxA2 biosynthesis, it does not prevent the formation and, most importantly, the actions of other eicosanoids, such as hydroxyeicosatetraenoic acids and isoprostanes, which can both directly stimulate the receptor for TxA2 and trigger proinflammatory reactions.15 Interestingly, antagonism or genetic deletion of the TxA2 receptor, called TP, reduces atherosclerosis in apoE-KO mice.16,17 Taken together, the data would support the hypothesis that suppression of TxA2 formation alone would not afford the most antiatherogenic effect because of the coincidental presence of nonconventional TP ligands, which could still favor a proinflammatory and proatherogenic vascular phenotype. Therefore, antagonism of the TP receptor associated with suppression of TxA2 biosynthesis could augment the beneficial anti-inflammatory effects of COX-1 inhibition and be a more effective therapeutic approach to modulate atherogenesis than suppression of COX-1 activation alone.
To this end, we treated LDLR KO mice with SC-560, a selective COX-1 inhibitor,18 BM-573, a TP antagonist,19 or a combination of the 2 drugs for 12 weeks during the development of atherogenesis. At the end of the treatments, we found that the combination therapy, by inducing a more potent anti-inflammatory effect, reduced atherogenesis more efficiently than either of the 2 drugs alone.
Materials and methods
Animals and experimental protocols
LDLR KO mice (back-crossed 10 times to C57BL/6 mice) were obtained from Jackson Laboratories (Bar Harbor, ME) at 6 weeks of age. All procedures and care of animals were approved by the Institutional Animal Care and Usage Committee of the University of Pennsylvania. Only male mice were used in the studies presented in this paper.
Starting at 8 weeks of age mice were fed a high-fat diet (normal chow supplemented with 0.15% cholesterol and 20% butter fat), divided into 4 groups of 10 mice each, and randomized to receive placebo, SC-560 (Cayman Chemical, Ann Arbor, MI; 15-mg/kg diet), BM-573 (10 mg/L), or a combination of the 2 drugs for 12 weeks. The doses used in our studies were based on previous published works.19,20 Urine was collected overnight in metabolic cages; blood samples were obtained by retro-orbital bleeding from animals fasted overnight, as previously described.9,10
Biochemical analysis
When possible all the analyses were performed in duplicate and a blind fashion. Serum TxB2, urinary 2,3-dinor-TxB2, 2,3-dinor 6-keto-PGF1α, and isoprostane iPF2α-III levels were all measured by specific and sensitive enzyme-linked immunosorbent assay (ELISA) kits (Cayman Chemical) as previously described.21,22 Plasma cholesterol and triglyceride levels were determined enzymatically using Sigma reagents (Sigma Chemical, St Louis, MO). Levels of monocyte chemoattractant protein 1 (MCP-1) were measured by a specific ELISA kit, as previously described (Endogen, Woburn, MA).10,11 Serum paraoxonase 1 (PON1) activity was determined with the use of paraoxon (O'-O-diethyl-O-P-nitrophenylphosphate; Sigma Chemical) as the substrate and measured by the increase in the absorbance at 412 nm due to the formation of 4-nitrophenol, as previously described. Briefly, 15μL serum was added to Tris/HCl buffer containing 1 mM CaCl2 and 1 mM paraoxon, and the generation of 4-nitrophenol was measured at 412 nm.22
Platelet aggregation studies
Platelet aggregation was studied as previously described.4 Briefly, anticoagulated blood was immediately centrifuged at 100g for 10 minutes at room temperature and platelet-rich plasma collected. The remaining fraction was centrifuged at 2000g to obtain platelet-poor plasma. Platelet aggregation was determined by light absorbance using a platelet aggregometer with constant magnetic stirring. Arachidonic acid (100 μM) and U-46619 (1 μM), the stable analog of TxA2 and specific TP agonist, were used as agents to induce an irreversible aggregation.
Preparation of mouse aortas and quantitation of atherosclerosis
After the final blood collection, mice were killed and the aortic tree was perfused for 10 minutes with ice-cold PBS containing 20 μM butylated hydroxytoluene (BHT) and 2 mM EDTA, pH 7.4, by inserting a cannula into the left ventricle and allowing free efflux from an incision in the vena cava.
Following removal of the surrounding adventitial tissue, the aorta was opened longitudinally from the aortic root to the iliac bifurcation, fixed in formal-sucrose (4% paraformaldehyde, 5% sucrose, 20 μM BHT and 2 mM EDTA, pH 7.4) then stained with Sudan IV. The extent of atherosclerosis was determined using the en face method. Quantitation was performed by capturing images of aortas with a Dage-MTI 3CCD 3 chips color video camera connected to a Leica MZ12 dissection microscope, as previously described.11,21
Atherosclerosis was also quantified in the aortic root cross-sections from fresh-frozen OCT-embedded hearts, as previously described.11,21 Briefly, alternate 10-μm frozen sections of the aortic root covering 300 μm of the proximal aorta, starting at the sinus, were fixed in acetone, rehydrated, and stained for atherosclerotic lesions. Images were captured digitally with a video camera connected to a Leica microscope and analyzed by computed image analysis (Image Pro Plus, Media Cybernetics, Silver Spring, MD). The acquisition of the images and analysis for both methods were always performed in a blinded fashion.
Histology and immunohistochemistry
Briefly, serial alternate 6-μm frozen sections of the aortic root covering the 300 μm of the proximal aorta, starting at the sinus, were fixed in 1% paraformaldehyde, rehydrated, and stained for collagen type I and III, as previously described.10 Sections were incubated for 90 minutes in 0.1% Sirius red F3BA (Polysciences, Warrington, PA) in saturated picric acid. After rinsing in 0.01 N HCl and in distilled water, sections were dehydrated with 70% ethanol and analyzed by polarization microscopy. For smooth muscle cells, monoclonal anti–human smooth muscle α-actin (clone 1A4, dilution 1:40, Sigma Chemical) was used as the primary antibody followed by a secondary peroxidase-conjugated rabbit antimouse antibody (P-0260, dilution 1:300, Dako, Carpenteria, CA). Antibody reactivity was detected with the use of a Nova red substrate kit (SK-4800, Vector Laboratories, Burlingame, CA). Immunostaining for macrophage content was performed as previously described.11,21 The avidin-biotin-alkaline phosphatase method (Vector Laboratories and Boehringer Mannheim, Mannheim, Germany), using rat monoclonal antibody to mouse macrophages (MOMA-2; Accurate Chemical Science, Westbury, NY) diluted in PBS 1:30 was used. Cross-sections were counterstained with hematoxylin.
Sections were also incubated with primary antibody against CD40L (Santa Cruz Biotechnology, Santa Cruz, CA). After washing in PBS, the slides were incubated with the secondary biotinylated antibody and the immunocomplex visualized using the diaminobenzidine chromogen (ABC Complex, Vector Laboratories). As control, no primary antibody was added to the same sections. Macrophage, smooth muscle cell, collagen and CD40L+ regions were quantified in sections by determination of the area that stained positive for the respective markers.11,21 Images of these sections were always captured and analyzed in a blinded fashion.
Statistical analysis
Results were expressed as mean ± SEM. Statistical analyses were performed by ANOVA and subsequently by Student unpaired 2-tailed t test, as appropriate and using a computerized software package (GraphPad Prism version 4.0, GraphPad, San Diego, CA). A value of P below .05 was always considered significant.
Results
Impact of COX-1 inhibition, TP blockade, or both on eicosanoid biosynthesis
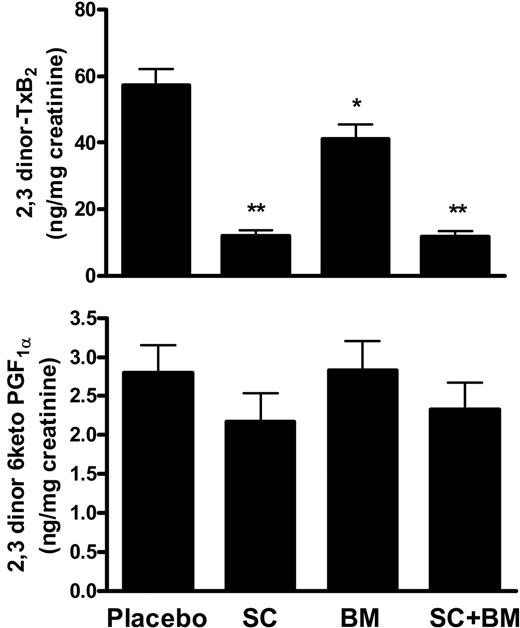
Starting at 8 weeks of age, LDLR KO mice were fed a high-fat diet for the entire study (12 weeks). Body weight and total plasma cholesterol and triglycerides levels were not different among animals when randomized to the 4 groups at the beginning of the study (not shown). As expected, animals on placebo achieved a significant increase in plasma cholesterol, triglycerides, and isoprostane iPF2α-III levels and body weights by the end of the study—that is, at 20 weeks of age (Table 1). Excretion of 2,3-dinor-TxB2, the major Tx metabolite in mice,4 also increased. This increment was already evident after 8 weeks on high-fat diet (4 ± 1.5 versus 31 ± 7 ng/mg creatinine; P < .001) and a further increase was observed by the end of the study (Figure 1A).
Metabolic effect in LDLR KO mice treated with SC-560, BM-573, or a combination of both drugs
| . | Placebo . | SC-560 . | BM-573 . | SC+BM . |
|---|---|---|---|---|
| Weight, g | 30 ± 1 | 29 ± 1.4 | 31 ± 1.2 | 30.5 ± 1.1 |
| Triglycerides, mg/dL | 251 ± 25 | 276 ± 21 | 267 ± 30 | 275 ± 28 |
| Cholesterol, mg/dL | 1030 ± 45 | 958 ± 51 | 972 ± 48 | 989 ± 55 |
| TxB2, ng/mL | 70 ± 15 | 8 ± 1.3* | 44 ± 8† | 9 ± 1.5* |
| IPF2a-III, pg/mL | 346 ± 32 | 352 ± 35 | 365 ± 29 | 125 ± 30‡ |
| Platelet aggregation U46619, LT% | 81 ± 5 | 76 ± 4 | 9 ± 2* | 8 ± 2* |
| Platelet aggregation AA, LT% | 80 ± 6 | 10 ± 2* | 7 ± 2* | 8 ± 1.5 |
| . | Placebo . | SC-560 . | BM-573 . | SC+BM . |
|---|---|---|---|---|
| Weight, g | 30 ± 1 | 29 ± 1.4 | 31 ± 1.2 | 30.5 ± 1.1 |
| Triglycerides, mg/dL | 251 ± 25 | 276 ± 21 | 267 ± 30 | 275 ± 28 |
| Cholesterol, mg/dL | 1030 ± 45 | 958 ± 51 | 972 ± 48 | 989 ± 55 |
| TxB2, ng/mL | 70 ± 15 | 8 ± 1.3* | 44 ± 8† | 9 ± 1.5* |
| IPF2a-III, pg/mL | 346 ± 32 | 352 ± 35 | 365 ± 29 | 125 ± 30‡ |
| Platelet aggregation U46619, LT% | 81 ± 5 | 76 ± 4 | 9 ± 2* | 8 ± 2* |
| Platelet aggregation AA, LT% | 80 ± 6 | 10 ± 2* | 7 ± 2* | 8 ± 1.5 |
Body weight and levels of total plasma triglycerides, cholesterol, isoprostane iPF2a-III, and TxB2, and the platelet aggregation responses to U-46619 and AA were measured in mice after 12 weeks on high-fat diet receiving placebo or the different drugs. Results are expressed as mean ± SEM.
LT indicates light transmission; AA, arachidonic acid.
*P < .001 versus placebo.
P =.02 versus placebo.
P < .01 versus SC-560 and BM-573.
Effects of COX-1 inhibition, TP blockade, or both on TxA2 and PGI2 biosynthesis in LDLR KO mice on a high-fat diet. (A) Whereas SC-560 treatment significantly suppressed, BM-573 only partially reduced urinary 2,3-dinor-TxB2 excretion. (B) Neither SC-560, nor BM-573, alone or in combination, had a significant effect on urinary 2,3-dinor-6-ketoPGF1α excretion. *P =.03 and **P < .01 versus placebo. Results are presented as mean ± SEM.
Effects of COX-1 inhibition, TP blockade, or both on TxA2 and PGI2 biosynthesis in LDLR KO mice on a high-fat diet. (A) Whereas SC-560 treatment significantly suppressed, BM-573 only partially reduced urinary 2,3-dinor-TxB2 excretion. (B) Neither SC-560, nor BM-573, alone or in combination, had a significant effect on urinary 2,3-dinor-6-ketoPGF1α excretion. *P =.03 and **P < .01 versus placebo. Results are presented as mean ± SEM.
A group of 10 LDLR KO mice (all males) were randomized to receive SC-560 (15-mg/kg diet). Assuming that each mouse eats 4 to 5 g chow diet/d, the estimated daily intake of the drug was calculated around 60 to 75 μg.
Compared with placebo, the drug had no effect on weight and plasma cholesterol, triglyceride, and iPF2α-III levels (Table 1). Confirming the compliance with the drug, serum levels of TxB2 and platelet aggregation induced by arachidonic acid, but not U-46619, a specific TP agonist, were suppressed (Table 1). Consistently, we observed that after 12 weeks on SC-560, urinary levels of 2,3-dinor-TXB2 were reduced by 75% to 80% compared with placebo (Figure 1A). By contrast, no significant changes were found in urinary excretion of the stable prostacyclin metabolite,4 2,3-dinor-6keto-PGF1α (Figure 1B).
A second group of mice received BM-573 (10 mg/L) for 12 weeks. Assuming that each mouse drinks 3 to 4 mL water/d, the estimated daily intake of the drug was calculated around 30 to 40 μg. This group of mice did not show any significant difference in weight or plasma cholesterol, triglyceride, and isoprostane iPF2α-III levels compared to the animals receiving vehicle (Table 1).
However, by the end of the study, we observed that they had a significant reduction in urinary 2,3-dinor-TxB2 levels (P =.03; Figure 1A) and serum TxB2 (P =.02; Table 1). Platelet aggregation induced by the specific TP receptor agonist U-46619 (1 μM) and arachidonic acid (100 μM) was suppressed (Table 1). Finally, compared with placebo, animals receiving the TP antagonist did not have any change in urinary levels of 2,3-dinor-6-ketoPGF1α (Figure 1B).
A third group of mice received both SC-560 and BM-573 at the same time. This therapeutic regimen did not have any significant effect on their weight or plasma cholesterol and triglycerides levels compared with vehicle (Table 1). In contrast, we observed that plasma levels of iPF2α-III were significantly reduced (Table 1). We also found that the combination of the 2 drugs resulted in suppression of urinary excretion of 2,3-dinor-TxB2, serum TxB2, and platelet aggregation induced by both U46619 and arachidonic acid, whereas 2,3-dinor-6keto PGF1α was unchanged compared with vehicle (Figure 1; Table 1).
Effect of of COX-1 inhibition, TP blockade, or both on vascular inflammation
Consistent with previous observations in mouse models of atherosclerosis,10,16 when the mouse was killed circulating levels of the inflammatory molecule MCP-1 were reduced in mice receiving SC-560 (184 ± 40 versus 128 ± 15 ng/mL; P < .05) and BM-573 alone (105 ± 20 ng/mL; P < .05) However, a much more significant reduction was observed in mice treated with the combination therapy (55 ± 12 ng/mL, P < 0.01, versus both SC-560 and BM-573 alone).
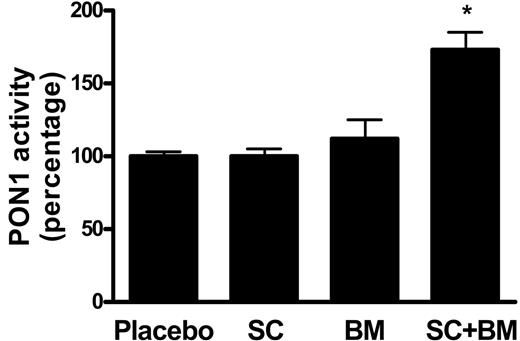
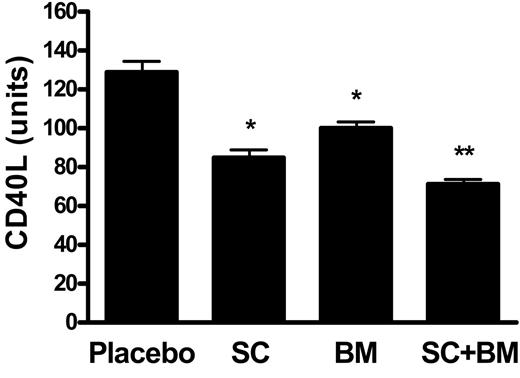
Next, we assayed the activity levels of serum paraoxonase 1 (PON1), an esterase associated with the high-density lipoprotein (HDL) that has potent anti-inflammatory and antioxidant activities.22 Serum PON1 activity was unchanged between mice receiving placebo and SC-560, whereas only a trend toward an increase was observed in BM-573–treated LDLR KO mice (Figure 2). By contrast, a statistically significant increase was found in mice receiving the combination therapy (Figure 2). At the end of the study we found that there was a marked expression of CD40L in LDLR KO mice fed a high-fat diet for 12 weeks. Mice treated with SC-560 had a significant reduction, which was also evident in the BM-573–treated group (Figure 3) However, mice receiving both drugs at the same time had a further significant reduction in CD40L levels compared with the group of mice treated with the single drug (Figure 3).
Effects of COX-1 inhibition, TP blockade, or a combination of both on serum activity levels of PON1. Compared with placebo, no significant difference was observed in LDLR KO mice fed a high-fat diet containing SC-560 or BM-573 for 12 weeks. By contrast, a statistically significant increase was found in mice receiving high-fat diet containing both drugs (*P < .01). Results are presented as mean ± SEM.
Effects of COX-1 inhibition, TP blockade, or a combination of both on serum activity levels of PON1. Compared with placebo, no significant difference was observed in LDLR KO mice fed a high-fat diet containing SC-560 or BM-573 for 12 weeks. By contrast, a statistically significant increase was found in mice receiving high-fat diet containing both drugs (*P < .01). Results are presented as mean ± SEM.
Effects of COX-1 inhibition, TP blockade, or a combination of both on CD40L plaque immunoreactivity. Compared with placebo, a significant decrease in CD40L immunoreactivity was observed in mice receiving SC-560 or BM-573 (*P < .001 versus placebo). However, a further significant reduction compared with those 2 groups was found in the group of mice receiving the combination therapy: *P < .01 versus SC or BM. Results are presented as mean ± SEM.
Effects of COX-1 inhibition, TP blockade, or a combination of both on CD40L plaque immunoreactivity. Compared with placebo, a significant decrease in CD40L immunoreactivity was observed in mice receiving SC-560 or BM-573 (*P < .001 versus placebo). However, a further significant reduction compared with those 2 groups was found in the group of mice receiving the combination therapy: *P < .01 versus SC or BM. Results are presented as mean ± SEM.
Effect of COX-1 inhibition, TP blockade, or both on atherogenesis and plaque cellular composition
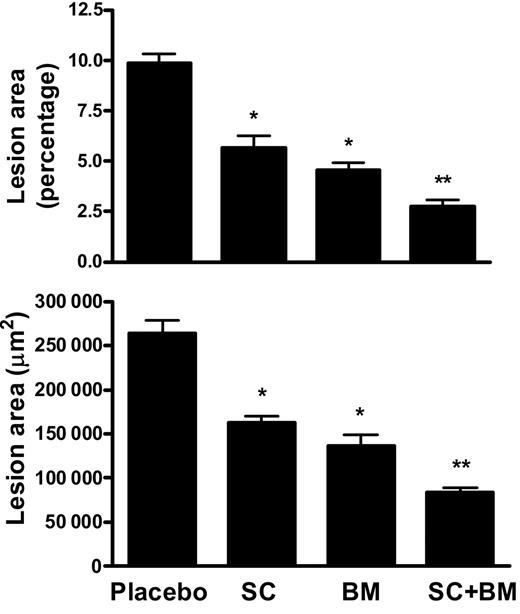
Mice were killed at the end of the study and their aortas analyzed for the extent of atherosclerosis. The aortic atherosclerotic lesion area was quantified by 2 independent methods, the en face method of the entire aortic tree and cross-section analysis of the proximal aorta. As expected, after 12 weeks on a high-fat diet, LDLR KO mice receiving placebo developed discrete aortic atherosclerotic lesions. At the end of the study, we observed that both SC-560– and BM-573–treated mice had a significant reduction in the atherosclerotic areas in their aortas (Figure 4). However, we found that mice receiving both SC-560 and BM-573 at the same time had a significant further reduction of their atherosclerotic lesion areas (P < .001 versus placebo; Figure 4A).
Effects of COX-1 inhibition, TP blockade, or a combination of both on atherogenesis in LDLR KO mice. (A) Quantitation of atherosclerosis by en face method. Percentage lesion formation in mice fed for 12 weeks a high-fat diet containing SC-560 or BM-573 or a combination of both. (B) Quantitation of lesion areas of aortic cross-sections from mice fed for 12 weeks a high-fat diet containing SC-560 or BM-573 or a combination of both. *P < .01 versus placebo; **P < .01 versus SC and BM. Results are presented as mean ± SEM.
Effects of COX-1 inhibition, TP blockade, or a combination of both on atherogenesis in LDLR KO mice. (A) Quantitation of atherosclerosis by en face method. Percentage lesion formation in mice fed for 12 weeks a high-fat diet containing SC-560 or BM-573 or a combination of both. (B) Quantitation of lesion areas of aortic cross-sections from mice fed for 12 weeks a high-fat diet containing SC-560 or BM-573 or a combination of both. *P < .01 versus placebo; **P < .01 versus SC and BM. Results are presented as mean ± SEM.
Consistently, cross-section analysis of the aortic sinuses of mice on placebo showed discrete lesion areas after 3 months on the high-fat diet (265 690 ± 39 008 μm2). LDLR KO mice treated with SC-560 or BM-573 had a significant reduction of the area when compared with the placebo group, which, however, was even more pronounced in mice receiving the combination therapy (Figure 4B).
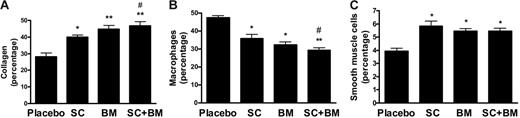
Immunocytochemical analyses of cross-sections of the proximal aorta were carried out to investigate whether any of those treatments had an impact on plaque composition. The atherosclerotic lesions of LDLR KO on placebo (high-fat diet for 3 months) contained about one third collagen and one third macrophage-derived foam cells, respectively (Figure 5). Twelve weeks on SC-560 or BM-573 resulted in a decrease in macrophages and an increase in smooth muscle cells and collagen content (Figure 5). However, the coincidental administration of the 2 drugs led to a further and significant decrease in macrophages and a significant increase in collagen within the aortic atherosclerotic lesions of these animals (Figure 5). As a result of those changes in cellularity, the ratio of macrophage to collagen (1.7 ± 0.3 versus 0.62 ± 0.1) and macrophages to smooth muscle cells (11.4 ± 1 versus 5 ± 0.8) were significantly reduced in the combination group (P < .001 for both). No immunostaining was detected in sections in which the primary antibody was omitted (not shown).
Effects of COX-1 inhibition, TP blockade, or a combination of both on cellular composition of atherosclerotic vascular lesions in the LDLR KO mice on a high-fat diet. Percentage areas of aortic root atherosclerotic lesions occupied by collagen (A), macrophages (B), and smooth muscle cells (C). * and ** versus placebo; # versus SC-560. Results are presented as mean ± SEM.
Effects of COX-1 inhibition, TP blockade, or a combination of both on cellular composition of atherosclerotic vascular lesions in the LDLR KO mice on a high-fat diet. Percentage areas of aortic root atherosclerotic lesions occupied by collagen (A), macrophages (B), and smooth muscle cells (C). * and ** versus placebo; # versus SC-560. Results are presented as mean ± SEM.
Discussion
The main finding of our study is the demonstration that in a setting where TxA2 biosynthesis is suppressed, the coincidental blockade of the TP increases the beneficial anti-inflammatory action of COX-1 inhibition and results in an additional antiatherogenic effect. This observation supports the hypothesis that in atherogenesis COX-1–independent ligands can still activate TP in vivo and modulate a proinflammatory and proatherogenic vascular phenotype.
Atherosclerosis is a chronic disease of the arterial wall, which involves a continuous interplay between components of the bloodstream and the endothelium. The generation of prostaglandins, specifically TxA2 and prostacyclin, is abnormal in human and in animal models of atherosclerosis.2-4 These bioactive lipid mediators can influence atherogenesis in several ways: by modulating inflammatory responses, expression of metalloproteinases, growth of smooth muscle cells, and platelet reactivity.23 Previous studies have shown that pharmacological inhibition or genetic deletion of COX-1–dependent TxA2 formation, as well as TP antagonism or TP genetic deletion, reduce atherogenesis in apoE KO mice.9-13,16,17 TxA2 and its PGH2 endoperoxide precursor represent the conventional ligands for the TP.24 However, this receptor can be activated by other COX-independent ligands in vitro and in vivo, among them the isoprostane iPF2α-III. Thus, recently we have shown that this bioactive compound, but not other oxidized lipids, directly accelerates atherosclerosis in 2 distinct mouse models, the apoE KO and LDLR KO mice.22 Interestingly, we also provided evidence that this effect was directly mediated by the activation of TP, but independent of de novo TxA2 biosynthesis because it was still evident after pharmacological suppression of TxA2 formation by low-dose aspirin.22 Our current study provides support for the novel concept that in atherosclerosis where both TxA2 and isoprostane are elevated, the suppression of the first alone would not afford the most benefit for the atherosclerotic patients because the coincidental presence of isoprostanes can still activate the thromboxane receptor and favor a proatherogenic vascular phenotype.
In line with hypothesis, a recent study showed that administration of a TP antagonist improved endothelial dysfunction in patients with coronary artery disease treated with aspirin.25
It could be argued that the effect of TP blockade observed in mice receiving a COX-1–selective inhibitor is related to inhibition of residual platelet TxA2 formation. However, we can rule out this possibility for 2 reasons. First, we found that arachidonic acid-induced platelet aggregation was abolished in mice treated with SC-560. Second, no additional suppressive effect on TxA2 biosynthesis was observed in the combination therapy group. However, we cannot completely exclude that in our setting “other” sources such as endothelium or other vascular cells are still capable to producing TxA2 through a transcellular mechanism.26
The biosynthesis of biologically active isoprostanes is increased during atherogenesis in mice, and suppression of their generation by exogenous antioxidant reduces atherosclerosis.27 In the present paper we confirmed that plasma isoprostane levels are elevated in the LDLR KO mice on a high-fat diet. Interestingly, we observed that whereas mice receiving SC-560 or BM-573 alone did not have any significant change in plasma iPF2α-III levels, the group of animals receiving both drugs at the same time had significant reduction. Taken together, these data would support the hypothesis that this effect is not secondary to a direct antioxidant activity of the drugs, because when they were administered alone no effect was observed. It is important to point out that the finding that COX-1 inhibition/TP blockade yields a greater reduction in atherosclerosis than TP alone may also suggest that COX-1–derived ligands promote atherogenesis through TP-independent mechanisms via modulation of potent vascular proinflammatory responses.
This hypothesis is supported by the observation that only in the combination group did we observe a dramatic reduction in MCP-1, an important proinflammatory cytokine involved in atherogenesis.28 In addition, we found that suppression of TxA2 biosynthesis in the presence of blockade of TP resulted in a significantly increased activity of one of the most potent endogenous anti-inflammatory and antioxidant systems, PON1, which has already been implicated in atherogenesis.29 Finally, we observed that the expression of CD40L, another proinflammatory cytokine known to modulate experimental atherogenesis,30 was reduced to a significantly greater extent in the combination group when compared with the mice receiving the single drug. These further reductions in inflammation and increase in antioxidant capacity, could in turn justify the further decrease in monocyte/macrophages and increase in collagen content of the atherosclerotic lesions. This hypothesis is based on recent published works showing that both inflammation and oxidative stress modulate matrix metalloproteinases in atherosclerosis.31,32
Limitations of the study
A limitation of our study is related to the use of an animal model of atherosclerosis, which only partially reproduces the human disease. Therefore, it is always with caution that any result in this kind of studies is extrapolated to humans. The second limitation is the fact that animals were constantly exposed to the drugs of interest, whereas humans would take any drug at a particular time point of their day.
Conclusions
In summary, we have provided the first in vivo evidence that combining blockade of TP with COX-1 inhibition results in a greater antiatherogenic effect than either of the 2 therapies alone in mice. This phenomenon occurs through TP independent cellular mechanisms, which modulate proinflammatory and pro-oxidative vascular responses. In conclusion, selective pharmacological strategies aimed at preventing the activation of this receptor during chronic treatment with COX-1 inhibitors (aspirin-like drugs) could represent a novel and more effective therapeutic approach in the secondary prevention of cardiovascular events during the progression or evolution of human atherosclerosis.
Authorship
Contribution: T.C. designed the study, performed atherosclerosis analyses, and drafted the manuscript; Y.Y. performed the study, biochemical measurements, and animal husbandry; T.D. performed biochemical measurements and animal husbandry; J.-M.D. designed the study, drafted the manuscript, and did statistical analyses; and D.P. designed the study, collected data, performed statistical analyses, and drafted the manuscript.
Conflict-of-interest disclosure: The authors have no competing financial interests.
Correspondence: Domenico Praticò, University of Pennsylvania, Department of Pharmacology, 3620 Hamilton Walk, John Morgan Bldg, Rm 125, Philadelphia, PA 19104; e-mail: domenico@spirit.gcrc.upenn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was support by grants from The CART Foundation and the National Institutes of Health (AG-22512).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal