Abstract
Activation of tyrosine kinase genes is a frequent event in human hematologic malignancies. Because gene activation could be associated with gene dysregulation, we attempted to screen for activating gene mutation based on high-level gene expression. We focused our study on the Janus kinase 2 (JAK2) gene in 90 cases of acute leukemia. This strategy led to the identification of a novel JAK2-acquired mutation in a patient with Down syndrome (DS) with B-cell precursor acute lymphoblastic leukemia (BCP-ALL). This mutation involves a 5–amino acid deletion within the JH2 pseudokinase domain (JAK2ΔIREED). Expression of JAK2ΔIREED in Ba/F3 cells induced constitutive activation of the JAK-STAT pathway and growth factor–independent cell proliferation. These results highlight the JAK2 pseudokinase domain as an oncogenic hot spot and indicate that activation of the JAK-STAT pathway may contribute to lymphoid malignancies and hematologic disorders observed in children with DS.
Introduction
Activating mutations of genes encoding protein tyrosine kinases are known to participate in malignant leukemogenesis in humans. Along with the well-known involvement of ABL1, KIT, and FLT3 genes, attention has been drawn to the JAK family members. These genes encode cytoplasmic protein tyrosine kinases associated with the intracellular region of cytokine receptors and essential to their activity. Chromosomal translocations were the first-described genetic lesions affecting JAK2, in both acute leukemia or myeloproliferative disease (MPD). They lead to the expression of fusion proteins containing the carboxy-terminus part of JAK2 and an amino-terminal portion of TEL,1,2 BCR,3 or PCM14–6 that generally possess constitutive kinase activity.
The most frequent-activating mutation affecting the JAK2 gene in human diseases is a valine-to-phenylalanine substitution at amino acid 617 (V617F) present in a subtype of MPD (reviewed in James et al7 ). Interestingly, the wild-type JAK2 copy is frequently lost in patients with polycythemia vera (PV), resulting from duplication of the mutated copy through mitotic recombination. The V617F mutation is located within the JAK homology 2 (JH2) pseudokinase, or inhibitory domain of the protein. It leads to constitutive JAK2 activation and confers growth factor hyperresponsiveness or independency to the Ba/F3 cell line. When assayed in bone marrow transplantation experiments, JAK2V617F recapitulates most of the clinical features of human PV.8–10 Contrasting with its high frequency in MPD, JAK2V617F is a rare event in acute myeloblastic leukemia (AML) that is essentially limited to cases of AML that develop following MPD.11–14
We hypothesized that somatic JAK2 mutations could be associated with increased expression of the mutated gene. Using quantitative reverse transcriptase–polymerase chain reaction (RT-PCR), we evaluated the expression of JAK2 in a series of 90 acute leukemias of myeloid or B-cell origin. We identified an activating JAK2 mutation in a patient with DS with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) associated with high expression of the mutated JAK2 allele.
Materials and methods
Quantitative RT-PCR
Total RNA was extracted using RNAble reagent (Eurobio, Courtaboeuf, France). Reverse transcription was carried out with 4 μg RNA using random hexamers and Moloney Murine Leukemia Virus Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. cDNAs were amplified using TaqMan Universal PCR Master Mix (Applied BioSystem, Foster City, CA) and 10 pmoles of primers and probes; reactions were performed on an ABI PRISM 7000 Sequence Detection System (Applied BioSystem) at 60°C for 40 cycles.
Patient's data (UPN3759)
Samples were obtained with informed consent in accordance with the Declaration of Helsinki. The INSERM review board gave approval for this study. UPN3759 is a 4-year-old child with Down syndrome. No transient myeloid disorder was documented prior to the BCP-ALL diagnosis. Ninety-two percent of blast cells were observed in the peripheral blood with a leukocytosis of 18 × 109/L, a thrombopenia of 9 × 109/L, and an anemia at 81 g/L (8.1 g/dL). Liver and spleen were slightly enlarged. No mediastinal or central nervous system infiltrations were detected. Immunophenotypic analyses of the peripheral-blood blasts demonstrated expression of B-cell markers (CD19, CD20, CD22, CD10, and cytoplasmic CD79a) along with TdT, HLA-DR, and CD34 in the absence of immunoglobulin expression. Myeloid markers (CD13, CD33, CD15, CD65, CD117/c-KIT, GPA, MPO, CD36, CD42, and CD61) and T-cell markers (CD2, CD3, and CD5) were not expressed. Molecular analyses characterized IGH and TCRG clonal rearrangements. The most frequent fusion transcripts associated with BCP-ALL (ie, BCR-ABL, TEL-AML1, E2A-PBX1, and MLL-AF4) were not found.
Plasmids
The JAK2 JH2 domain 5–amino acid (IREED) deletion construct was obtained by PCR using a human JAK2 wild-type cDNA template. All cDNAs were tagged at their amino terminus with a FLAG epitope, verified by sequence analyses, and subcloned into the MSCV-IRES-GFP retroviral vector.
Cell cultures
Murine IL-3–dependent lymphoid pro-B Ba/F3 cells were grown with 5% WEHI-conditioned medium (WCM) as a source of IL-3, and Ba/F3 cells expressing the erythropoietin receptor (Ba/F3-EPO-R) were cultured in the presence of 1 U/mL murine erythropoietin (Promocell, Heildelberg, Germany). Cells were electroporated and GFP-positive cells were sorted by flow cytometry after 6 days of culture. For cytokine-independent growth assays, cells were washed twice in PBS and seeded at 105 cells/mL. Serum starvation was performed as described.15 Cell growth was monitored using CellTiter 96 Proliferation assay (Promega, Madison, WI) or a Vi-cell counter (Beckman-Coulter, Fullerton, CA).
Results and discussion
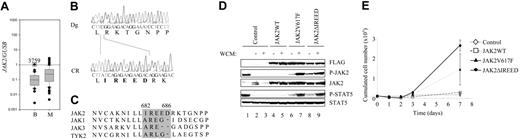
Using quantitative RT-PCR assay we evaluated the expression of JAK2 in a series of 90 acute leukemias of myeloid or B-cell origin (Figure 1A). Nucleotide sequence analyses of the JH2 region in the 10 patients expressing the highest levels of JAK2 identified a novel mutation in a 4-year-old child with DS with BCP-ALL (UPN3759). The JAK2 nucleotide sequence determined from RNA and genomic DNA of this patient only detected a mutated copy (Figure 1B). No structural abnormality of the JAK2 locus could be observed by standard karyotyping or fluorescence in situ hybridization (FISH) analysis (data not shown), suggesting the presence of the mutated gene on both chromosomes. Accordingly, the analyses of single nucleotide polymorphism (SNP) located within exons 4 and 17 of JAK2 indicated a loss of heterozygosity at the diagnostic stage (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). The mutated JAK2 encodes a protein lacking 5 amino acids (682 to 686) (JAK2ΔIREED) (Figure 1C). The JAK2 mutation described here occurred in a patient with BCP-ALL, indicating that JAK2 mutations are recurrent in this leukemic type.17 The activity of JAK2ΔIREED and JAK2V617F were compared in IL-3–dependent lymphoid Ba/F3 cells. Activation of intracellular signaling pathways was investigated by detecting tyrosine-phosphorylated JAK2 and STAT5 proteins in flow-sorted GFP-positive cells. Constitutive tyrosine phosphorylation of JAK2 and STAT5 was observed in Ba/F3-JAK2V167F and Ba/F3-JAK2ΔIREED cells, demonstrating the activation of the JAK-STAT pathway (Figure 1D). The ERK and AKT pathways were also activated (data not shown). As shown in Figure 1E, JAK2ΔIREED was able to transform Ba/F3 cells to growth factor independency. Transformation was facilitated when the mutated JAK2 was associated with EPO-R expression18–22 (data not shown). Interestingly, in the Ba/F3 cellular context, JAK2ΔIREED was sensitive to the JAK inhibitor I (Figure S2). Pilot bone marrow transplant (BMT) assays in mice showed that JAK2ΔIREED was able to induce a MPD in recipients. The salient features were elevation of platelet, granulocytic, and red blood cell counts observed 2 months after transplantation, similar to what is observed with JAK2V617F.8–10 The EPOR, MPL, and GCSFR genes were, however, not transcribed in the patient's cells, as judged from quantitative PCR experiments (data not shown). The cytokine receptor chain expressed in the leukemic cells and likely to be associated with mutated JAK2, therefore, remains to be identified.
A mutated JAK2 is overexpressed in a BCP-ALL sample and transforms Ba/F3 cells. (A) Quantitative RT-PCR analysis of JAK2 expression in leukemic samples. Human Taqman sets were located in the 3′ region of the tyrosine kinase domain, conserved in all JAK2 gene rearrangements. JAK2 primers and probes were forward (GTGTTCCATTTGATAGAACTTTTGAAGA), probe (5′-FAM-AGATTACCAAGACCAGATGGATGCCCAGA-TAMRA-3′), and reverse (ATTATTGTTCCAGCATTCTGTCATGA). The quality of the cDNA was evaluated using GUSB expression as described.16 The relative expression level of tyrosine kinase genes was calculated using the ΔCt calculation. The ratio of gene expression relative to the ubiquitously expressed gene GUSB was calculated for each sample. Two groups of samples are compared: BCP-ALL (n = 26) and AML (n = 61). Three ill-classified acute leukemia samples are not shown. Results are presented as a box plot graph using a logarithmic scale. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Narrow horizontal bars above and below the box indicate the 90th and 10th percentiles. Outliers appear as dots, and the UPN3759 sample is shown. (B) Sequence analysis of the JH2 domain JAK2ΔIREED mutation in the patient with DS with BCP-ALL showing a combined deletion of 16 nucleotides and the addition of 1 nucleotide. Sequence shown was obtained from DNA at diagnosis (Dg; top) and at complete remission (CR; bottom). During remission the sequence was identical to the wild-type JAK2 sequence. (C) The R683 amino acid, deleted in the JAK2 genomic sequence of the UPN3759 sample (gray box), is a conserved residue of the 4 human JAK proteins. (D) The JAK2ΔIREED mutant activates the JAK/STAT pathway. Western blotting analyses of JAK2 and STAT5 proteins in IL-3–dependent control cells or transformed JAK2 Ba/F3 cells. Transduced cells were sorted by flow cytometry for GFP expression and grown in the presence (+) or absence (−) of WEHI-conditioned medium (WCM). Lane 1 corresponds to wild-type Ba/F3 cells starved and stimulated by IL-3. Extraction and Western blotting analyses were performed using standard protocols. Antibodies against phospho-STAT5 (Y694) and phospho-JAK2 (Y1007/Y1008) were from Cell Signaling Technology (Danvers, MA). The JAK2 (C20) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), the Flag M2 antibody was from Sigma Aldrich (St Louis, MO), and the STAT5 immune serum was previously described.15 Cell lysates (100 μg) were used. (E) Sorted GFP-positive cells were incubated in media without IL-3 for 7 days, and the numbers of total viable cells were scored. Error bars indicate SDs.
A mutated JAK2 is overexpressed in a BCP-ALL sample and transforms Ba/F3 cells. (A) Quantitative RT-PCR analysis of JAK2 expression in leukemic samples. Human Taqman sets were located in the 3′ region of the tyrosine kinase domain, conserved in all JAK2 gene rearrangements. JAK2 primers and probes were forward (GTGTTCCATTTGATAGAACTTTTGAAGA), probe (5′-FAM-AGATTACCAAGACCAGATGGATGCCCAGA-TAMRA-3′), and reverse (ATTATTGTTCCAGCATTCTGTCATGA). The quality of the cDNA was evaluated using GUSB expression as described.16 The relative expression level of tyrosine kinase genes was calculated using the ΔCt calculation. The ratio of gene expression relative to the ubiquitously expressed gene GUSB was calculated for each sample. Two groups of samples are compared: BCP-ALL (n = 26) and AML (n = 61). Three ill-classified acute leukemia samples are not shown. Results are presented as a box plot graph using a logarithmic scale. The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Narrow horizontal bars above and below the box indicate the 90th and 10th percentiles. Outliers appear as dots, and the UPN3759 sample is shown. (B) Sequence analysis of the JH2 domain JAK2ΔIREED mutation in the patient with DS with BCP-ALL showing a combined deletion of 16 nucleotides and the addition of 1 nucleotide. Sequence shown was obtained from DNA at diagnosis (Dg; top) and at complete remission (CR; bottom). During remission the sequence was identical to the wild-type JAK2 sequence. (C) The R683 amino acid, deleted in the JAK2 genomic sequence of the UPN3759 sample (gray box), is a conserved residue of the 4 human JAK proteins. (D) The JAK2ΔIREED mutant activates the JAK/STAT pathway. Western blotting analyses of JAK2 and STAT5 proteins in IL-3–dependent control cells or transformed JAK2 Ba/F3 cells. Transduced cells were sorted by flow cytometry for GFP expression and grown in the presence (+) or absence (−) of WEHI-conditioned medium (WCM). Lane 1 corresponds to wild-type Ba/F3 cells starved and stimulated by IL-3. Extraction and Western blotting analyses were performed using standard protocols. Antibodies against phospho-STAT5 (Y694) and phospho-JAK2 (Y1007/Y1008) were from Cell Signaling Technology (Danvers, MA). The JAK2 (C20) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), the Flag M2 antibody was from Sigma Aldrich (St Louis, MO), and the STAT5 immune serum was previously described.15 Cell lysates (100 μg) were used. (E) Sorted GFP-positive cells were incubated in media without IL-3 for 7 days, and the numbers of total viable cells were scored. Error bars indicate SDs.
Mutations of the GATA1 gene have been identified as an early event in the transient myeloid disorders and acute megakaryoblastic leukemia observed in patients with DS.23 The GATA1 gene was not expressed in the lymphoid blasts of our patient, and no mutation was observed in the 5′ part of the gene (data not shown). This patient was also negative for the most frequent oncogenetic events associated with BCP-ALL (BCR-ABL, MLL-AF4, E2A-PBX1, TEL-RUNX1 fusion genes). Consequently, the only identified additional proleukemic event in the present case is the trisomy 21. Our results underscore a possible leukemic cooperation between constitutional trisomy 21 and activation of the JAK/STAT pathway as suggested by Walters et al.24
Authorship
Contribution: S.M., R.B.-M., C.S., and I.R.-W. performed research and analyzed the data; M.D., K.B., and E.A.M. provided vital reagents; R.B., J.-L.V., and W.V. provided vital reagents and reviewed the manuscript; E.D. performed research, designed the research, analyzed data, and wrote the manuscript; and O.A.B. and V.P.-L. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
E.D. and V.P.-L. share senior authorship.
Correspondence: Virginie Penard-Lacronique, INSERM E210, Hôpital Necker, 149 rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: penard@necker.fr; and Eric Delabesse, Laboratoire d'Hématologie and INSERM U563, Hôpital Purpan, Toulouse, France; e-mail: delabesse@chu-toulouse.fr.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank F. Moreau-Gachelin and R. Monni for valuable help, F. Delhommeau and D. Pisani for BaF3 and BaF3-EPO-R, Jérôme Mégret for cell sorting, and C. Lavau for critical reading of the manuscript.
This work was supported by INSERM, Ligue Nationale Contre le Cancer - Comité de Paris (équipe labellisée, O.A.B. and W.V.) and INCa grants PL038 and PL054. S.M. was a recipient of FRM and SFH fellowships.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal