Abstract
Mutations in the cold-induced autoinflammatory syndrome 1 (CIAS1) gene are associated with a spectrum of autoinflammatory diseases, including familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and chronic infantile neurologic, cutaneous, articular syndrome, also known as neonatal-onset multisystem inflammatory disease. CIAS1 encodes cryopyrin, a protein that localizes to the cytosol and functions as pattern recognition receptor. Cryopyrin also participates in nuclear factor-κB regulation and caspase-1–mediated maturation of interleukin 1β. In this study, we showed that disease-associated mutations in CIAS1 induced rapid cell death of THP-1 monocytic cells. The features of cell death, including 7-AAD staining, the presence of cellular edema, and early membrane damage resulting in lactate dehydrogenase (LDH) release, indicated that it was more likely to be necrosis than apoptosis, and was effectively blocked with the cathepsin B–specific inhibitor CA-074-Me. CA-074-Me also suppressed induced by disease-associated mutation lysosomal leakage and mitochondrial damage. In addition, R837, a recently identified activator of cryopyrin-associated inflammasomes, induced cell death in wild type CIAS1-transfected THP-1 cells. These results indicated that monocytes undergo rapid cell death in a cathepsin B–dependent manner upon activation of cryopyrin, which is also a specific phenomenon induced by disease-associated mutation of CIAS1.
Introduction
Chronic infantile neurologic, cutaneous, articular syndrome (CINCA; MIM #697115; Mendelian Inheritance in Man [MIM]; http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM), also known as neonatal-onset multisystem inflammatory disease, is characterized by recurrent fever, an urticarial rash beginning in the neonatal period, arthropathy characterized by epiphyseal patellar overgrowth, growth retardation, chronic meningitis, papilledema, and hearing loss.1,2 The gene responsible for the syndrome is known as cold-induced autoinflammatory syndrome 1 (CIAS1), and is associated with 2 less severe but phenotypically similar syndromes, familial cold autoinflammatory syndrome (FCAS; MIM #120100) and Muckle-Wells syndrome (MWS; MIM #191900).3-7 CIAS1, also designated as PYPAF1 and NALP3, is expressed in polymorphonuclear cells, monocytes, chondrocytes, and activated T cells.6,8,9 Its product is cryopyrin, which contains an amino-terminal pyrin domain (PYD), a centrally located nucleotide-binding oligomerization domain (NOD), also called a NACHT domain, and several carboxy-terminal leucine-rich repeats (LRRs). Cryopyrin localizes to the cytosol and is believed to function as a pattern-recognition receptor.
Animals and plants have several types of so-called pattern-recognition receptors that distinguish characteristic molecular structures of microorganisms and activate innate immune responses. In mammals, Toll-like receptors (TLRs) are a well-characterized type of pattern-recognition receptor that recognize pathogen-associated molecular patterns via their extracellular LRRs.10 Cryopyrin belongs to another class of mammalian pattern-recognition receptors that are homologous to plant disease resistance–associated NOD-LRR proteins (NLRs).11,12 More than 20 genes encoding NLRs have been identified in the human genome, many of which contain a caspase-activating recruitment domain (CARD), or a PYD, in their amino-terminal region. Among the human NLRs, NOD1/CARD4 and NOD2/CARD15 were found to recognize the structure of a distinct bacterial peptidoglycan, namely γ-d-glutamyl-meso-diaminopimelic acid and muramyl dipeptide (MDP), respectively, via their intracellular LRRs. Recognition results in activation of nuclear factor κB (NF-κB) and caspase-9.13-15 Several other members of the NLR family induce caspase-1–mediated maturation of interleukin (IL)–1β,16-20 suggesting that these molecules also function as cytoplasmic receptors for the immune response. In addition to participating in NF-κB regulation,21,22 cryopyrin activates caspase-1, and is involved in the conversion of the proform of IL-1β into the mature and biologically active molecule, along with an adaptor protein called apoptosis-associated speck-like protein containing a CARD (ASC), which also contains a PYD.21,23 ASC was originally identified as a protein that generates speck-like aggregations in apoptotic HL-60 cells treated with chemotherapeutic agents.24 Thus, ASC has been implicated in apoptosis,25 but its role in disease-associated CIAS1 function and cell death has not been preciously described.
Programmed cell death is essential for proper development and maintenance of multicellular organisms. Caspases, a family of cysteine-aspatatic acid proteases that reside in an inactive zymogen form in the cytosol of virtually every cell, have long been considered the essential killer enzymes in all programmed cell death. However, in addition to caspases, increasing evidence suggests a role for lysosomal proteases in cell death, and in the clearance of infected cells.26,27 Lysosomes contain a number of proteases, among them the cysteine protease cathepsin B and the aspartic protease cathepsin D, which are the most abundant and have most often been linked to cell death.28-33
Recently, we found that monocytes in patients with CINCA underwent rapid necrosis-like cell death after the induction of the CIAS1 gene with lipopolysaccharide (M.S., R.N., N.K., A.F., H.T., T.H., Y.M., and T.N., manuscript submitted). In this study, we explored the mechanism, and found that disease-associated mutations of CIAS1 provoked rapid cell death of THP-1 monocytes. Cell death did not exhibit the typical features of apoptosis, but appeared to be more like necrosis. Interestingly, several features of cell death induced by disease-associated CIAS1 mutation were effectively blocked with the cathepsin B inhibitor CA-074-Me. In addition, a small synthetic antiviral molecule R837, recently identified as an activator of cryopyrin-associated inflammasome, induced cell death in wild-type CIAS1-transfected THP-1 cells. These results indicate that cryopyrin, when activated, participates in rapid necrosis-like cell death in a lysosomal cathepsin B–dependent manner.
Materials and methods
Reagents
The caspase inhibitors zVAD-fmk, zYVAD-cho, zIETD-fmk, and zLEHD-fmk were purchased from R&D Systems (Minneapolis, MN). The caspase-3 inhibitor zDEVD-fmk was obtained from Peptide Institute (Osaka, Japan), and the cathepsin B inhibitor CA-074-Me ([L-3-trans-(propylcarbamoyl) oxirane-2-carbonyl]-l-isoleucyl-l-proline methyl ester) was obtained from Calbiochem (Darmstadt, Germany). Nigericin and actinomycin D were purchased from Sigma-Aldrich (St Louis, MO). The small synthetic antiviral molecule imiquimod R837 was purchased from InvivoGen (San Diego, CA). Anti–caspase-1, anti–caspase-8, anti-Bid, and the apoptosis sampler kit containing rabbit-derived polyclonal antibodies (Abs) against caspase-3 and the corresponding cleaved form of caspase-3 were obtained from Cell Signaling Technology (Beverly, MA). Caspase-3 activity was measured using a Colorimetoric CaspACE Assay System (Promega, Madison, WI).
Plasmids
Expression plasmids for CIAS1 and ASC in the pEF-BOS vector background have been previously described.34 CIAS1 was also cloned into the pIRES2-EGFP vector (Invitrogen, Carlsbad, CA). Mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), as described previously.35 NOD2/CARD15 and its disease-associated mutation, an asparagine-to-lysine substitution at amino acid (aa) 670 (N670K) in p3xFLAG-CMV, were previously described.36 cDNAs encoding carboxy-terminal green fluorescent protein (GFP)–tagged CIAS1 and its mutants were generated and subcloned into pcDNA5/TO (Invitrogen). The ability of each construct to induce NF-κB activation was assessed by a dual luciferase reporter assay in HEK293 human embryonic kidney cells, as previously described.35
Cell lines
Human monocytic THP-1 cells were maintained in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum. Expression plasmids were introduced into THP-1 cells using cell line nucleofector kit V (Amaxa Biosystems, Cologne, Germany) according to the manufacturer's instructions with equipment program no. V-0l. Treatment of the cells with 10 ng/mL phorbol 12-myristate 13-acetatae (PMA) dramatically improved gene expression and reduced spontaneous cell death induced by gene transfection without affecting cell death induced by the mutants of CIAS1 (data not shown). Therefore, we added PMA (10 ng/mL; Sigma-Aldrich) to the cells immediately following gene transfection. THP-1 cells were also incubated with 50 μM proteinase inhibitors where indicated. Because the expression of cryopyrin-GFP required more than 1 hour of incubation after transfection (Figure 1C), proteinase inhibitor was added to the culture medium just after gene transfection. The concentration of CA-074-Me (50 μM) used in this study was determined by monitoring the suppression of intracellular cathepsin B activity in THP-1 cells using a cathepsin B fluorogenic substate (Calbiochem; data not shown).
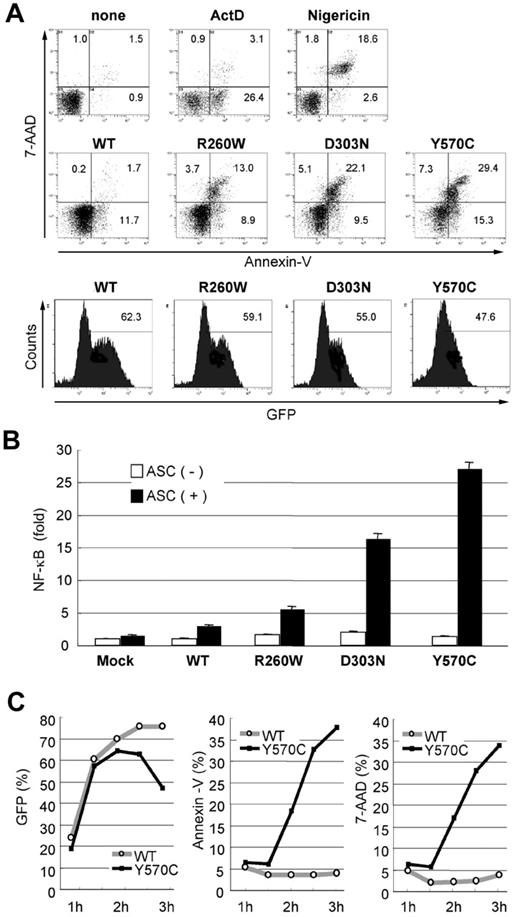
Autoinflammatory disease-associated mutations in CIAS1 induce rapid cell death. (A) As the controls for cell death, 1 × 106 THP-1 cells were treated with 1 μg/mL actinomycin D (ActD) and 20 μM nigericin. 0.5 μg of plasmid encoding GFP-CIAS1 or the disease-associated mutants (R260W, D303N, Y570C) were introduced into 1 × 106 THP-1 cells. After transfection (3 hours), cells were analyzed by flow cytometry, and the expression of CIAS1 was monitored by measuring GFP fluorescence. Cell death was assessed as positive annexin V staining (apoptosis) or double positive for annexin V and 7-AAD (necrosis). The number in each quadrant shows the percentage of cells. (B) HEK293 cells were cotransfected with 20 ng of WT-CIAS1 or the indicated mutants in the presence or absence of 20 ng of ASC. The ability to induce NF-κB activation was assessed by a dual luciferase reporter assay in HEK293 cells. Disease-associated mutants induced ASC-dependent NF-κB activation spontaneously. Values represent the mean of normalized data (mock without ASC = 1) of triplicate cultures, and error bars indicate SD. (C) Expression of GFP-CIAS1 and annexin V and 7-AAD staining were monitored by flow cytometry. Expression of GFP was observed at 1 hour and 30 minutes after transfection, at which point annexin V/7-AAD–positive cells started to appear in the Y570C-transfected cell population. The percentage of Y570C-transfected cells that were annexin V/7-AAD positive increased in a time-dependent manner. Representative data from 3 independent analyses of similar results are shown.
Autoinflammatory disease-associated mutations in CIAS1 induce rapid cell death. (A) As the controls for cell death, 1 × 106 THP-1 cells were treated with 1 μg/mL actinomycin D (ActD) and 20 μM nigericin. 0.5 μg of plasmid encoding GFP-CIAS1 or the disease-associated mutants (R260W, D303N, Y570C) were introduced into 1 × 106 THP-1 cells. After transfection (3 hours), cells were analyzed by flow cytometry, and the expression of CIAS1 was monitored by measuring GFP fluorescence. Cell death was assessed as positive annexin V staining (apoptosis) or double positive for annexin V and 7-AAD (necrosis). The number in each quadrant shows the percentage of cells. (B) HEK293 cells were cotransfected with 20 ng of WT-CIAS1 or the indicated mutants in the presence or absence of 20 ng of ASC. The ability to induce NF-κB activation was assessed by a dual luciferase reporter assay in HEK293 cells. Disease-associated mutants induced ASC-dependent NF-κB activation spontaneously. Values represent the mean of normalized data (mock without ASC = 1) of triplicate cultures, and error bars indicate SD. (C) Expression of GFP-CIAS1 and annexin V and 7-AAD staining were monitored by flow cytometry. Expression of GFP was observed at 1 hour and 30 minutes after transfection, at which point annexin V/7-AAD–positive cells started to appear in the Y570C-transfected cell population. The percentage of Y570C-transfected cells that were annexin V/7-AAD positive increased in a time-dependent manner. Representative data from 3 independent analyses of similar results are shown.
Flow cytometry
Transfection efficiency of GFP fusion protein constructs was assessed by flow cytometry (Epics XL-MCL; Beckman Coulter, Miami, FL). Cell death was analyzed using an annexin V–PE apoptosis detection kit (BD Pharmingen, San Diego, CA), and was classified as positive for annexin V (apoptosis), or double-positive for annexin V and 7-AAD (necrosis).
Assessment of lysosomal and mitochondrial integrity
For the evaluation of small scale of lysosomal leakage (ssΔLL) and loss of mitochondrial inner transmembrane potential (Δψm), cells were incubated with 1 μg/mL acridine orange (Invitrogen) and 1 μM MitoTracker DeepRed 633 (Invitrogen), respectively, in the culture medium for 15 minutes at 37°C, then washed in PBS. Cells were assessed by flow cytometry, and also observed with a confocal laser-scanning microscope (LSM510; Carl Zeiss, Jena, Germany) equipped with a 63× /1.2 water objective lens. LSM510 version 3.2 software was used to process images. For assessment of ssΔLL, we used CIAS1 and its variants without GFP tags, and evaluated nuclei by green fluorescence following acridine orange staining.
Measurement of IL-1β and LDH
For cytokine analysis, cells were cultured with or without lipopolysaccharide (LPS, 10 ng/mL; Sigma-Aldrich) and supernatants were collected. An enzyme-linked immunosorbent assay (ELISA) for IL-1β (eBioscience, San Diego, CA) was performed according to the manufacturer's protocol. Lactate dehydrogenase (LDH) that was spontaneously released from damaged cells into the culture supernatant was assessed using the CytoTox96 nonradioactive cytotoxicity assay kit from Promega.
Electron microscopy
Cells were fixed in 2% glutaraldehyde in phosphate buffer. Routine procedures for observation by electron microscopy were performed by SRL Inc (Tokyo, Japan).
Western blot analysis
Plasmid encoding wild-type (WT)–CIAS1 or Y570C mutant (1 μg) was introduced into 2 × 106 THP-1 cells. After transfection (3 hours), cells were lysed in M-PER mammalian protein extraction reagent (Pierce, Rockford, IL) containing protease inhibitor cocktail. The concentration of protein in the cell lysates was measured using the Protein Assay Dye Reagent (BioRad, Hercules, CA). Denatured proteins in BioRad sample buffer were separated on a 12% polyacrylamide gel under reducing conditions with 5% 2-mercaptoethanol. After electrophoresis, proteins were transferred onto a membrane, and the membrane was incubated with primary Ab, followed by incubation with secondary Ab conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA). Immunoreactive proteins were detected using an ECL Plus detection system (Amersham Biosciences, Piscataway, NJ).
Results
Disease-associated mutations in CIAS1 induce rapid cell death in human THP-1 monocytic cells
Mutation of CIAS1 is associated with FCAS, MWS, and CINCA, which represent a spectrum of diseases, and have been grouped together as cryopyrin-associated periodic syndrome (CAPS). Among these diseases, FCAS is the mildest, and CINCA is the most severe. In the current study, as the disease-associated mutations of CIAS1, we used the arginine-to-tryptophan substitution at aa 260 (R260W), which was identified in FCAS and MWS; the aspartic acid-to-asparagine substitution at aa 303 (D303N), which was identified in MWS and CINCA; and the tyrosine-to-cysteine substitution at aa 570 (Y570C), which was identified in the most severe cases of CINCA associated with brain atrophy and episodes of epilepsy.37 We first monitored cell death after transfection of the indicated CIAS1 mutants using annexin V as an indicator of phosphatidylserine exposure, and 7-AAD staining as an indicator of membrane damage. In THP-1 cells transfected with disease-associated mutants of CIAS1, the number of cells that were positive for both annexin V and 7-AAD dramatically increased as early as 3 hours after transfection. This profile of cell death was not observed in cells transfected with WT-CIAS1 (Figure 1A), indicating that disease-associated mutations of CIAS1 induced rapid cell death. Interestingly, the percentages of annexin V– or 7-AAD–positive cells were comparable with the level of ASC-dependent NF-κB activation by each of the disease-associated variants (Figure 1B).
When the expression of GFP-cryopyrin was monitored by flow cytometry, GFP began to become evident in cells at 1 hour and 30 minutes after transfection in both WT-CIAS1– and Y570C-transfected cells (Figure 1C). The expression of GFP-fusion protein was almost comparable between Y570C- and WT-transfected cells, up to 2 hours after transfection. At this point, the number of annexin V/7-AAD–positive cells increased only in Y570C-transfected THP-1 cells in a time-dependent manner (Figure 1C). Moreover, the percentage of annexin V– or 7-AAD–positive cells was almost comparable during the entire incubation period examined, suggesting that cells directly became annexin V/7-AAD–double-positive shortly after the initiation of gene expression. The efficiency of transfection was a approximately 75% in WT-CIAS1–transfected cells 3 hours after transfection, at which point 40% of Y570C-transfected cells were either annexin V or 7-AAD positive. At this point, the expression of GFP-fusion protein was reduced in Y570C-transfected cells (Figure 1C), most likely reflecting the decrease in cell viability.
To exclude the possibility that cell death induced by transfection of Y570C was due to the influence of the GFP moiety, WT-CIAS1 and the disease-associated mutants of CIAS1 lacking the epitope tag were cloned into pIRES2-EGFP. Using this expression system, we confirmed that Y570C, but not WT-CIAS1, induced rapid cell death in THP-1 cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). When we examined the another mammalian NLR NOD2/CARD15, and 2 of its disease-associated mutants, R334W and N670K, both of which are ligand-independent activating mutations observed in patients with Blau syndrome (MIM #186580) or early-onset sarcoidosis (MIM #609464),36,38 neither WT nor disease-associated mutants of NOD2/CARD15 induced cell death (data not shown), indicating that the rapid cell death we observed was a specific phenomenon associated with CAPS-associated CIAS1 mutations.
The cell death induced by Y570C is necrosis-like cell death
Disease-associated mutations in CIAS1 induced rapid cell death, which exhibited features more similar to the necrosis seen in nigericin-treated cells rather than the apoptosis observed in actinomycin D–treated cells (Figure 1A). We were interested in evaluating other distinctive features of cell death, in addition to those observed using flow cytometry, that were induced by Y570C transfection. The amount of LDH released into the culture supernatant from Y570C-transfected cells was 28% at 2.5 hours and 45% at 3 hours after transduction, compared with total intracellular LDH content (which was set to 100%). This was significantly higher than that released by WT-transfected cells (8% at 2.5 hours and 3 hours), indicating that there was a rapid release of LDH from damaged cells (Figure 2A).
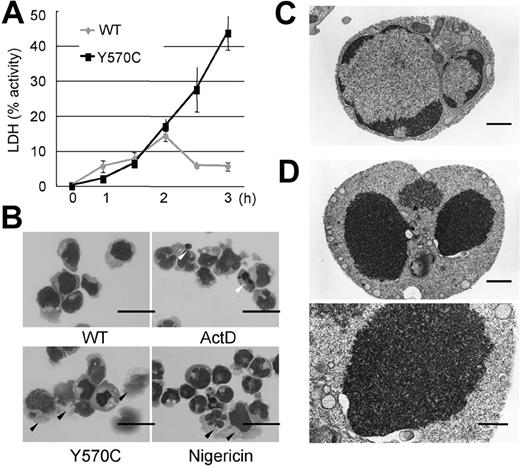
Distinctive features of cell death after Y570C transfection. (A) LDH release from damaged cells, as a percentage of total intracellular LDH content, which was set as 100%. The amount of LDH released into the culture supernatant from Y570C-transfected cells was significantly more than that released by WT-CIAS1–transfected cells. Error bars indicate SD (n = 3). (B) Cytospin preparations stained with Giemsa of the indicated cell populations and observed by inverted microscopy using an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a 40×/0.85 objective lens, an Olympus DP70 camera, and DP-controller version 1.1 software. Cells treated with 1 μg/mL actinomycin D (ActD) showed typical nuclear condensation with the formation of apoptotic bodies (white arrowheads), and slight cytoplasmic shrinkage. Some of the nuclei in nigericin-treated cells were swollen and stained weakly with Giemsa. In addition, nigericin-treated cells had larger cytoplasms, and were easily destroyed by the cytospin preparation process (black arrowheads). In Y570C-transfected cells, obvious nuclear condensation and formation of apoptotic bodies was rare, and their phenotypic appearance was similar to nigericin-treated necrotic cells. The scale bar represents 20 μm. (C) Electron microscopy of WT-CIAS1–transfected cells. The scale bar represents 1 μm. (D) Electron microscopy of Y570C-transfected cells revealed loss of the nuclear membrane cavity, and fusion of chromatin with the cytosol, as well as obscured structures of cytosolic organelles. The top scale bar represents 1 μm; the bottom scale bar represents 500 nm. Representative data from 3 independent analyses of similar results are shown.
Distinctive features of cell death after Y570C transfection. (A) LDH release from damaged cells, as a percentage of total intracellular LDH content, which was set as 100%. The amount of LDH released into the culture supernatant from Y570C-transfected cells was significantly more than that released by WT-CIAS1–transfected cells. Error bars indicate SD (n = 3). (B) Cytospin preparations stained with Giemsa of the indicated cell populations and observed by inverted microscopy using an Olympus BX51 microscope (Olympus, Tokyo, Japan) equipped with a 40×/0.85 objective lens, an Olympus DP70 camera, and DP-controller version 1.1 software. Cells treated with 1 μg/mL actinomycin D (ActD) showed typical nuclear condensation with the formation of apoptotic bodies (white arrowheads), and slight cytoplasmic shrinkage. Some of the nuclei in nigericin-treated cells were swollen and stained weakly with Giemsa. In addition, nigericin-treated cells had larger cytoplasms, and were easily destroyed by the cytospin preparation process (black arrowheads). In Y570C-transfected cells, obvious nuclear condensation and formation of apoptotic bodies was rare, and their phenotypic appearance was similar to nigericin-treated necrotic cells. The scale bar represents 20 μm. (C) Electron microscopy of WT-CIAS1–transfected cells. The scale bar represents 1 μm. (D) Electron microscopy of Y570C-transfected cells revealed loss of the nuclear membrane cavity, and fusion of chromatin with the cytosol, as well as obscured structures of cytosolic organelles. The top scale bar represents 1 μm; the bottom scale bar represents 500 nm. Representative data from 3 independent analyses of similar results are shown.
Cytospin preparations stained with Giemsa of WT-CIAS1– and Y570C-transfected cells are shown in Figure 2B. When cells were treated with actinomycin D, typical nuclear condensation and the formation of apoptotic bodies (white arrowheads in Figure 2B) was observed, along with slight shrinkage of the cytoplasm. In contrast, some of the nuclei in nigericin-treated cells were swollen, and were weakly positive for Giemsa staining. The cytoplasm in these cells was larger, and easily destroyed during cytospin preparation (black arrowheads in Figure 2B). In Y570C-transfected cells, obvious nuclear condensation and formation of apoptotic bodies was rare. Rather, the phenotypic appearance of these cells was similar to nigericin-treated necrotic cells (black arrowheads in Figure 2B). Examination of the cells using inverted microscopy showed that, after Y570C transfection, the cytoplasm of some cells became larger, with reduced reflection against visual light, and their nuclei more clearly identifiable, features which are associated with cellular edema (white arrowheads in Figure 3B). These features were also observed in nigericin-treated cells (data not shown). In addition, electron microscopy revealed the loss of the nuclear membrane cavity, and fusion of chromatin with the cytosol (Figure 2D), as well as obscured structures of cytosolic organelles, in agreement with the assessment of cellular edema based on inverted microscopy and the analysis of cytospin preparations. Thus, the features of rapid cell death induced by the CINCA-associated Y570C mutation were more like necrosis than apoptosis.
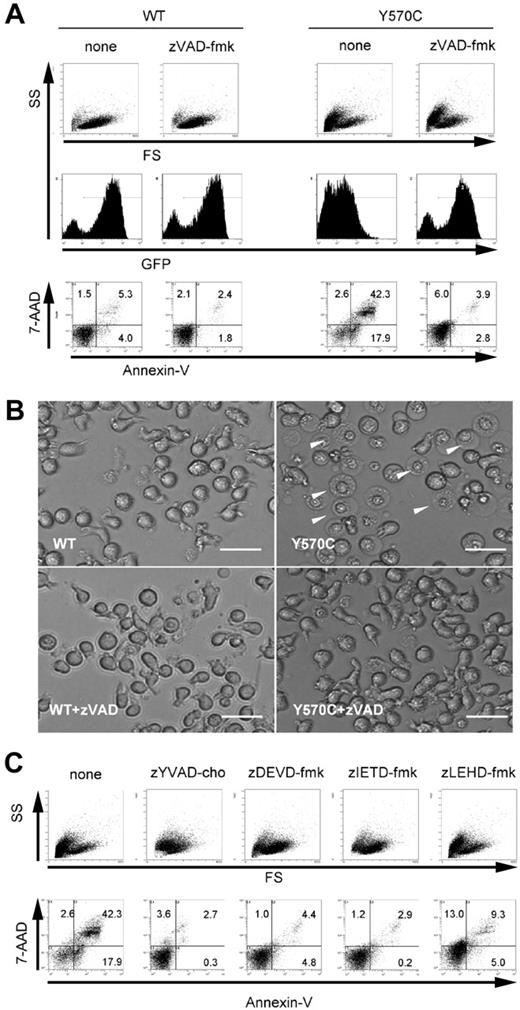
Suppressive effect of specific proteinase inhibitors on Y570C-induced cell death. (A) zVAD-fmk (50 μM) was added to THP-1 cells just after transfection. After transfection (3 hours), the cells were analyzed by flow cytometry. The expression of CIAS1 was monitored by GFP. Cells undergoing cell death appear as the cells smaller in size on the forward side-scatter (FS) axis, and cells positive for annexin V/7-AAD. The number in each quadrant shows the percentage of cells. (B) Transfected THP-1 cells were cultured in the presence or absence of 50 μM zVAD-fmk and observed by inverted microscopy using an Olympus IX70 microscope equipped with a 20×/0.40 objective lens and an Axiocam camera (Carl Zeiss). Axiovision version 3.0 software was used to process images. In some cells transfected with Y570C in the absence of zVAD-fmk treatment, the nucleus was more clearly delineated, and the cytoplasm was bigger in size but was less reflective of visual light (arrowheads), indicative of cells undergoing cell death. The scale bar represents 20 μm. (C) 50 μM of each caspase-specific inhibitor (caspase-1 inhibitor zYVAD-cho, caspase-3 inhibitor zDEVD-fmk, caspase-8 inhibitor zIETD-fmk, and caspase-9 inhibitor zLEHD-fmk) was added to THP-1 cells immediately after Y570C transfection, and cells were analyzed for cell death as for panel A. Representative data from 3 independent analyses of similar results are shown.
Suppressive effect of specific proteinase inhibitors on Y570C-induced cell death. (A) zVAD-fmk (50 μM) was added to THP-1 cells just after transfection. After transfection (3 hours), the cells were analyzed by flow cytometry. The expression of CIAS1 was monitored by GFP. Cells undergoing cell death appear as the cells smaller in size on the forward side-scatter (FS) axis, and cells positive for annexin V/7-AAD. The number in each quadrant shows the percentage of cells. (B) Transfected THP-1 cells were cultured in the presence or absence of 50 μM zVAD-fmk and observed by inverted microscopy using an Olympus IX70 microscope equipped with a 20×/0.40 objective lens and an Axiocam camera (Carl Zeiss). Axiovision version 3.0 software was used to process images. In some cells transfected with Y570C in the absence of zVAD-fmk treatment, the nucleus was more clearly delineated, and the cytoplasm was bigger in size but was less reflective of visual light (arrowheads), indicative of cells undergoing cell death. The scale bar represents 20 μm. (C) 50 μM of each caspase-specific inhibitor (caspase-1 inhibitor zYVAD-cho, caspase-3 inhibitor zDEVD-fmk, caspase-8 inhibitor zIETD-fmk, and caspase-9 inhibitor zLEHD-fmk) was added to THP-1 cells immediately after Y570C transfection, and cells were analyzed for cell death as for panel A. Representative data from 3 independent analyses of similar results are shown.
Necrosis, as the conceptual counterpart to apoptosis, occurs after exposure to high concentrations of detergents, oxidants, ionophores, or high intensities of pathologic insults, and is prevented only by removal of the stimuli. In contrast, programmed cell death is defined as a phenomenon that can blocked by an inhibitor of any signal pathway or activity (for example, caspases) within target cells.27 Therefore, we examined the effect of a pancaspase inhibitor to inhibit cell death by disease-associated CIAS1 mutation. In the presence of 50 μM zVAD-fmk, the increase in cells that were double positive for annexin V and 7-AAD was suppressed (Figure 3A), even though the cell morphology profile represented by forward- and side-scatter window was not rescued by zVAD-fmk treatment. However, the inhibitory effect of the pancaspase inhibitor was also observed under inverted microscopy, in which the number of cells with a necrotic morphology after Y570C transfection was dramatically decreased by treatment with zVAD-fmk (Figure 3B).
Cell death induced by the disease-associated CIAS1 mutant is inhibited by a cathepsin B inhibitor
Because Y570C-induced cell death was inhibited by zVAD-fmk, we next examined the effect of specific inhibitors of each caspase. Caspase activation can occur via several routes, including the extrinsic pathway, characterized by death receptor–mediated recruitment and activation of apical caspase-8, and the intrinsic pathway, characterized by assembly of cytosolic Apaf-1 and mitochondrial cytochrome c, and subsequent activation of apical caspase-9.39 Both of these pathways converge at the level of activation of executioner caspase-3.40 Caspase-1, a member of the family of inflammatory caspases, is also known as IL-1β converting enzyme.8 As shown in Figure 3C, the number of annexin V/7-AAD–double-positive cells observed after Y570C transfection in the absence of inhibitor (42.3%) unexpectedly decreased in the presence of each of the caspase inhibitors tested, including caspase-1 inhibitor zYVAD-cho (2.7%), caspase-3 inhibitor zDEVD-fmk (4.4%), caspase-8 inhibitor zITED-fmk (2.9%), and caspase-9 inhibitor zLEHD-fmk (9.3%). However, none of the caspase inhibitors rescued the cell morphology profile represented by the forward- and side-scatter window.
Although we could not identify the specific caspase-dependent cascade activated in Y570C-induced rapid cell death, it has been reported that zVAD-fmk and other caspase inhibitors are also potent cathepsin B inhibitors.41 We next examined effect of the cathepsin B-specific inhibitor CA-074-Me on Y570C-induced cell death. CA-074-Me (50 μM) effectively inhibited ability of Y570C to induce cell death, as assessed by positive annexin V and 7-AAD staining (Figure 4A), and also rescued the cell morphology profile in the flow cytometry window representing forward and side scatter. Thus, disease-associated CIAS1 mutation–induced cell death involves a cathepsin B–dependent pathway.
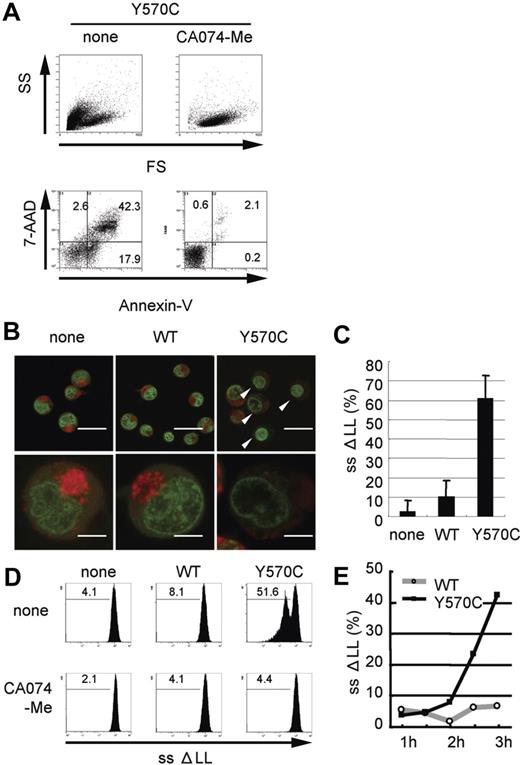
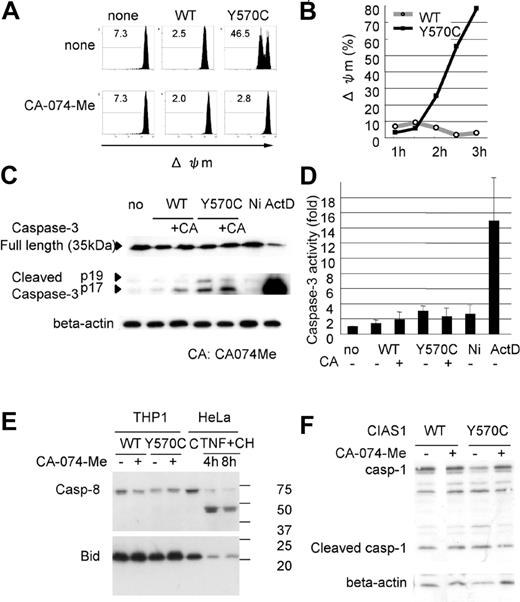
Cathepsin B-specific inhibitor suppresses Y570C-induced cell death and Y570C-transfection induces lysosomal leakage. (A) Y570C-transfected THP-1 cells were analyzed by flow cytometry 3 hours after transfection. Cells undergoing death are represented as cells that are smaller in size on the FS axis, and positive for annexin V/7-AAD. The cathepsin B–specific inhibitor CA-074-Me (50 μM) was added to THP-1 cells just after Y570C transfection. The number in each quadrant shows the percentage of cells. (B) Small-scale lysosomal leakage (ssΔLL) following transfection of WT-CIAS1 and Y570C lacking the GFP epitope tag was assessed by incubating cells with 1 μg/mL of acridine orange and monitoring fluorescence using confocal laser microscopy. In Y570C-transfected cells, cytosolic red fluorescence was reduced, accompanied by a dimming of the green-fluorescent structures of the nuclei. The top scale bar represents 20 μm; the bottom represents 5 μm. (C) The percentage of the cells with ssΔLL 3 hours after transfection with either WT-CIAS1 or Y570C. Error bars indicate SD (n = 6). (D) Analysis of ssΔLL by flow cytometry in cells transfected with the indicated constructs, in the presence or absence of 50 μM cathepsin B–specific inhibitor CA-074-Me. The cathepsin B–specific inhibitor CA-074-Me (50 μM) suppressed ssΔLL in Y570C-transfected cells. ssΔLL represents the loss of red fluorescence, and each number shows the percentage calculated in the region under the bar. (E) The percentage of cells with ssΔLL assessed by flow cytometry. Transfection with Y570C resulted in a time-dependent increase in ssΔLL in THP-1 cells. Representative data from 3 independent analyses of similar results are shown.
Cathepsin B-specific inhibitor suppresses Y570C-induced cell death and Y570C-transfection induces lysosomal leakage. (A) Y570C-transfected THP-1 cells were analyzed by flow cytometry 3 hours after transfection. Cells undergoing death are represented as cells that are smaller in size on the FS axis, and positive for annexin V/7-AAD. The cathepsin B–specific inhibitor CA-074-Me (50 μM) was added to THP-1 cells just after Y570C transfection. The number in each quadrant shows the percentage of cells. (B) Small-scale lysosomal leakage (ssΔLL) following transfection of WT-CIAS1 and Y570C lacking the GFP epitope tag was assessed by incubating cells with 1 μg/mL of acridine orange and monitoring fluorescence using confocal laser microscopy. In Y570C-transfected cells, cytosolic red fluorescence was reduced, accompanied by a dimming of the green-fluorescent structures of the nuclei. The top scale bar represents 20 μm; the bottom represents 5 μm. (C) The percentage of the cells with ssΔLL 3 hours after transfection with either WT-CIAS1 or Y570C. Error bars indicate SD (n = 6). (D) Analysis of ssΔLL by flow cytometry in cells transfected with the indicated constructs, in the presence or absence of 50 μM cathepsin B–specific inhibitor CA-074-Me. The cathepsin B–specific inhibitor CA-074-Me (50 μM) suppressed ssΔLL in Y570C-transfected cells. ssΔLL represents the loss of red fluorescence, and each number shows the percentage calculated in the region under the bar. (E) The percentage of cells with ssΔLL assessed by flow cytometry. Transfection with Y570C resulted in a time-dependent increase in ssΔLL in THP-1 cells. Representative data from 3 independent analyses of similar results are shown.
Disease-associated CIAS1 mutation induces lysosomal leakage
Because cathepsin B is a lysosomal enzyme, we evaluated lysosomal functional integrity in Y570C-transfected cells by measuring the level of ssΔLL. Acridine orange preferentially accumulates in lysosomes, resulting in the appearance of red fluorescence after excitation with blue light,42 whereas when this cationic dye is bound to DNA, it exhibits green fluorescence. In order to evaluate this green fluorescence, we used CIAS1 and its variants without GFP in this study. When WT-CIAS1–transfected cells, as well as nontransfected cells, were incubated with acridine orange and examined by confocal laser microscopy, we observed a bright accumulation of red fluorescence in the lysosomes (Figure 4B). In these cells, we also observed fine structures in the nuclei that exhibited green fluorescence. In contrast, cytosolic red fluorescence was reduced in Y570C-transfected cells (white arrowheads in Figure 4B). In addition, green fluorescent structures in the nuclei became dim in cells that had also lost lysosome-associated red fluorescence. The percentage of the cells exhibiting ssΔLL after Y570C transfection (Figure 4C) was almost comparable with the percentage of cells that became annexin V/7-AAD positive in experiments performed side by side with GFP-tagged proteins (data not shown). Using flow cytometry, we found that the percentage of Y570C-transfected cells with ssΔLL increased in a time-dependent manner (Figure 4D), reaching 51.6% at 3 hours after transfection (Figure 4D). ssΔLL was also observed after transfection with other disease-associated mutations of CIAS1 (Figure S2). This was also comparable to the results of annexin V/7-AAD staining. The induction of ssΔLL by Y570C transfection was effectively blocked with CA-074-Me (Figure 4D), suggesting that there may be paracrine/autocrine amplification pathway of cathepsin B–induced lysosomal leakage.
Disease-associated CIAS1 mutation induces mitochondria damage
To further evaluate the mechanism of cell death induced by CAPS-associated CIAS1 mutation, we assessed mitochondrial function using flow cytometry. Transfection with Y570C, but not WT-CIAS1, induced Δψm, indicating the presence of mitochondrial damage (Figure 5A-B). Interestingly, the percentage of cells with Δψm at 3 hours after transfection was most similar to GFP expression level, as represented in Figure 1C, and was greater than the number of cells that became annexin V/7-AAD positive, or showed ssΔLL after Y570C transfection (Figure 4E). Δψm was also observed after transfection with other disease-associated mutation of CIAS1 (Figure S2). Treatment with CA-074-Me completely suppressed Δψm (Figure 5A), indicating that cathepsin B worked upstream from mitochondrial damage in CIAS1-induced cell death.
Y570C-transfection induces mitochondrial damage. (A) Loss of mitochondrial inner transmembrane potential (Δψm) was assessed by flow cytometry using 1 μM of MitoTracker DeepRed 633. Δψm is represented as the loss of deep red fluorescence. The cathepsin B–specific inhibitor CA-074-Me (50 μM) suppressed Δψm in Y570C-transfected cells. Each number shows the percentage calculated in the region under the bar. (B) The percentage of cells with Δψm increased in a time-dependent manner following transfection with Y570C. (C) Full-length caspase-3 was detected by using a caspase-3 antibody, and cleaved caspase-3 was detected by using a cleaved caspase-3–specific antibody. Y570C-tranfection resulted in slight cleavage of caspase-3. However, this level of cleavage was far less than that induced by actinomycin D (ActD) treatment, but similar to the level induced by nigericin treatment. (D) After transfection with the WT-CIAS1 or Y570C mutant (3 hours), THP-1 cells were harvested and caspase-3 activity was measured according to the manufacturer's protocol. THP-1 cells treated with 1 μg/mL ActD and 20 μM nigericin (Ni) were used as controls. The fold increases in caspase-3 activity are the means of the normalized data (no treatment = 1) of triplicate cultures, and error bars indicate SD. (E) Neither cleaved caspase-8 nor Bid cleavage was observed upon Western blot analysis of cells following Y570C transfection. Bid cleavage can be assessed by the loss of full length of Bid. HeLa cells without treatment or treated with 20 ng/mL TNF-α and 10 μg/mL cycloheximide (TNF + CH) were used as positive controls for protein cleavage. (F) Y570C transfection did not induce caspase-1 cleavage at least 3 hours after transfection. Representative data from 3 independent analyses of similar results are shown.
Y570C-transfection induces mitochondrial damage. (A) Loss of mitochondrial inner transmembrane potential (Δψm) was assessed by flow cytometry using 1 μM of MitoTracker DeepRed 633. Δψm is represented as the loss of deep red fluorescence. The cathepsin B–specific inhibitor CA-074-Me (50 μM) suppressed Δψm in Y570C-transfected cells. Each number shows the percentage calculated in the region under the bar. (B) The percentage of cells with Δψm increased in a time-dependent manner following transfection with Y570C. (C) Full-length caspase-3 was detected by using a caspase-3 antibody, and cleaved caspase-3 was detected by using a cleaved caspase-3–specific antibody. Y570C-tranfection resulted in slight cleavage of caspase-3. However, this level of cleavage was far less than that induced by actinomycin D (ActD) treatment, but similar to the level induced by nigericin treatment. (D) After transfection with the WT-CIAS1 or Y570C mutant (3 hours), THP-1 cells were harvested and caspase-3 activity was measured according to the manufacturer's protocol. THP-1 cells treated with 1 μg/mL ActD and 20 μM nigericin (Ni) were used as controls. The fold increases in caspase-3 activity are the means of the normalized data (no treatment = 1) of triplicate cultures, and error bars indicate SD. (E) Neither cleaved caspase-8 nor Bid cleavage was observed upon Western blot analysis of cells following Y570C transfection. Bid cleavage can be assessed by the loss of full length of Bid. HeLa cells without treatment or treated with 20 ng/mL TNF-α and 10 μg/mL cycloheximide (TNF + CH) were used as positive controls for protein cleavage. (F) Y570C transfection did not induce caspase-1 cleavage at least 3 hours after transfection. Representative data from 3 independent analyses of similar results are shown.
In some models of programmed cell death, it has been suggested that cathepsins act independently of caspases, whereas in other models, cathepsin-mediated cell death is caspase dependent.26,27 In our experiments with each caspase-specific inhibitor, a specific caspase-dependent cascade was not evident. When Y570C was transfected into THP-1 cells, Western blotting showed that the cleavage of caspase-3 was slightly induced. However, cleavage was far less induced by Y570C transfection than by actinomycin D treatment (Figure 5C), even though there were no dramatic differences in the percentage of annexin V–positive cells (either PI-negative or -positive) between Y570C-transfected cells and actinomycin D–treated cells. Moreover, when caspase-3 enzyme activity was evaluated by using a colorimetric substrate, no increase in caspase-3 activity was observed after Y570C transfection (Figure 5D). On the other hand, when nigericin-treated THP-1 cells underwent necrosis, slight cleavage of caspase-3 was evident on the Western blots (Figure 5C), indicating that caspase-3 cleavage by disease-associated CIAS1 mutants may be accompanied by necrosis-like rapid cell death.
Apoptosis induced by forced oligomerization of ASC was reported to be mediated by caspase-8.43 We observed no evidence of caspase-8 cleavage after Y570C transfection in our study (Figure 5E). Furthermore, it has been reported that caspase-1–dependent IL-1β activation is important in the necrosis-like cell death observed during Salmonella infection. However, Y570C transfection did not induce an apparent increase in caspase-1 cleavage (Figure 5F) within the time period of Y570C-induced rapid cell death.
Proapoptotic caspases are not likely to be directly activated by lysosomal proteases.44-46 To identify the mechanism of lysosome-triggered cell death, we initiated a search for the cellular substrates of cathepsin B. One of the most likely cytosolic targets is Bid, a proapoptotic BH3-only Bcl-2 family member.47,48 Bid has been reported to be a substrate of lysosomal proteases,46 and in vitro studies indicate that lysosomal proteases can mediate cytochrome c release through Bid cleavage.44 However, we did not observe Bid cleavage after Y570C transfection (Figure 5E), even though THP-1 cells contained high levels of full-length Bid.
R837, an activator of the cryopyrin-associated inflammasome, induces rapid cell death
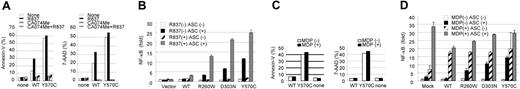
Disease-associated mutations of CIAS1 are thought to mimic active conformational changes in cryopyrin induced by microbial ligands. Recently, bacterial RNA and the small antiviral compound R837 were reported to activate caspase-1 through cryopyrin.49 Addition of R837 to WT-CIAS1–transfected THP-1 cells induced rapid cell death, which was effectively suppressed by CA-074-Me (Figure 6A). R837-induced activation of NF-κB was confirmed using a reporter assay in HEK293 cells (Figure 6B). The basal level of NF-κB activity in mock-trasnsfected cells was set as 1. ASC-dependent NF-κB activation by WT-CIAS1 (2.12 ± 0.02 fold; n = 3) increased approximately 2-fold with R837 treatment (5.12 ± 0.25 fold; n = 3). Interestingly, the ASC-dependent NF-κB activity of each of the disease-associated mutants also increased approximately 2-fold after R837 treatment (Figure 6B), even though R837 treatment did not increase the percentage of cells that were annexin V/7-AAD positive (Figure 6A).
R837, activator of cryopyrin-associated inflammasome, induces rapid cell death. (A) Addition of 10 μg/mL imiquimod R837 to WT-CIAS1–transfected THP-1 cells induced rapid cell death, as assessed by annexin V– and 7-AAD–positive staining. This effect was effectively suppressed by CA-074-Me (CA074) treatment. (B) ASC-dependent-activation of NF-κB induced by R837 was assessed using a gene reporter assay in HEK293 cells in the presence or absence of 10 μg/mL R837. NF-κB activity in cells expressing each of the disease-related CIAS1 mutants increased approximately 2-fold after R837 treatment. Values represent the mean of normalized data (mock without R837 = 1) of triplicate cultures, and error bars indicate SD. (C) Addition of 2 μg/mL of MDP to WT-CIAS1– and Y570C-transfected THP-1 cells had only a marginal effect on cell death, as assessed by the percentage of cells that were positive for annexin V and 7-AAD. (D) ASC-dependent activation of NF-κB induced by MDP was assessed using a gene reporter assay in HEK293 cells in the presence or absence of 2 μg/mL MDP. Although MDP induced NF-κB activation, it did not show ASC dependency. Values represent the means of the normalized data (mock without MDP = 1) of triplicate cultures, and error bars indicate SD. Representative data from 3 independent analyses of similar results are shown.
R837, activator of cryopyrin-associated inflammasome, induces rapid cell death. (A) Addition of 10 μg/mL imiquimod R837 to WT-CIAS1–transfected THP-1 cells induced rapid cell death, as assessed by annexin V– and 7-AAD–positive staining. This effect was effectively suppressed by CA-074-Me (CA074) treatment. (B) ASC-dependent-activation of NF-κB induced by R837 was assessed using a gene reporter assay in HEK293 cells in the presence or absence of 10 μg/mL R837. NF-κB activity in cells expressing each of the disease-related CIAS1 mutants increased approximately 2-fold after R837 treatment. Values represent the mean of normalized data (mock without R837 = 1) of triplicate cultures, and error bars indicate SD. (C) Addition of 2 μg/mL of MDP to WT-CIAS1– and Y570C-transfected THP-1 cells had only a marginal effect on cell death, as assessed by the percentage of cells that were positive for annexin V and 7-AAD. (D) ASC-dependent activation of NF-κB induced by MDP was assessed using a gene reporter assay in HEK293 cells in the presence or absence of 2 μg/mL MDP. Although MDP induced NF-κB activation, it did not show ASC dependency. Values represent the means of the normalized data (mock without MDP = 1) of triplicate cultures, and error bars indicate SD. Representative data from 3 independent analyses of similar results are shown.
On the other hand, addition of 2 μg/mL of MDP had only a marginal effect on cell death (Figure 6C). MDP also induced activation of NF-κB, but it did not show ASC dependency (Figure 6D). This is in contrast to a previous report that MDP induced IL-1β secretion from THP-1 cells as well as peripheral monocytes purified from a patient with CINCA.50
Discussion
More than 20 disease-associated mutations of CIAS1 have been described in patients with FCAS, MWS, and CINCA, most of which are found within the centrally located NOD.37,51,52 Of note, missense mutations similar to those found in NOD2/CARD1551,53 have been identified in patients with Blau syndrome, another autosomal-dominant autoinflammatory syndrome, and early-onset sarcoidosis, a set of sporadic granulomatous disorders that are phenotypically similar to Blau syndrome. Amino acids affected by the R260W mutation in CIAS1 and the R334W mutation in NOD2/CARD15 are at analogous sequence positions, suggesting a common molecular mechanism for their role in the development of autoinflammatory disease. In addition to NF-κB activation and IL-1β production in PMA-treated THP-1 cells, in this study we showed that disease-associated mutations of CIAS1 induced rapid cell death in THP-1 cells. Interestingly, CAPS-associated mutations in CIAS1 induced a type of cell death that had several characteristic features of necrosis. Cells became annexin V/7-AAD double-positive shortly after the initiation of gene expression. Using inverted-microscopy and analysis of cytospin preparations, we demonstrated that Y570C-transfected cells had cellular edema without apparent nuclear condensation. These results were confirmed by electron microscopy. CAPS-associated mutations in CIAS1 also provoked early LDH release, resulting in membrane damage. The protein encoded by CIAS1, cryopyrin, is homologous to plant disease resistance–associated NLRs, which mediate the hypersensitivity response, including metabolic alterations, production of antipathogen molecules, and localized cell death at the site of pathogen invasion.54 Therefore, the rapid cell death induced within a few hours by activating CIAS1 may be a suitable response for protection against invading microorganisms. In addition, the cell death induced by CIAS1 mutation was more characteristic of necrosis than typical apoptosis, and therefore may be more likely to evoke inflammation, a very reasonable response for “ringing the bell” in response to intruders.
Cell death induced by disease-associated CIAS1 mutations was accompanied by lysosomal leakage, and loss of mitochondrial inner transmembrane potential. Interestingly, all of these effects were most significantly suppressed with the cathepsin B–specific inhibitor CA-074-Me. This indicates that disease-associated CIAS1 mutation-induced cell death involves a cathepsin B–dependent pathway. Until recently, cathepsins were believed to be primarily involved in nonselective intracellular protein degradation in lysosomes, and their function outside lysosomes was largely discounted because of their instability at a neutral pH. However, cathepsins have transient activity in the cytosol, and as such, represent an enormous destructive potential. There are several examples of moderate lysosomal damage, often referred to as ssΔLL, leading to programmed cell death.55,56 Unfortunately, the mechanism of cathepsin B release into the cytosol upon mutational activation of CIAS1 remains unclear. It is possible that the method of gene transfection used in these studies induced lysosomal damage, and supported necrosis-like cell death of THP-1 monocytes. However, rapid cell death was not observed after the transfection with WT-CIAS1 or Blau syndrome/early-onset sarcoidois-associated NOD2/CARD15 under the same conditions, strongly suggesting that the cell death induced by CAPS-associated mutations in CIAS1 was a specific phenomenon of the CIAS1 mutants. On the other hand, our data indicated that cathepsin B participated in lysosomal leakage upon activation of CIAS1, since the induction of ssΔLL by disease-associated CIAS1 mutations was effectively suppressed by CA-074-Me.
IL-1β reportedly plays an important role in the necrosis-like cell death observed during Salmonella infection. We found that the amount of IL-1β in cell-culture supernatants of PMA-treated THP-1 cells was very low, and almost comparable between Y570C- and WT-CIAS1–transfected cells at 4 hours after transfection (data not shown), a point at which rapid cell death had already been observed in Y570C-transfected cells. In addition, we could not confirm the activation of caspase-1 by Western blot after transfection of Y570C. Among patients with autoinflammatory disorders, the remarkable response of patients with MWS/CINCA to the recombinant human IL-1 receptor antagonist anakinra suggests that IL-1β has a fundamental role in the pathogenesis of inflammation associated with mutations in CIAS1.57 However, we believe that the contribution of caspase-1 and IL-1β may be less potent in CIAS1-induced cell death.
The disease-associated mutations in CIAS1 are thought to mimic active conformational changes induced by microbial ligands. Through intramolecular interactions of their LRRs, cryopyrin and NOD2/CARD15 are maintained in an inactive conformation. This conformation is relieved by ligand recognition through the LRRs. Bacterial RNA and the small antiviral compound R837, as well as the ATP and gout-associated uric acid crystals, were recently reported to activate caspase-1 through cryopyrin.49,58,59 In the current study, we demonstrated that R837 induced rapid cell death of WT-CIAS1–transfected THP-1 cells. R837 functions as a ligand for TLR7 and TLR8 in humans. Since THP-1 cells express endogenous TLR7, there remains a possibility that the rapid cell death observed after the incubation of cells with R837 was not only cryopyrin mediated, but also TLR7 mediated. However, as shown in Figure 6A, CIAS1-WT–transfected THP-1 cells were more sensitive to R837 than untransfected cells, suggesting that R837-induced cell death is mediated by cryopyrin. Although R837 is considered to be an activator of the cryopyrin-associated inflammasome, it is also possible that R837, like ATP, stimulates members of the P2X and P2Y family of purine receptors.60
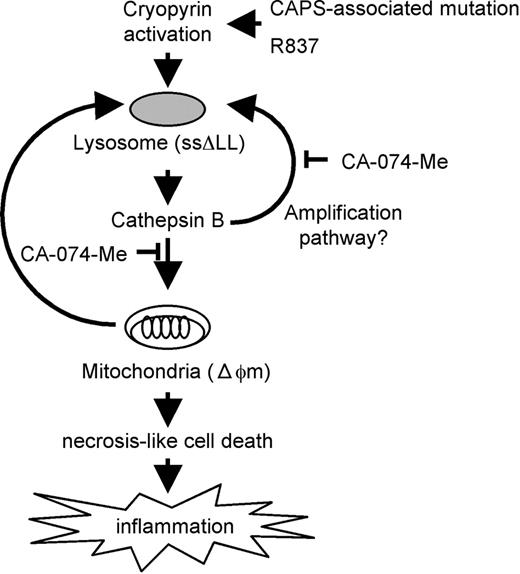
Based on our results, we present the following model for cell death induced by activating mutations of CIAS1 (Figure 7). The precise trigger for the release of cathepsin B remains unknown, but various insults, such as oxidative stress,61,62 can destabilize the lysosomal membrane, thereby releasing lysosomal proteases into the cytosol. In fact, CIAS1 was designated in association with FCAS, which is characterized by urticarial wheals, pain and swelling of joints, chills, and fever after exposure to cold stress. Following release of cathepsins into the cytosol, the initial response is magnified by the activation of cryopyrin, resulting in more lysosomal leakage. Released cathepsin B causes mitochondrial membrane dysfunction, but here as well, the mechanism remains to be fully elucidated, although it does not appear to involve Bid. Cytochrome c release from damaged mitochondria facilitates apoptosome formation, along with Apaf-1 activation and subsequent caspase-9, executioner caspase-3 and PARP activation. However, caspase-3 activation was not evident in our experiments, indicating that caspase-3 cleavage may be accompanied by necrosis-like rapid cell death. Cryopyrin-associated cell death may occur in a caspase-independent pathway.
Activation of cryopyrin induces cathepsin B–dependent rapid cell death of human THP-1 monocytic cells. Activation of cryopyrin is induced by the CAPS-associated mutation or R837. It induces necrosis-like cell death, which is accompanied by lysosomal leakage (ssΔLL) and loss of mitochondrial inner transmembrane potential (Δψm). All of these effects are effectively suppressed by the cathepsin B–specific inhibitor CA-074-Me, indicating that the cell death involves a cathepsin B–dependent pathway. Unfortunately, the mechanism of cathepsin B release into the cytosol upon activation of CIAS1 remains unclear; however, there may be an amplification pathway that cathepsin B participated in further lysosomal leakage upon activation of CIAS1. Activation of cryopyrin induces cathepsin B–dependent rapid necrosis-like cell death, which has the potential to evoke inflammation.
Activation of cryopyrin induces cathepsin B–dependent rapid cell death of human THP-1 monocytic cells. Activation of cryopyrin is induced by the CAPS-associated mutation or R837. It induces necrosis-like cell death, which is accompanied by lysosomal leakage (ssΔLL) and loss of mitochondrial inner transmembrane potential (Δψm). All of these effects are effectively suppressed by the cathepsin B–specific inhibitor CA-074-Me, indicating that the cell death involves a cathepsin B–dependent pathway. Unfortunately, the mechanism of cathepsin B release into the cytosol upon activation of CIAS1 remains unclear; however, there may be an amplification pathway that cathepsin B participated in further lysosomal leakage upon activation of CIAS1. Activation of cryopyrin induces cathepsin B–dependent rapid necrosis-like cell death, which has the potential to evoke inflammation.
In summary, cryopyrin, when activated, mediates rapid necrosis-like cell death in a lysosomal cathepsin B–dependent manner. Because cryopyrin is estimated to function as an intracellular pattern recognition receptor, cell death induced by putative activating mutations in CIAS1 is more like necrosis-like cell death, which has the potential to evoke inflammation, a very reasonable response.
Authorship
Contribution: A.F. performed the research and wrote the paper; N. Kambe and R.N. designed the research, analyzed the data, and helped in writing the paper; M.S. and H.T. performed and discussed the research; N. Kanazawa and S.A. analyzed and discussed the data; J.S. and T.S. contributed to plasmid constructions and provided helpful discussions; and T.H., T.N., and Y.M. managed and discussed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Naotomo Kambe, Department of Dermatology, Kyoto University Graduate School of Medicine, 606-8507 Japan; e-mail: nkambe@kuhp.kyoto-u.ac.jp.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Prof Nobuhiko Katunuma (Institute for Health Science, Tokushima Bunri University, Tokushima, Japan) for the fruitful discussion about lysosomal cathepsins, and Dr Yuji Horiguchi (Osaka Redcross Hospital, Osaka, Japan) for the electron microscopic observation.
This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports, and Culture, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal