Abstract
Ferroportin (Fpn) (IREG1, SLC40A1, MTP1) is an iron transporter, and mutations in Fpn result in a genetically dominant form of iron overload disease. Previously, we demonstrated that Fpn is a multimer and that mutations in Fpn are dominant negative. Other studies have suggested that Fpn is not a multimer and that overexpression or epitope tags might affect the localization, topology, or multimerization of Fpn. We generated wild-type Fpn with 3 different epitopes, GFP, FLAG, and c-myc, and expressed these constructs in cultured cells. Co-expression of any 2 different epitope-tagged proteins in the same cell resulted in their quantitative coimmunoprecipitation. Treatment of Fpn-GFP/Fpn-FLAG–expressing cells with crosslinking reagents resulted in the crosslinking of Fpn-GFP and Fpn-FLAG. Western analysis of rat glioma C6 cells or mouse bone marrow macrophages exposed to crosslinking reagents showed that endogenous Fpn is a dimer. These results support the hypothesis that the dominant inheritance of Fpn–iron overload disease is due to the dominant-negative effects of mutant Fpn proteins.
Introduction
Ferroportin (Fpn) is the only identified iron exporter responsible for the entry of iron into plasma and is present on all physiologically relevant iron-exporting tissues: placenta, macrophages, hepatocytes, and intestinal duodenum. Fpn is the receptor for hepcidin, a polypeptide hormone made by the liver in response to iron stores and inflammation.1 Binding of hepcidin to Fpn leads to the internalization and degradation of Fpn.2 The interaction of Fpn and hepcidin can explain most of the genetic iron overload diseases; inadequate production of hepcidin in response to body iron burden will lead to continued iron export.
There is one genetic form of iron overload disease, termed Fpn disease, or hereditary hemochromatosis type IV, that is not the result of defective hepcidin production. Rather, the disorder is due to mutations in Fpn.3 Studies examining the behavior of mutant Fpn in culture cells showed that Fpn either does not reach the cell surface, or cell surface Fpn is not responsive to hepcidin.4–7 As opposed to the other iron overload diseases, which show recessive inheritance, this disorder is dominant.3 Analysis of the mutations that lead to this disorder have revealed that all mutations identified to date are missense mutations; no nonsense mutations have been identified.8 Further, mice heterozygous for a targeted gene deletion of Fpn do not show iron loading.9 Based on these observations, we suggested that Fpn is a multimer and that the mutant allele might affect the behavior of the wild-type allele, resulting in a dominant-negative phenotype.4 Recent studies suggesting that Fpn is not a multimer6,10,11 led us to reevaluate this issue. In this communication we present data on the topology of Fpn and on its multimeric structure.
Materials and methods
Cells, plasmids, and media
A mouse Fpn cDNA was cloned into the Xho1 sites of the cytomegalovirus (CMV)–containing vectors pEGFP-N1 (Clontech, Mountain View, CA), pCMV-Tag4B-FLAG (Stratagene, La Jolla, CA) or pCMV-Tag5–c-myc (Stratagene). Amino-terminally FLAG-tagged Zebrafish Fpn in pCMV2B-FLAG was a generous gift from Dr Adriana Donovan (Harvard Medical School). HEK293T cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum, and transfected with pFpn-EGFP-N1, pCMV4BFpn-FLAG, or pCMV-Tag5Fpn–c-myc using Nucleofector technology (Amaxa, Gaithersburg, MD), according to the manufacturer's directions and as previously described.4 Transfection efficiency was approximately 90% for single transfection and 80% for cotransfection.
Enzyme-linked immunosorbent assay (ELISA)
Control and transfected HEK293T-Fpn cells were incubated with 10 μM ferric ammonium citrate (FAC) for 24 hours and cellular protein was exacted as described.4 C6 rat glioma cells were maintained in DMEM. Bone marrow macrophages were isolated from C57/B6 mouse femurs and cultured in L-cell–conditioned medium (DMEM) with 10% fetal bovine serum for 6 to 7 days, and adherent cells were transferred to Petri dishes for additional passages. Bone marrow macrophages were iron loaded with 10 μM FAC for 18 to 24 hours to induce Fpn expression at the plasma membrane.
Ferritin ELISA
Ferritin levels from mammalian extracts were determined by an ELISA assay (Laguna Scientific, Laguna, CA) according to the manufacturer's instructions. Protein concentrations were determined by bicinchoninic acid assay (Pierce, Rockford, IL).
Immunofluorescence
Immunofluorescence was performed as previously described,2 with the following changes: after formaldehyde fixations, cells were incubated in phosphate-buffered saline (PBS), and 1% bovine serum albumin (BSA) with or without 0.1% saponin (Sigma, St Louis, MO). In the absence of detergent/permeabilization, only epitopes that are extracellular will be detected by immunofluorescence. In the presence of detergent/permeabilization, extracellular as well as intracellular epitopes are detected and this procedure was used to determine the location of the amino and carboxyl termini of Fpn. Images were captured on an Olympus BX51 microscope (Olympus, Tokyo, Japan) using an Olympus U-CMAD-Z camera and a 60×/1.3 NA oil objective with images acquired using Picture Frame 2.5 software. Captured images were combined in Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA) to generate multiple-image figures for publication. Green images are GFP and red images are Alexa 594 reagent (Invitrogen, Carlsbad, CA).
Immunoprecipitation and Western blot analysis
Immunoprecipitation of Fpn-GFP and Fpn-FLAG was performed as previously described.2,4 Briefly, cells were harvested and lysed in 1% Triton X-100, 150 mM NaCl, 50 mM Tris-HCl, and 50 mM EDTA pH 7.2, with 1X protease inhibitor cocktail (Roche, Milan, Italy) at 0°C for 60 minutes. Samples were centrifuged at 14 000g for 30 minutes and supernatants incubated with mouse anti-FLAG (1:1000, Sigma) and protein A/G resin (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Resin was pelleted, washed extensively, and Fpn-FLAG eluted with 200 μg/mL FLAG peptide (Sigma). Eluted samples were diluted in SDS sample buffer without β mercaptothanol or dithiothreitol, heated to 37°C for 10 minutes, and run on 10% SDS–polyacrylamide gel electrophoresis (PAGE) prior to Western blot analysis. Western analysis was performed as described previously4 using rabbit anti-GFP (1:10 000, Abcam no. ab6556, Cambridge, MA), mouse anti-FLAG (1:10 000, Sigma), mouse anti–c-myc (1:1000, Covance), rabbit anti–rat Fpn (1:500, Alpha Diagnostics), rabbit anti–rat DMT1 (1:500, Alpha Diagnostics, Berkeley, CA), rabbit anti–mouse Fpn (1:1000, Dr David Haile, University of Texas–San Antonio, or Dr Nancy Andrews, Harvard Medical School, Boston, MA), or goat anticeruloplasmin (1:1000, Sigma) followed by either peroxidase-conjugated goat anti–rabbit IgG (1:10 000, Jackson ImmunoResearch Labs, Bar Harbor, ME), goat anti–mouse IgG (1:10 000, Jackson ImmunoResearch Labs), or peroxidase-conjugated donkey anti–goat IgG (1:5 000, Santa Cruz Biotechnology). Western blots were developed using the chemiluminescence detection reagent Western Lightning (PerkinElmer Life Sciences, Shelton, CT) as per the manufacturer's instructions.
Crosslinking
Crosslinking was performed using the cleavable crosslinker ethylene blycolbis (succinimidylsuccinate; EGS; Pierce). Crosslinking and cleaving were performed using the manufacturer's instructions with the following modification: cells were washed with PBS 3 times prior to incubation with 1.5 mM EGS for 60 minutes at 4°C and cleavage was performed at room temperature or 37°C for 60 minutes.
Results
Topology of the amino and carboxyl terminals of Fpn
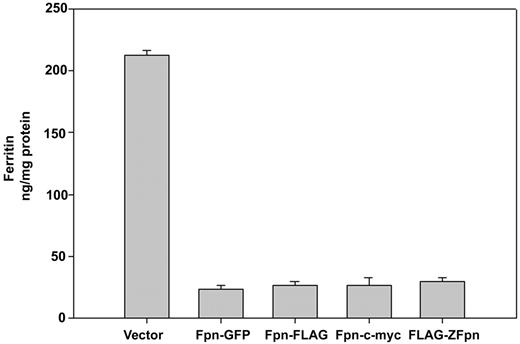
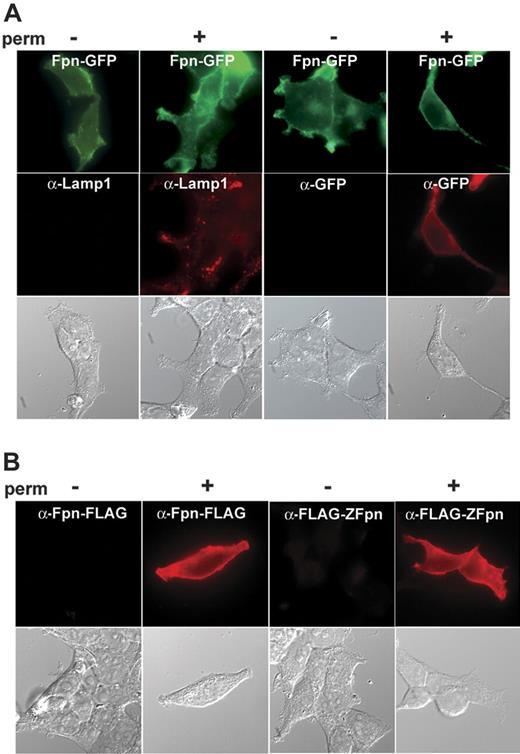
Our previous studies relied on the use of Fpn with a carboxyl terminal GFP.2,4 Expression of Fpn-GFP led to cellular iron export and decreased iron stores, showing that the protein was functional. Expression of Fpn lowers ferritin levels. We measured ferritin levels in FAC-loaded cells expressing Fpn-GFP, Fpn-FLAG, Fpn–c-myc, and FLAG-Zfpn. Addition of GFP, FLAG, or c-myc epitopes does not affect the ability of the epitope-tagged Fpn to transport iron (Figure 1). One study has reported that the carboxyl terminus of Fpn is intracellular,12 while other studies have suggested that the carboxyl terminus is extracellular.5,10 We examined the topology of carboxyl epitopes using immunofluorescence. HEK293T cells were transfected with a plasmid containing Fpn-GFP. Both intact and permeabilized cells were incubated with antibodies against GFP. As a control for plasma membrane integrity, cells were also incubated with an antibody specific to the lysosomal protein Lamp-1. As shown in Figure 2A, immunofluorescent detection of Fpn became apparent only when cells were permeabilized. Similar results were obtained in cells expressing Fpn–c-myc or Fpn-FLAG (data not shown). These results suggest that independent of the nature of the epitope, the carboxyl terminal of epitope-tagged Fpn is intracellular. We also examined whether the amino terminus of Fpn is intracellular or extracellular using the same permeabilization technique. Again, the amino terminal FLAG epitope-tagged zebrafish Fpn was detected only when cells were permeabilized (Figure 2B).
Epitope-tagged Fpn is functional. HEK293T cells were transiently transfected using the Amaxa nucleofector with empty vectors or vectors containing mouse Fpn with the designated carboxyl terminal epitopes, or zebrafish Fpn with an amino terminal FLAG. After 24 hours, cells were cultured with FAC (10 μM iron) for 24 hours and ferritin levels determined by ELISA. Error bars represent the standard deviation of 3 separate experiments in duplicate.
Epitope-tagged Fpn is functional. HEK293T cells were transiently transfected using the Amaxa nucleofector with empty vectors or vectors containing mouse Fpn with the designated carboxyl terminal epitopes, or zebrafish Fpn with an amino terminal FLAG. After 24 hours, cells were cultured with FAC (10 μM iron) for 24 hours and ferritin levels determined by ELISA. Error bars represent the standard deviation of 3 separate experiments in duplicate.
Topology of the amino and carboxyl terminals of Fpn. HEK293T cells were plated on glass coverslips and transfected with pFpn-GFP or pFLAG-Zebrafish-Fpn (FLAG-ZFpn). Eighteen to 24 hours after transfection, cells were fixed using 3.7% formaldehyde in PBS and permeabilized (perm) (+) or not (-) using 0.1% saponin in PBS/BSA for 20 minutes. Cells were incubated with primary antibodies (mouse anti-Lamp1, rabbit anti-GFP, or mouse anti-FLAG) followed by Alexa 594–conjugated goat anti–mouse IgG or goat anti–rabbit IgG. (A) Shows the Fpn-GFP signal and the immunodetection of GFP only upon permeabilization with Lamp1 as a positive control for permeabilization. (B) Shows that the carboxyl and amino terminal FLAG epitopes on Fpn are detected only upon permeabilization.
Topology of the amino and carboxyl terminals of Fpn. HEK293T cells were plated on glass coverslips and transfected with pFpn-GFP or pFLAG-Zebrafish-Fpn (FLAG-ZFpn). Eighteen to 24 hours after transfection, cells were fixed using 3.7% formaldehyde in PBS and permeabilized (perm) (+) or not (-) using 0.1% saponin in PBS/BSA for 20 minutes. Cells were incubated with primary antibodies (mouse anti-Lamp1, rabbit anti-GFP, or mouse anti-FLAG) followed by Alexa 594–conjugated goat anti–mouse IgG or goat anti–rabbit IgG. (A) Shows the Fpn-GFP signal and the immunodetection of GFP only upon permeabilization with Lamp1 as a positive control for permeabilization. (B) Shows that the carboxyl and amino terminal FLAG epitopes on Fpn are detected only upon permeabilization.
Multimerization of Fpn
Evidence for the multimeric structure of Fpn was based on coimmunoprecipitation studies in which cells were transfected with plasmids expressing 2 different epitope forms of Fpn. It is possible that the epitopes used might influence the ability to coimmunoprecipitate Fpn. To address this issue we cotransfected HEK293T cells with plasmids containing either Fpn–c-myc or Fpn-FLAG. At 24 hours after transfection, cells were lysed and Fpn immunoprecipitated using antibodies specific to c-myc or FLAG. Coimmunoprecipitation of both transfected epitope-tagged Fpn was observed regardless of the epitope used (Figure 3A and De Domenico et al4 ). No coimmunoprecipitation was detected when extracts of cells expressing only Fpn-FLAG were mixed with extracts of cells expressing either Fpn-GFP or Fpn–c-myc before immunoprecipitation, nor when cells expressing only Fpn-FLAG, Fpn-GFP, or Fpn–c-myc were mixed prior to extraction (data not shown). Further, preadsorption of extracts with pansorbin or protein A/G beads prior to addition of antibody did not affect the ability to coimmunoprecipitate Fpn epitope-tagged proteins (data not shown). Examination of the amount of Fpn-FLAG and Fpn–c-myc in the initial input and in the eluate showed that most of the Fpn-FLAG protein was captured by the immunoprecipitation and that almost half of the Fpn–c-myc protein was also captured by the immunoprecipitation of Fpn-FLAG. The amount of Fpn–c-myc in the flow-through was reduced relative to extracts not immunoprecipitated. This is predicted if a significant amount of Fpn–c-myc is present as a heteromultimer with Fpn-FLAG. We would expect to deplete the Fpn–c-myc population by 33% by coimmunoprecipitation using Fpn-FLAG. Previously, we demonstrated that the immunoprecipitation of Fpn epitopes was specific, as the epidermal growth factor receptor was not found in the Fpn immunoprecipitates.4 However, endogenous levels of epidermal growth factor receptor are low. We confirmed the specificity of the immunoprecipitation by transiently expressing DMT1-GFP in cells expressing Fpn-FLAG. DMT1-GFP did not coimmunoprecipitate with Fpn-FLAG (data not shown).
Immunoprecipitation and crosslinking of Fpn demonstrate Fpn is a multimer. (A) HEK293T cells were transfected with pFpn-FLAG and pFpn–c-myc. Twenty-four hours after transfection cells were solubilized in lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.2, 50 mM EDTA, 1% Triton X-100 with 1X protease inhibitor cocktail) and Fpn-FLAG immunoprecipitated as described in “Materials and methods.” The input, flow-through (FT), and eluate were examined for the presence of Fpn-FLAG and Fpn–c-myc by SDS-PAGE and Western blot analysis. The volumes of the input and flow-through analyzed were twice that of the eluate. (B) HEK293T cells were transfected with pFpn-GFP and pFpn-FLAG. Twenty-four hours after transfection cells were placed at 0°C and incubated with 1.5 mM crosslinking reagent EGS for 60 minutes. The crosslinking reagent was quenched by addition of cell growth medium, cells were solubilized in lysis buffer as in panel A, and the lysates were incubated in the presence or absence of the cleaving reagent, 1.0 M hydroxylamine-HCl, for 60 minutes at room temperature. The extracts were examined for the presence of crosslinked high-molecular-mass Fpn-GFP and Fpn-FLAG by 4% SDS-PAGE and Western blot analysis (left panel) or 8% SDS/PAGE and Western blot analysis after cleavage of the crosslinking.
Immunoprecipitation and crosslinking of Fpn demonstrate Fpn is a multimer. (A) HEK293T cells were transfected with pFpn-FLAG and pFpn–c-myc. Twenty-four hours after transfection cells were solubilized in lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.2, 50 mM EDTA, 1% Triton X-100 with 1X protease inhibitor cocktail) and Fpn-FLAG immunoprecipitated as described in “Materials and methods.” The input, flow-through (FT), and eluate were examined for the presence of Fpn-FLAG and Fpn–c-myc by SDS-PAGE and Western blot analysis. The volumes of the input and flow-through analyzed were twice that of the eluate. (B) HEK293T cells were transfected with pFpn-GFP and pFpn-FLAG. Twenty-four hours after transfection cells were placed at 0°C and incubated with 1.5 mM crosslinking reagent EGS for 60 minutes. The crosslinking reagent was quenched by addition of cell growth medium, cells were solubilized in lysis buffer as in panel A, and the lysates were incubated in the presence or absence of the cleaving reagent, 1.0 M hydroxylamine-HCl, for 60 minutes at room temperature. The extracts were examined for the presence of crosslinked high-molecular-mass Fpn-GFP and Fpn-FLAG by 4% SDS-PAGE and Western blot analysis (left panel) or 8% SDS/PAGE and Western blot analysis after cleavage of the crosslinking.
It is possible that multimerization occurs in solution once the proteins have been extracted from the membrane, although control experiments have suggested that this did not occur.4 To further rule out this possibility we used crosslinking reagents. Cells cotransfected with plasmids containing Fpn-FLAG and Fpn-GFP were incubated at 0°C with the permeable cleavable homofunctional crosslinking agent EGS. After exposure to the crosslinker, cells were lysed and extracts analyzed by Western blot for both Fpn-GFP and Fpn-FLAG. Using 4.0% SDS-PAGE, we could resolve all 3 expected Fpn multimers. Both Fpn-FLAG and Fpn-GFP showed a higher molecular mass migration pattern upon crosslinking (Figure 3B). Two bands were detected using anti-GFP antibodies, one at the predicted size of the Fpn-GFP/Fpn-GFP dimer (200 kDa) and one at the predicted size of the Fpn-GFP/Fpn-FLAG dimer (170 kDa). Similarly, antibodies directed against the FLAG epitope showed 2 bands, one at the predicted size of the Fpn-FLAG/Fpn-GFP dimer (170 kDa) and one at the predicted size of the Fpn-FLAG/Fpn-FLAG dimer (140 kDa). When the crosslinker was cleaved using hydroxylamine-HCl, the anti-GFP antibody detected one band at the predicted mass of Fpn-GFP, whereas the anti-FLAG antibody detected one band at the predicted mass of Fpn-FLAG on 8.0% SDS-PAGE.
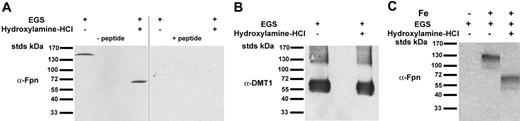
All of the Fpn constructs were expressed using a CMV promoter, and it is possible that multimerization of Fpn is observed only because of overexpression and that at endogenous levels Fpn is not multimeric. To examine whether endogenous Fpn was multimeric, we took advantage of the rat glioma cell line C6, which expresses Fpn at levels that can be detected with a commercially available antibody.13 C6 cells were exposed to the crosslinking agent EGS at 4°C, lysed, and the cell extract analyzed by SDS-PAGE and Western blotting. Endogenous Fpn migrates at a predicted molecular mass of 67 kDa. The migration pattern of Fpn extracted from cells that had been exposed to the crosslinking reagent was approximately 140 kDa (Figure 4A). That the antibody detected endogenous Fpn was shown by the loss of both bands when antibody was pretreated with the recombinant Fpn-specific peptide (Figure 4A) as well as the loss of the specific protein band upon incubation of cells with hepcidin, the predicted consequence being the internalization and degradation of Fpn (data not shown). Treatment of samples with hydroxylamine resulted in Fpn migrating at the predicted molecular mass of 67 kDa. To insure that the crosslinking of Fpn was specific for Fpn, we examined whether another membrane protein, DMT1, was crosslinked upon treatment with EGS. The migration of DMT1 was unaltered after crosslinking, suggesting that DMT1 is not a multimer (Figure 4B). We also examined the multimeric state of Fpn in mouse bone marrow macrophages, as macrophages are a major exporter of iron. Previous reports have suggested that Fpn exists at low levels in intracellular compartments in macrophages and is present on the plasma membrane only upon exposure to iron.14 We therefore incubated bone marrow macrophages with iron for 18 hours to express Fpn on the plasma membrane. Both iron-exposed and control cells were incubated with the crosslinking agent EGS, and cell lysates were analyzed by SDS-PAGE and Western blotting using antibodies directed against mouse Fpn. The levels of mouse Fpn in non–iron loaded cells were below the level of detection (Figure 4C). The migration pattern of Fpn extracted from cells that had been exposed to the crosslinking reagent was approximately 140 kDa, suggesting that Fpn is a dimer. Treatment of samples with hydroxylamine resulted in mouse Fpn migrating at a predicted monomer mass of 67 kDa, similar to that seen in the rat glioma cell line.
Crosslinking of endogenous Fpn. C6 rat glioma cells were treated with EGS as in Figure 3B. The extracts were examined for the presence of endogenous (A) Fpn or (B) DMT1 using antibody to either Fpn or DMT1 (Alpha Diagnostics), with the specificity of the Fpn antibody determined using blocking peptide. (C) Bone marrow macrophages were isolated from C57/B6 mice and cultured in L-cell–conditioned medium plus DMEM with 10% FBS. Following adherence (6-7 days), cells were split and plated onto 60-mm plates in the presence or absence of 10 μM iron for 18 to 24 hours. Cells were removed from plates, placed at 0°C, and incubated with 1.5 mM crosslinking reagent EGS for 60 minutes. The crosslinking reagent was quenched by addition of cell growth medium, cells were solubilized in lysis buffer, and the lysates were incubated in the presence or absence of the cleaving reagent, 1.0 M hydroxylamine-HCl, for 60 minutes at room temperature. The extracts were examined for Fpn by 10% SDS-PAGE and Western blot analysis using rabbit anti–mouse Fpn (1:1000) followed by peroxidase-conjugated goat anti–rabbit IgG antibodies. Fpn is a dimer, whereas DMT1 is a monomer.
Crosslinking of endogenous Fpn. C6 rat glioma cells were treated with EGS as in Figure 3B. The extracts were examined for the presence of endogenous (A) Fpn or (B) DMT1 using antibody to either Fpn or DMT1 (Alpha Diagnostics), with the specificity of the Fpn antibody determined using blocking peptide. (C) Bone marrow macrophages were isolated from C57/B6 mice and cultured in L-cell–conditioned medium plus DMEM with 10% FBS. Following adherence (6-7 days), cells were split and plated onto 60-mm plates in the presence or absence of 10 μM iron for 18 to 24 hours. Cells were removed from plates, placed at 0°C, and incubated with 1.5 mM crosslinking reagent EGS for 60 minutes. The crosslinking reagent was quenched by addition of cell growth medium, cells were solubilized in lysis buffer, and the lysates were incubated in the presence or absence of the cleaving reagent, 1.0 M hydroxylamine-HCl, for 60 minutes at room temperature. The extracts were examined for Fpn by 10% SDS-PAGE and Western blot analysis using rabbit anti–mouse Fpn (1:1000) followed by peroxidase-conjugated goat anti–rabbit IgG antibodies. Fpn is a dimer, whereas DMT1 is a monomer.
It is possible that endogenous Fpn multimerizes with another protein, as opposed to being a homomultimer. One candidate is GPI-linked ceruloplasmin, as previous studies have suggested that Fpn-mediated iron export is dependent on the presence of ceruloplasmin.13 Although ceruloplasmin could be detected in C6 cells, no ceruloplasmin was detected in crosslinked Fpn-immunoprecipitated samples under these experimental conditions (data not shown). These studies confirm that Fpn is a multimer and support the idea that Fpn is a dimer.
Discussion
Iron overload disease due to mutations in Fpn is distinguished from other forms of genetic iron overload disease, as this disorder is dominant whereas the others (HFE, TfR-2, hemojuvelin, and HAMP) are recessive.15 Further, Fpn iron overload disease shows 2 different phenotypes. Ferroportin disease can manifest as either typically hereditary hemochromatosis with iron deposition in hepatocytes and high transferrin saturation or it can manifest as macrophage/Kupffer cell iron loading and low transferrin saturation.15 We and others have determined the molecular basis for the different phenotypes observed in ferroportin disease.4–6,12,16 Some Fpn mutations result in a constitutively active Fpn molecule (N144H) present on the plasma membrane that does not respond to hepcidin binding. The consequence of an inability to hepcidin-induce Fpn degradation is that transferrin saturation increases and hepatocytes become iron loaded. Another class of Fpn mutations results in an Fpn molecule that is localized intracellularly, probably in the endoplasmic reticulum (Δ162V). The consequence of this type of mutation is decreased export of iron from macrophages, resulting in low transferrin saturation. While it might be expected that intestinal cells should show an impairment in iron transport as well, the ability to increase expression of Fpn in response to iron need may overcome the decreased export. Macrophages, given their iron load due to erythrophagocytosis, may be exporting iron at maximum capacity.
The molecular characterization of Fpn mutants can provide an explanation for most of the patient phenotypes; however, the critical question is what is the explanation for the dominant inheritance of the disorder? Two possibilities exist: haploinsufficiency and a dominant-negative effect. Data that argue against haploinsufficiency are that no nonsense mutations have been identified.15 It is quite striking that, with one exception, only missense mutations have been reported. The one exception is a mutation in the noncoding region of Fpn near the putative iron-responsive element (IRE).17 Based on studies of a mutation within the 5′ IRE of mouse Fpn, this mutation would be expected to act as a gain-of-function, resulting in increased Fpn expression.18 The second line of evidence that argues against haploinsufficiency is that mice heterozygous for a targeted gene deletion of Fpn do not show any evidence of pathologic iron accumulation.9 An increase in liver iron staining was observed in aged heterozygous mice but none of the usual indicators of iron overload disease were observed. The mice, although not anemic, did show decreased cellular hemoglobin and decreased cell volume, indicative of iron-restricted erythropoiesis. It could be argued that mice do not show Fpn iron overload; however, the other human genetic iron overload disorders are faithfully recapitulated in mice.19
The alternative hypothesis to explain the dominant nature of Fpn iron overload is that most mutations are gain-of-function and act as dominant-negative mutations. Our initial data supported this view by demonstrating that Fpn was multimeric and the behavior of the mutant protein affected the behavior of the multimeric complex.4 Depending on the behavior of the mutant protein, there was either reduced expression on the cell surface or defective internalization in response to hepcidin. A study in which wild-type and mutant Fpn were expressed in oocytes also concluded that Fpn was a multimer.20 Studies, however, suggested that Fpn was not multimeric and that multimerization was an artifact of either the epitope or overexpression of Fpn.5,10 Here we show that 3 different epitope-tagged Fpn, all of which are functional proteins, have the carboxyl terminal on the cytosolic surface and that a specific epitope did not affect the coimmunoprecipitation. Much of the debate about multimers relies on the specificity of coimmunoprecipitation and Fpn overexpression. To obviate these issues we used crosslinking agents. We evaluated a number of crosslinking agents and, similar to Pignatti et al,11 we found that treatment of cells with dithiobis (succinimidyl)propionate (DSP) did not affect the molecular mass of Fpn. However, treatment of cells with EGS, which has a longer spacer arm, led to changes in the molecular mass of Fpn. Exposure of both C6 glioma cells and mouse bone marrow macrophages to EGS resulted in a doubling of the molecular mass of endogenous Fpn, whereas the size of endogenous DMT1 was unaffected. EGS crosslinking of 2 different epitope-tagged Fpn expressed in the same cell led to identification of all 3 multimeric forms of Fpn. These data suggest that multimerization of Fpn occurs prior to extraction, showing that multimerization is not a consequence of detergent extraction or overexpression of epitope-tagged molecules. The degree of multimerization is quantitatively significant. In cells coexpressing Fpn-FLAG and Fpn–c-myc, most of one epitope-tagged Fpn-FLAG is found in a complex with the other epitope-tagged Fpn–c-myc. If multimers were the result of overexpression, then we might expect an equilibrium between multimers and monomers. We did not observe any significant degree of monomeric Fpn. One possible explanation for published results, which did not detect Fpn multimerization, may be that the level of cotransfection of epitope-tagged Fpn constructs was low, thereby precluding the ability to detect multimers by coimmunoprecipitation. Most importantly, we demonstrate that endogenous Fpn is multimeric and our data suggest that Fpn is a dimer. In total, our results suggest that Fpn is a dimer and indicate that multimerization of Fpn can explain the dominant inheritance of ferroportin disease.
Authorship
Contribution: I.D.D. and D.M.W. performed experiments, analyzed data, and wrote the manuscript; G.M. analyzed data; and J.K. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jerry Kaplan, Department of Pathology, School of Medicine, University of Utah, Salt Lake City, UT 84132; e-mail: jerry.kaplan@path.utah.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank David Haile and Nancy Andrews for their generous gift of antibodies. We would like to thank Alain Townsend, Hal Drakesmith, and Lisa Schimanski for sharing their reagents with us and for their helpful discussions.
This work was supported by National Institutes of Health grant DK070 947 (J.K.).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal