Zou and colleagues use retrovirus infection and fetal liver transplantation to engineer platelets in mice expressing αIIbβ3 and use point mutations to dissect inside-out and outside-in signaling in vivo.
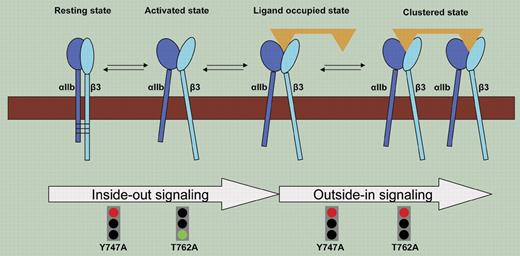
As if integrin nomenclature were not complicated enough, with all its alphas and betas, we must now be cognizant of the subtleties of an additional layer of terminologies, “inside-out” and “outside-in” signaling. There is, of course, a logic to integrate these additional terms into our integrin vocabulary: they define distinct and biologically important aspects of integrin-mediated cellular responses. αIIbβ3 is the integrin that mediates platelet aggregation, and its ability to form a stable thrombus in vivo depends upon both inside-out and outside-in signaling. Engagement of fibrinogen or von Willebrand factor by the extracellular domain of αIIbβ3 is necessary for platelet aggregation and requires a conformational transition, the consequence of inside-out signaling. As platelets aggregate, occupied αIIbβ3 integrins cluster and trigger outside-in signaling that stabilizes the aggregate and supports responses, including platelet spreading and clot retraction. Hence, inside-out and outside-in signaling constitute the 2 elements of the bidirectional signaling across αIIbβ3 (see figure) and represent potential antithrombotic therapeutic targets, and their dissection is indispensable to our understanding of platelet biology.
Distinction between inside-out and outside-in signaling across integrin αIIbβ3.
Distinction between inside-out and outside-in signaling across integrin αIIbβ3.
The inside-out and outside-in signaling pathways ultimately trace to cytoplasmic tails (CTs) of αIIbβ3, which serve as receivers and transmitters of bidirectional signaling within the receptor. While the CTs of the αIIb and β3 subunits are short, they are structurally complex and interact with numerous binding partners. Ordinarily, the contribution of specific structural elements or individual amino acids can be dissected by straightforward mutagenesis. However, platelets are anucleated; and alternative approaches have had to be invoked to examine inside-out and outside-in signaling. These approaches have included the following: (1) expression of αIIbβ3 in heterologous cells; (2) transfection of the primary megakaryocytes; (3) introduction of membrane-permeable peptides into platelets; and (4) generation of knock-in mice expressing mutant αIIbβ3. Each of these approaches has been used successfully to probe integrin signaling, but each approach has inherent limitations.
By infecting fetal liver cells from β3-deficient mice with retrovirus engineered to encode wild-type β3-subunit and transplanting these cells into irradiated recipients, Zou and colleagues show that these cells can successfully express αIIbβ3 and rescue both inside-out and outside-in signaling in blood platelets. This strategy is then used to introduce specific mutations into the mouse platelets. Inside-out signaling is evaluated by the capacity of the platelets to bind soluble fibrinogen, and outside-in signaling is assessed by platelet spreading. A single point mutation in the midregion of the β3 CT, β3Y747A, prevents restoration of both inside-out and outside-in signaling, while a point mutation at the extreme C-terminus of β3, β3T762A, results in selective loss of outside-in signaling. These results are generally consistent with those derived from the other approaches we outlined and fortify the conclusion that different sites within the β3 CT can mediate different aspects of the bidirectional signaling.
The experimental approach for in vivo expression in platelets described by Zou et al here and by others1 allows analyses of αIIbβ3 activation in platelets in vivo and has high throughput potential. Fetal liver transplantation could undoubtedly be applied to other platelet proteins provided that a deficient mouse background is available. Low, variable, and unstable expression of the platelet protein may set limitations on this approach. Nonetheless, the Zou et al paper sets the precedent for a new strategy to dissect inside-out and outside-in signaling across αIIbβ3 in vivo.
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal