Abstract
The FLT3 receptor tyrosine kinase is expressed in more than 90% of acute myelogeneous leukemias (AMLs), up to 30% of which carry an internal tandem duplication (ITD) within the FLT3 gene. Although varying duplication sites exist, most FLT3-ITDs affect a single protein domain. We analyzed the FLT3-ITD of an AML patient for encoding HLA class I–restricted immunogenic peptides. One of the tested peptides (YVDFREYEYY) induced in vitro autologous T-cell responses restricted by HLA-A*0101 that were also detectable ex vivo. These peptide-reactive T cells recognized targets transfected with the patient's FLT3-ITD, but not wild-type FLT3, and recognized the patient's AML cells. Our results demonstrate that AML leukemic blasts can in principle process and present immunogenic FLT3-ITD neoepitopes. Therefore, FLT3-ITD represents a potential candidate target antigen for the immunotherapy of AML.
Introduction
The fibroblast-macrophage stimulating factor receptor (FMS)–like tyrosine kinase receptor 3 (FLT3) is involved in the proliferation, survival, and differentiation of early hematopoietic progenitor cells. More than 90% of acute myelogenous leukemias (AMLs) express FLT3. At the same time, FLT3 represents the most common mutated gene in AML. Two major types of mutation have been described. These are internal tandem duplications (FLT3-ITDs) within the juxtamembrane domain and point mutations within the kinase domain.1-3 FLT3-ITDs occur in 25% to 30% of AMLs and lead to leukemic transformation by constitutive phosphorylation and uncontrolled activation of the tyrosine kinase.4
Targeting FLT3 in AML by small molecules is a novel and promising therapeutic approach in AML.5,6 We reasoned that, in particular, FLT3-ITD–positive AML cells might be specifically targeted also by the T-cell system. Although FLT3-ITDs largely vary in length with duplications of 3 to more than 400 bp,7 about 60% of them occur between codons 591 and 601 and are in frame.1,8 It is likely that duplication regions encode immunogenic neoepitopes that can be presented by individual HLA alleles. Using a reverse-immunology approach, we demonstrated that an individual FLT3-ITD can indeed be immunogenic and can induce autologous antileukemia T-cell responses.
Patients, materials, and methods
Patients, lymphocytes, and cell lines
Sixty-nine-year-old patient J.C. (an anonymous designation) was diagnosed with an FLT3-ITD–positive AML (French-American-British [FAB] M5a). Standard chemotherapy with cytarabine and idarubicin induced a complete remission (CR), but the patient died from recurrent disease 4 months later. Leukapheresis cells (JC-AML cells) and bone marrow cells were obtained at diagnosis. In JC-AML cells, the white blood cell count was 224 × 109/L (224 000/μL), 89% of which were monoblasts. In flow cytometry, 99% stained positive for CD33 and 73% for FLT3 (not shown). Peripheral blood mononuclear cells (JC-PBMCs) were collected in first CR after reconstitution. Sample collection was performed with informed consent. Approval was obtained from the Mainz University Hospital Institutional Review Board. The patient carried the HLA–class I (HLA-I) alleles HLA-A*0101, -A*0201, -B*0801, -B*4402, -Cw*0501, and -Cw*0702. K562/HLA cells were stably transfected with the aforementioned HLA-A and -B alleles as published.9 Epstein-Barr virus (EBV)–transformed B lymphocytes of healthy donor A.K. (AK-EBV-B) were cultivated as detailed before.10 Mature dendritic cells (EF-mDCs) were generated from PBMCs of HLA-I–matched healthy donor E.F. as described.11
FLT3-PCR and in vitro transcription of FLT3-mRNA
RNA was isolated from JC-AML cells for reverse transcriptase–polymerase chain reaction (RT-PCR). Full-length wild-type FLT3 (wtFLT3) and JC-FLT3-ITD were amplified with primers 5′-atagctagcaccatgccggcgttggcgcgcgac-3′ and 5′-gcgggatccggctacgaatcttcgacctgagcct-3′, cloned into the vector pIREShyg3 (Clontech Laboratories, Mountain View, CA), and sequenced. The patient's FLT3-ITD sequence, JC-FLT3-ITD, is shown in Figure 1. For in vitro transcription the plasmids were linearized with BamHI (New England Biolabs, Frankfurt am Main, Germany) permitting full-length transcription of the insert and subsequent polyadenylation of the mRNA as described.12 Polyadenylated mRNA was stored at −80°C.
Peptides
Peptides encoded by the JC-FLT3-ITD were predicted with public domain algorithms.13-16 They were synthesized in an automated peptide synthesizer EPS221 (Abimed, Langen, Germany) following the Fmoc/tBu strategy. Synthesis products were analyzed by high-performance liquid chromatography (HPLC) (Varian star, Darmstadt, Germany) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (GSG-future, Bruchsal, Germany). Peptides of less than 80% purity were purified by HPLC.
In vitro stimulation of JC-FLT3-ITD–specific T cells
In vitro stimulation (IVS) was performed as published17 with slight modifications. JC-PBMCs were seeded in 96-well plates (5 × 104 per well) in AIM-V supplemented with 5% human serum, IL-2 (20 U/mL), IL-7 (10 ng/mL), IL-4 (10 ng/mL), and synthetic JC-FLT3-ITD peptides (5 μg/mL) and restimulated on day 7. One of the IVS-responder populations was cloned by limiting dilution. Feeders were allogeneic AK-EBV-B lymphocytes (irradiated, 2 × 104 per well). Stimulators were EF-DCs (irradiated, 1 × 103 per well) transfected with JC-FLT3-ITD mRNA (1 μg per 0.2 × 105 DCs) using TransMessenger Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Restimulations were performed in 7-day intervals.
ELISPOT assays
IFN-γ and granzyme B (GrB) enzyme-linked immunospot (ELISPOT) assays were performed as described.12,18 Spots were evaluated with computer-assisted video image analysis. Briefly, image acquisition was performed with an AxioCam MRc camera attached to a Zeiss Axio Imager M1 microscope via a 0.63× adapter. Spots were counted with a 3.15× magnification at a resolution of 1388×1040 pixels. The software was KS ELISPOT version 4.8. Targets were K562/HLA cells electroporated with mRNA at 300 μF and 250 V or loaded with synthetic peptides (10 μg/mL).
Results and discussion
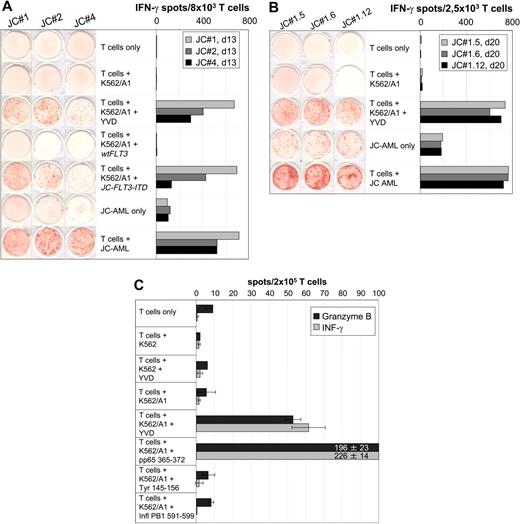
The FLT3-ITD identified in JC-AML cells (JC-FLT3-ITD) encoded a duplication of amino acids 591 to 599 (Figure 1). The same duplication event has already been found in another AML patient,19 which is in line with the rather limited location of FLT3-ITD occurrence.1,8 Five peptides with high combined prediction scores were selected (Figure 1) and used for IVS with JC-PBMCs collected in CR. T-cell responses against peptide YVDFREYEYY (YVD/A1), predicted to bind to HLA-A*0101, could be repeatedly generated. The peptide was only recognized in association with HLA-A*0101 but not with any other HLA-A or -B allele of patient J.C. (not shown). YVD/A1-reactive IVS responders recognized K562/A1 transfected with full-length JC-FLT3-ITD mRNA but not K562/A1 transfected with wtFLT3 mRNA (Figure 2A). This indicated that the peptide YVD/A1 was endogenously processed and proved the existence of JC-FLT3-ITD–specific T cells.
Prediction of HLA-binding peptides encoded by the FLT3-ITD sequence identified in AML cells of patient J.C. (JC-FLT3-ITD). Peptides from the ITD protein region (boxed) were predicted with public domain algorithms13-16 combining predictions for proteasomal processing, transporter associated with antigen processing (TAP) transport, and binding to the patient's HLA-A and -B alleles. Five peptides, ranked either first or second by the algorithms, were chosen for the in vitro stimulation of JC-PBMCs. The amino acid sequences of these peptides, their respective presenting HLA-I alleles, and their prediction scores and ranks are indicated (n.a. indicates prediction not available for this allele/epitope length).
Prediction of HLA-binding peptides encoded by the FLT3-ITD sequence identified in AML cells of patient J.C. (JC-FLT3-ITD). Peptides from the ITD protein region (boxed) were predicted with public domain algorithms13-16 combining predictions for proteasomal processing, transporter associated with antigen processing (TAP) transport, and binding to the patient's HLA-A and -B alleles. Five peptides, ranked either first or second by the algorithms, were chosen for the in vitro stimulation of JC-PBMCs. The amino acid sequences of these peptides, their respective presenting HLA-I alleles, and their prediction scores and ranks are indicated (n.a. indicates prediction not available for this allele/epitope length).
AML-reactive CD8+ T cells of patient J.C. specifically recognized the HLA-A*0101–restricted peptide YVDFREYEYY (YVD/A1) encoded by JC-FLT3-ITD. (A) Specificity of IVS-responder populations. JC-PBMCs collected in CR after induction chemotherapy were stimulated with peptide YVD/A1 in independent IVS. IVS responders were tested on day 13 in a 20-hour IFN-γ ELISPOT assay for recognition of unloaded, YVD/A1-loaded, wtFLT3 mRNA–transfected, or JC-FLT3-ITD mRNA–transfected K562/A1 cells (1 × 105 per well) as well as for recognition of autologous AML cells (1 × 105 per well). The results obtained with IVS JC#1, JC#2, and JC#4 are shown as representative examples. (B) Specificity of T-cell clones derived from IVS JC#1. JC#1 responders were cloned on day 14 by limiting dilution using HLA-I–compatible EF-DCs transfected with JC-FLT3-ITD mRNA as stimulators. T-cell clones were tested 20 days later against unloaded or YVD/A1-loaded K562/A1 cells (1 × 105 per well) as well as against autologous AML cells (1 × 105 per well) in a 20-hour IFN-γ ELISPOT assay. Three representative clones, JC#1.5, JC#1.6, and JC#1.12, are shown. (C) Reactivity against YVD/A1 detectable in ex vivo CD8+ T cells. CD8+ T cells were positively selected from JC-AML cells with immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and tested in a 30-hour IFN-γ and in a 30-hour GrB ELISPOT assay against unloaded or peptide-loaded untransfected K562 cells (1 × 105 per well) as well as against unloaded or peptide-loaded K562/A1 cells (1 × 105 per well). Peptides were YVD/A1- and known HLA-A1–binding peptides from HCMV pp65, tyrosinase, and influenza A basic polymerase 1 (pp65 364 to 373, SEHPTFTSQY; tyrosinase 146 to 156, SSDYVIPIGTY; PB1 591 to 599, VSDGGPNLY, respectively). The pp65 peptide served as a positive control; tyrosinase and influenza A peptides were negative controls. Data are means of duplicates. Notably, YVD/A1 recognition required the presence of HLA-A1 on K562 cells, because peptide-loaded but untransfected K562 cells did not induce spot formation in any test.
AML-reactive CD8+ T cells of patient J.C. specifically recognized the HLA-A*0101–restricted peptide YVDFREYEYY (YVD/A1) encoded by JC-FLT3-ITD. (A) Specificity of IVS-responder populations. JC-PBMCs collected in CR after induction chemotherapy were stimulated with peptide YVD/A1 in independent IVS. IVS responders were tested on day 13 in a 20-hour IFN-γ ELISPOT assay for recognition of unloaded, YVD/A1-loaded, wtFLT3 mRNA–transfected, or JC-FLT3-ITD mRNA–transfected K562/A1 cells (1 × 105 per well) as well as for recognition of autologous AML cells (1 × 105 per well). The results obtained with IVS JC#1, JC#2, and JC#4 are shown as representative examples. (B) Specificity of T-cell clones derived from IVS JC#1. JC#1 responders were cloned on day 14 by limiting dilution using HLA-I–compatible EF-DCs transfected with JC-FLT3-ITD mRNA as stimulators. T-cell clones were tested 20 days later against unloaded or YVD/A1-loaded K562/A1 cells (1 × 105 per well) as well as against autologous AML cells (1 × 105 per well) in a 20-hour IFN-γ ELISPOT assay. Three representative clones, JC#1.5, JC#1.6, and JC#1.12, are shown. (C) Reactivity against YVD/A1 detectable in ex vivo CD8+ T cells. CD8+ T cells were positively selected from JC-AML cells with immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and tested in a 30-hour IFN-γ and in a 30-hour GrB ELISPOT assay against unloaded or peptide-loaded untransfected K562 cells (1 × 105 per well) as well as against unloaded or peptide-loaded K562/A1 cells (1 × 105 per well). Peptides were YVD/A1- and known HLA-A1–binding peptides from HCMV pp65, tyrosinase, and influenza A basic polymerase 1 (pp65 364 to 373, SEHPTFTSQY; tyrosinase 146 to 156, SSDYVIPIGTY; PB1 591 to 599, VSDGGPNLY, respectively). The pp65 peptide served as a positive control; tyrosinase and influenza A peptides were negative controls. Data are means of duplicates. Notably, YVD/A1 recognition required the presence of HLA-A1 on K562 cells, because peptide-loaded but untransfected K562 cells did not induce spot formation in any test.
IVS-responder populations containing YVD/A1-reactive T cells also recognized JC-AML cells (Figure 2A). We cloned IVS JC#1 using HLA-I–matched EF-DCs transfected with JC-FLT3-ITD as stimulators. Ten of 19 T-cell clones recognized YVD/A1 and JC-AML equally well (Figure 2B), which demonstrated that JC-FLT3-ITD was expressed and processed by JC-AML cells leading to the presentation of YVD/A1 on their cell surface. When YVD/A1 was titrated, half-maximal recognition was observed at 20 nM (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article), which is in the range of moderate avidity T cells.
YVD/A1-specific T cells were detectable ex vivo at similar frequencies in the patient's peripheral blood lymphocytes (Figure 2C) and in bone marrow lymphocytes (not shown) both drawn at diagnosis. This indicated an in vivo expansion of these T cells. In addition, ex vivo CD8+ T cells secreted GrB in response to YVD/A1, which demonstrated their cytotoxic ability.18 It is widely accepted that progressive malignant disease in the presence of antitumor T cells is not an oxymoron considering the complexity of resistance mechanisms already discovered.20
Recently, Scholl and colleagues showed the potency of FLT3-ITD–encoded peptides and related mimotopes to bind to HLA-I molecules.21 However, whether these peptides were processed and recognized by T cells was not explored. Analogous to JC-FLT3-ITD, fusion proteins occurring in leukemia subsets have been found to induce T-cell responses (eg, ETV6/AML1 22 and TEL/AML1 23 ). As newly synthesized FLT3-ITD is retained in the endoplasmic reticulum because of inefficient folding and chaperoning,24 it appears likely that FLT3-ITD molecules are preferentially degraded after synthesis and introduced into the HLA-I–presenting pathway. This would favor T-cell recognition in case peptides are immunogenic.25
It has been shown that FLT3-ITDs are present in leukemia stem cells.26 Targeting of leukemogenic molecules with T cells might improve the chances to eliminate also the quiescent fraction of leukemia stem cells as compared with small inhibitory molecules.27
To fully recognize the potential of FLT3-ITD as an immunotherapeutic target, it will be necessary to study in a next step a cohort of FLT3-ITD–positive patients for anti–FLT3-ITD T-cell responses and to include CD8+ as well as CD4+ T-cell responses via all individual HLA-I and -II alleles.
Authorship
Contribution: C.G. performed the experiments and drafted the manuscript as part of her PhD thesis; F.H. performed FLT3-ITD sequencing and cloning; S.T. and M.P.R. performed peptide predictions; F.K.S. performed PCR-based FLT3-ITD screening and prepared clinical samples; C.M.B. generated stable K562/HLA transfectants; C.H. contributed to the design of the project; T.F. contributed to the design of the project and to the writing of the manuscript; and T.W., C.G.'s thesis supervisor, designed the project and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Wölfel, III Medizinische Klinik, Johannes Gutenberg-Universität, D-55101 Mainz, Germany; e-mail: t.woelfel@3-med.klinik.uni-mainz.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
C.G. was a fellow of the research training group 1043, “antigen-specific immunotherapy,” (project A3) and a PhD candidate at the Johannes Gutenberg-Universität in Mainz; T. W. was supported by the collaborative research initiative SFB 432 (project A1); and both projects were funded by the Deutsche Forschungsgemeinschaft. This work was further supported by a MAIFOR grant provided by the Medical Faculty of the Johannes Gutenberg-Universität (F.H. and T.F.); SFB 490 (project E6) (S.T.); and the Deutsche Krebshilfe (grant 70-2427-HuI) (C.H.). The authors thank S. Stevanovic (Department of Immunology, Tübingen) for peptide synthesis. They acknowledge the contributions by members of the Tumor Vaccination Center, particularly technical assistance by B. Schuch, M. Brkic, and K. Bechtold and FACS analysis by A. Konur. The authors also thank S. Debo and D. Eberts for technical assistance and M. Fatho and V. Lennerz for helpful discussions.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal