Abstract
To prospectively assess the applicability of reduced-intensity conditioning hematopoietic stem cell transplantation (RIC-HSCT), we wrote a protocol in which all untreated patients 50 years or older with acute myeloid leukemia (AML) and unfavorable cytogenetics would be evaluated during induction for a possible RIC-HSCT in first complete remission (CR1). Ninety-nine of 259 patients entered CR. Fifty-three of the 99 were seen by the Transplant Service with the remainder not seen because of illness, lack/unavailability of siblings, refusal, or, primarily, unclear reasons (21 patients). A donor was identified for 26 patients (21 sibling, 5 unrelated) with RIC-HSCT performed in 14 (13 sibling). Results in consulted patients suggested that 50% or fewer of the 85 patients who did not undergo transplantation were potential transplant candidates. We attempted to find one or more chemotherapy pair-mates for each patient who underwent transplantation based on cytogenetics, age, and a relapse-free survival (RFS) time that was more than or equal to the time from CR1 to RIC-HSCT in the patient who underwent transplantation. Thirty-two of the 39 matches favored (longer RFS) RIC-HSCT and 7, chemotherapy. The probability that the corresponding beta distribution was different than expected (ie, that RIC-HSCT was superior) was 0.99 (P = .004). Results were similar with respect to survival. While RIC-HSCT thus seems of interest, methods are needed to extend its applicability.
Introduction
Leukemia cell cytogenetics are the principal predictor of relapse-free survival time (RFS) in acute myeloid leukemia (AML).1-3 Remissions in patients with abnormal karyotypes, except inv(16), t(15;17), or t(8;21), are almost invariably brief and, as a result, median survivals average less than 1 year from complete remission date. Allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR1) may extend RFS in such patients.4-8 However, the risk of transplant-related morbidity and mortality increases with age, such that conventional myeloablative conditioning is associated with considerable risk in patients older than 50 to 60 years. Since the median age of patients with AML and abnormal cytogenetics achieving first CR is 60 years, myeloablative HSCT is applicable to only a minority of these patients.
Reduced-intensity conditioning (RIC) regimens allow older and debilitated patients to undergo HSCT.9-15 Several studies have shown outcomes and complication rates comparable with myeloablative HSCT in younger patients.16-18 However, as with myeloablative transplantations, questions have arisen as to the relevance of RIC-HSCT to AML in CR1. In particular, to the extent that various selection biases lead to selection of only the “best “ older patients for RIC-HSCT, the results of the procedure may be irreproducible in the vast majority of older patients in CR1.
To address this issue prospectively, we designed a study in which all patients older than 50 years presenting to the M. D. Anderson Leukemia Service for treatment of newly-diagnosed AML or high-risk myelodysplastic syndrome (MDS; > 10% marrow blasts) characterized by a cytogenetic abnormality, or insufficient metaphases for analysis, would be seen by Transplant Service physicians within 1 to 2 weeks of start of treatment. If the latter thought RIC-HSCT might plausibly be done in CR1, they initiated a search for potential donors and proceeded to perform RIC-HSCT. Our primary goal here is to report how often patients underwent transplantation and, within the limits of retrospective analysis, the reasons they did not. We also compare RFS and survival in various subsets of our patients.
Patients, materials, and methods
Two-hundred fifty-nine patients with the characteristics noted in the preceding paragraph and excluding those with inv(16), t(8;21), or t(15;17) received induction therapy in the Department of Leukemia from 2001 through 2003. Ninety-six percent received ara-C, most commonly combined with idarubicin, and 4% received other cytotoxic agents (gemtuzumab ozogamycin, troxacitabine, cloretazine). Seventy percent were treated and managed in HEPA-filtered rooms, with the inpatient setting facilitating consultation with the Transplant Service. The median age was 65 years, and 26% had a Zubrod performance status of 2 to 4. The most common cytogenetic abnormalities (48% of patients) involved chromosomes 5 and/or 7 (hereafter called −5/−7 and including −5, −7, 7q−, or 5q− alone or, more often, in a complex pattern); 4% of patients had insufficient metaphases for analysis. Fifty-eight percent had an antecedent hematologic disorder (AHD), defined as a documented abnormality in blood count for 1 or more months before M. D. Anderson presentation. Because most of our patients with an AHD have not had marrow examined before coming here, we typically do not know the cause of the AHD. Nonetheless, based on patients with an AHD who have had marrows examined before arrival here, we suspect that most AHDs reflect prior MDS; the incidence of AHD in the patients in the present study is not dissimilar to the incidence in comparable patients seen here in the 1990s (49%). Reflecting their age, cytogenetics, and AHD status, only 99 (38%) of the 259 patients entered CR, all after receiving one course of treatment. In principle, these 99 were candidates for RIC-HSCT provided they had an HLA-matched or one-antigen–mismatched sibling donor, or an HLA-matched unrelated donor.
Treatments
Preparative regimens for RIC-HSCT consisted of either fludarabine 30 mg/m2 daily for 4 days and melphalan 100 mg/m2 for 1 day, or fludarabine 25 mg/m2 daily for 5 days and melphalan 70 mg/m2 daily for 2 days. Tacrolimus (0.015 mg/kg) and methotrexate 5 mg/m2 intravenously on days 1, 3, 6, and 11 after transplantation were used for graft-versus-host disease (GVHD) prophylaxis. Tacrolimus was given by continuous infusion from day −2 to maintain a level between 5 to 15 ng/mL and given orally after engraftment. All patients received standard supportive care and antibiotic prophylaxis. Patients were monitored for cytomegalovirus antigenemia and treated preemptively with ganciclovir or foscarnet if reactivation was observed. Median CD34 cell dose was 3.97 × 106 (range, 1.17-5.93 × 106).
Patients who entered CR1 but did not undergo transplantation continued to receive their induction chemotherapy at reduced doses for a median of 2 cycles (range, 0-8 cycles). Such postremission therapy was typically given on an outpatient basis.
Approval was obtained from The University of Texas M. D. Anderson Cancer Center's institutional review board for these studies. Informed consent was provided in accordance with the Declaration of Helsinki.
Analysis
Differences in RFS and survival were quantified by the log-rank test. Because a beneficial (harmful) effect ascribed to RIC-HSCT relative to chemotherapy could merely reflect an effect of differences in prognostic covariates, we matched patients who underwent transplantations (TPs) with prognostically comparable controls who received chemotherapy rather than underwent RIC-HSCT. Criteria for matching were as follows: cytogenetics (−5/−7 vs normal vs other), age (within 2 years), presence of an AHD, and, to account for the “guarantee time” accorded TPs resulting from the time spent in CR1 before the transplantation was performed, an RFS (or survival time) in the chemotherapy patient at least as long as the time from CR1 to transplantation date in the TP. Because matches where RFS (or survival) in the chemotherapy patient was the same as the interval from CR1 to transplantation in the transplant pair-mate might inherently favor the latter (since the former might be alive but moribund), we repeated the analysis using chemotherapy patients only if their RFS (or survival) time was 4 or more weeks longer than the time from CR1 to transplantation in the TPs. Because selection of only one pair-mate per TP might result, by chance, in selection of a particularly “favorable” (“unfavorable”) pair-mate, we identified, subject to the constraints noted in the second sentence of this paragraph, as many pair-mates as possible for each TP. For each match between a chemotherapy patient and a TP, we determined whether RFS (or survival) was longer in the TP than in the chemotherapy pair-mate. If so, the TP was considered the “winner,” and if not the TP was considered the “loser”; cases where both the TP and a pair-mate were each alive in first CR (or alive in the case of survival) were considered “ties.” Summing the results gave a certain number of wins and a certain number of losses for the TPs. If RIC-HSCT and continued chemotherapy were equivalent, the number of wins would be expected to equal the number of losses. To address whether the observed number of wins and losses was different than the expected number, we considered each of the expected and the observed wins and losses to follow a beta distribution, denoted as β (a, b), with a the number of wins and b the number of losses. Beta distributions are convenient for representing prior opinion.19 Calling the expected beta distribution Y and the observed beta distribution X, we computed the probability that X would be more than Y (ie, that the distributions were different) using the program Inequality Calculator available at M. D. Anderson's Department of Biostatistics and Applied Mathematics's website (http://biostatistics.mdanderson.org/SoftwareDownload/).
Results
Transplant consults
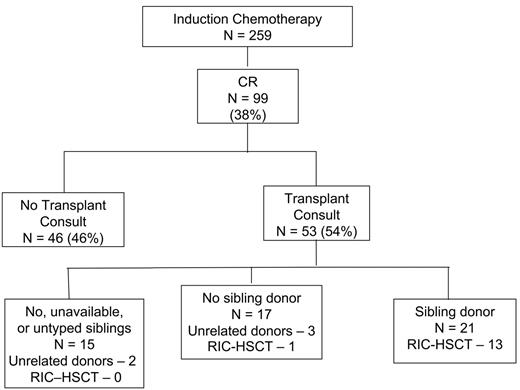
Fifty-three of the 99 patients who entered CR received a Transplant Service consult within 1 month of CR date; these patients almost invariably were seen within the first 2 weeks of initiation of induction chemotherapy. Another 8 patients were seen more than 1 month after CR date because of relapse, but clearly fall outside the bounds of what was intended. Fourteen of the 46 patients who did not have a transplant consult were considered “too ill” for RIC-HSCT; 10 of these 14 had a deterioration in their condition between the start of treatment and the date of CR. Five of the 46 not seen by the Transplant Service had no living biologic siblings, 3 had only ill siblings, 2 refused to consider the possibility of RIC-HSCT in CR1, and 1 had lung cancer. The reasons for failure to be seen by the Transplant Service were unclear in the remaining 21 of the 46 patients who did not receive a consult that would have permitted them to undergo transplantation in CR1.
Columns 2 and 3 of Table 1 compare the 53 patients who received a Transplant Service consult and the 46 who did not. The latter were older (P = .002, Mann-Whitney test), reflecting the inclusion of ill patients and patients without available siblings; the age of 21 of the 46 who did not receive a consult for unclear reasons was similar to the 53 who received a transplant consult (P = .16). The 46 were also more likely than the 53 to have a pretreatment performance status higher than Zubrod 1 (P = .04, Fisher exact test), although the same is not true for the 21 described in the preceding paragraph only 3 of whose pretreatment performance status was higher than Zubrod 1.
Patient characteristics
| . | No transplant consult . | Transplant consult . | Transplant consult; underwent RIC-HSCT . | Transplant consult; donor available/no RIC-HSCT . | Transplant consult; other* . |
|---|---|---|---|---|---|
| No. | 46 | 53 | 14 | 12 | 27 |
| Median age, y | 68 | 62 | 57 | 59 | 65 |
| Pretreatment performance status above Zubrod 1, no. (%) | 13 (28) | 6 (11) | 2 (14) | 2 (17) | 2 (7) |
| −5/−7 cytogenetics, no. (%) | 24 (52) | 20 (38) | 2 (14) | 4 (33) | 14 (52) |
| Antecedent hematologic disorder, no. (%) | 20 (44) | 27 (51) | 6 (43) | 4 (33) | 17 (63) |
| MDS, no. (%) | 11 (24) | 10 (19) | 2 (14) | 2 (18) | 6 (22) |
| . | No transplant consult . | Transplant consult . | Transplant consult; underwent RIC-HSCT . | Transplant consult; donor available/no RIC-HSCT . | Transplant consult; other* . |
|---|---|---|---|---|---|
| No. | 46 | 53 | 14 | 12 | 27 |
| Median age, y | 68 | 62 | 57 | 59 | 65 |
| Pretreatment performance status above Zubrod 1, no. (%) | 13 (28) | 6 (11) | 2 (14) | 2 (17) | 2 (7) |
| −5/−7 cytogenetics, no. (%) | 24 (52) | 20 (38) | 2 (14) | 4 (33) | 14 (52) |
| Antecedent hematologic disorder, no. (%) | 20 (44) | 27 (51) | 6 (43) | 4 (33) | 17 (63) |
| MDS, no. (%) | 11 (24) | 10 (19) | 2 (14) | 2 (18) | 6 (22) |
Includes patients with no/unavailable/untyped siblings (n = 13) and patients with no sibling donors (n = 14).
Donor availability and transplantation rate
Three of the 53 patients seen by the Transplant Service were uninterested in RIC-HSCT after discussing the procedure with the transplant physician. Forty-four of the remaining 50 had living biologic siblings, although 6 of these 44 were not typed because of age, infirmity, or unavailability (4 cases), or for unclear reasons (2 cases). Thus, siblings were unavailable or untyped in 15 of the 53 patients seen by the Transplant Service (Figure 1). Twenty-one of the 38 patients whose siblings were typed had an HLA-identical or one-antigen–mismatched donor. An HLA-matched unrelated donor was found for 5 patients who did not have a known HLA-compatible sibling donor (Figure 1). The donors for these 5 were identified at 1, 1, 3, 5, and 7 months from CR date; in each case, the patient was still in CR when the donor was found. Thus, a “donor” was identified for 49% (26/53) of the patients seen by the Transplant Service and a sibling donor for 40% (21/53). Columns 4 to 6 of Table 1, which divides the 53 patients seen by the Transplant Service into 3 groups, indicate that, not surprisingly, patients for whom donors were identified (81% of whom were siblings) were younger than patients for whom no donors were identified.
Patient flow. Although HLA-matched unrelated donors were found for 2 of the 15 patients with no, unavailable, or untyped siblings, no RIC-HSCTs were performed in this group. HLA-matched unrelated donors were found for 3 patients whose siblings were not HLA matched; one RIC-HSCT was performed.
Patient flow. Although HLA-matched unrelated donors were found for 2 of the 15 patients with no, unavailable, or untyped siblings, no RIC-HSCTs were performed in this group. HLA-matched unrelated donors were found for 3 patients whose siblings were not HLA matched; one RIC-HSCT was performed.
Fourteen of the 26 patients with a donor underwent RIC-HSCT in CR1. The donor was a sibling in 13 of the 14 patients who underwent transplantation and was unrelated in one patient. The 13 patients who underwent RIC-HSCT with a transplant from a sibling donor (Figure 1) represent 62% (13/21) of all patients with a known sibling donor. However, only one of 5 with an HLA-matched unrelated donor underwent RIC-HSCT. The 14 patients who underwent transplantation in CR1 represent only 26% of patients seen by the Transplant Service, 14% of all patients in CR1, and 5% of the 259 patients 50 years and older with abnormal cytogenetics or insufficient metaphases who were given induction chemotherapy at MDACC from January 1, 2000, to December 31, 2003. Another 4 patients underwent RIC-HSCT in CR2. Time from CR1 to transplantation ranged from 1 to 32 weeks, with a median of 11 weeks. Median time from diagnosis to transplantation was 15.5 weeks.
Reasons for failure to undergo transplantation in CR1
Table 2, a combination of data from patients who were, and were not, seen by the Transplant Service, indicates that 20 of the 85 patients who did not undergo RIC-HSCT were too ill for, or refused to consider, the procedure. Another 18 patients lacked healthy, available sibling donors who might have been typed, and 12 had siblings typed without identification of a donor and without subsequent identification of an unrelated donor. Twenty-two of these 30 (18 + 12) patients received a transplant consult. One of the 22 was uninterested in a search for a matched unrelated donor, one could not obtain insurance clearance for a search, 7 had an unsuccessful search (5 of these 7 relapsing within 6 months), and 13 had no search undertaken. The age distribution of the 7 (median, 68 years) and the 13 (median, 65 years) did not differ. Including the 5 patients in whom an unrelated donor was identified (preceding paragraph), 12 searches were performed among the 35 patients who lacked healthy available potential sibling donors.
Reasons for failure to undergo RIC-HSCT (85 patients)
| . | No. patients . |
|---|---|
| “Too ill” | 15* |
| Refused | 5 |
| No siblings | 11 |
| Siblings “too ill” or unavailable for HLA typing | 7 |
| Siblings typed without identification of donor† | 12 |
| Unclear‡ | 35 |
| . | No. patients . |
|---|---|
| “Too ill” | 15* |
| Refused | 5 |
| No siblings | 11 |
| Siblings “too ill” or unavailable for HLA typing | 7 |
| Siblings typed without identification of donor† | 12 |
| Unclear‡ | 35 |
Including one patient with lung cancer.
Seventeen patients fell into this category, but 4 of the 17 had an unrelated donor identified (but did not undergo RIC-HSCT), and 1 of the 17 underwent an unrelated RIC-HSCT. The 4 are included in the “unclear group”.
See last paragraph of “Reasons for failure to undergo transplantation in CR1”.
The principal reason RIC-HSCT was not performed was “unclear” (Table 2). The 35 patients in this category include 21 who did not receive a transplant consult, 2 in whom the transplant consult did not type living biologic siblings, 8 with known living HLA-matched sibling donors, and 4 with HLA-matched unrelated donors.
RFS with RIC-HSCT and chemotherapy
Median time to engraftment (ANC > 500/μL) was 14 days (range, 11-21 days). All patients engrafted; 12 patients achieved full, and 2 mixed, donor chimerism Acute GVHD developed in 5 patients (all grades 1-2) and chronic GVHD, in 8.
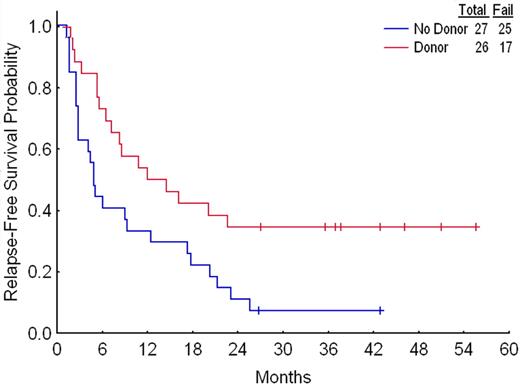
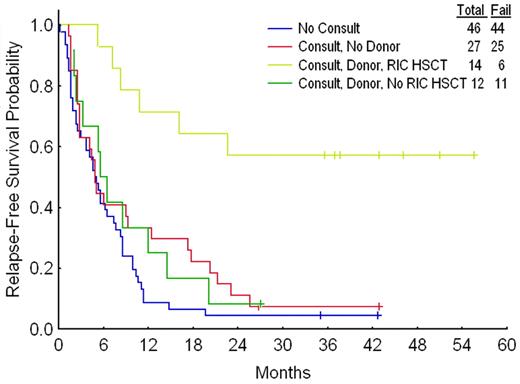
A donor–versus–no donor comparison showed that the former had superior RFS (Figure 2); the same was true regardless of whether the “no donor” group included only patients known not to have an HLA-compatible sibling or, as in Figure 2, the latter plus patients with no, unavailable, or untyped siblings. The donor–no donor difference was essentially entirely due to the outcome in patients with a donor who underwent RIC-HSCT (Figure 3). Twenty-four of the 85 patients who did not undergo transplantation were used for matching, enabling matches to be found for 12 of the 14 patients who underwent TPs. More than one match was found for 11 of these 12. For example, TP1 was 62 years old, had −5/−7 and an AHD, and underwent transplantation 19 weeks from CR date. Three of the 85 patients who did not undergo transplantation were eligible to be matched to TP1 based on the criteria described in “Patients and methods”; thus each was 61 to 64 years old, had −5/−7, had an AHD, and had an RFS time of 19 or more weeks. Proceeding in this fashion and allowing the same control patient to be used more than once produced a total of 43 separate matches (second column in Table 3) Each of the 3 matches for TP1 had a shorter RFS time than TP1 (third column in Table 3); thus TP1 was considered to have “won” each of these matches. TP2 had longer RFS than 3 of its 4 matches and shorter RFS than the fourth match (fourth column in Table 3); thus TP2 was considered to have won 3 and lost one match. TP3 is still alive in first CR at 241 weeks from CR date. While 3 of TP3's 4 matches have relapsed at fewer than 241 weeks, the fourth remains alive in first CR at 117 weeks; since it is thus plausible that RFS time will eventually be longer in this patient than in TP3, we considered the result of this match to be a tie (Table 3 column 5). Summing the results for each of TP1 to TP12 gave 32 matches in which RFS was longer with RIC-HSCT, 7 in which RFS was longer with chemotherapy, and 4 ties. If chemotherapy and RIC-HSCT were identical, one might expect RIC-HSCT to have 21.5 wins and 21.5 losses in the 43 matches, rather than the observed 32 wins and 7 losses. The program Inequality Calculator (“Patients and methods”) indicated that the probability that the observed and expected beta distributions ([32, 7] and [21.5, 21.5], respectively) were different, corresponding to the probability that RIC-HSCT was superior, was 0.999. For comparison, a standard Fisher exact test comparing 32 of 39 and 21 of 42 gives a P value of .004, also strongly suggesting that the null hypothesis of no difference is incorrect. Including only matches where RFS in the chemotherapy patient was at least 4 weeks greater than the time from CR1 to transplantation date in the transplant pair-mate gave 27 matches favoring RIC-HSCT, 7 matches favoring chemotherapy, and 3 inconclusive matches. Assuming a priori that among the 37 matches, 18.5 should have favored RIC-HSCT and 18.5 chemotherapy if the 2 treatments were identical, the probability that the observed and expected beta distributions were different was 0.996; the corresponding P value was .01. Furthermore, the 2 patients who underwent RIC-HSCT for whom matches could not be found each remain alive in CR1 at slightly less than 3 years from transplantation date.
Relapse-free survival according to donor status. Results were similar if the 13 patients with no, unavailable, or untyped siblings are removed from the no-donor group.
Relapse-free survival according to donor status. Results were similar if the 13 patients with no, unavailable, or untyped siblings are removed from the no-donor group.
Relapse-free survival according to consult, donor, and transplant status. Results were similar if the 13 patients with no, unavailable, or untyped siblings and the 14 with no sibling donors are considered separately.
Relapse-free survival according to consult, donor, and transplant status. Results were similar if the 13 patients with no, unavailable, or untyped siblings and the 14 with no sibling donors are considered separately.
RFS in patients who underwent RIC-HSCT and in matched controls
| Transplant patient . | No. matches . | No. matches in which RFS was longer in transplant patient . | No. matches in which RFS was longer in chemotherapy patient . | No. matches in which neither transplantation nor chemotherapy was superior . |
|---|---|---|---|---|
| 1 | 3 | 3 | 0 | 0 |
| 2 | 4 | 3 | 1 | 0 |
| 3 | 4 | 3 | 0 | 1 |
| 4 | 6 | 6 | 0 | 0 |
| 5 | 1 | 0 | 1 | 0 |
| 6 | 4 | 3 | 0 | 1 |
| 7 | 5 | 4 | 0 | 1 |
| 8 | 2 | 2 | 0 | 0 |
| 9 | 4 | 3 | 1 | 0 |
| 10 | 3 | 1 | 2 | 0 |
| 11 | 4 | 3 | 0 | 1 |
| 12 | 3 | 1 | 2 | 0 |
| All | 43 | 32 | 7 | 4 |
| Transplant patient . | No. matches . | No. matches in which RFS was longer in transplant patient . | No. matches in which RFS was longer in chemotherapy patient . | No. matches in which neither transplantation nor chemotherapy was superior . |
|---|---|---|---|---|
| 1 | 3 | 3 | 0 | 0 |
| 2 | 4 | 3 | 1 | 0 |
| 3 | 4 | 3 | 0 | 1 |
| 4 | 6 | 6 | 0 | 0 |
| 5 | 1 | 0 | 1 | 0 |
| 6 | 4 | 3 | 0 | 1 |
| 7 | 5 | 4 | 0 | 1 |
| 8 | 2 | 2 | 0 | 0 |
| 9 | 4 | 3 | 1 | 0 |
| 10 | 3 | 1 | 2 | 0 |
| 11 | 4 | 3 | 0 | 1 |
| 12 | 3 | 1 | 2 | 0 |
| All | 43 | 32 | 7 | 4 |
Survival with RIC-HSCT and chemotherapy
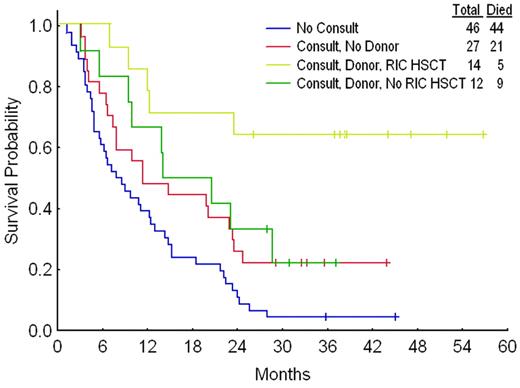
As with RFS (Figure 3), survival was longest in patients who received a transplant (Figure 4). However, in contrast to RFS, survival was shorter in the 46 patients who did not receive a transplant consult than in the 39 who did receive a consult but did not undergo transplantation. Using the matching criteria described above gave 49 matches, regardless of whether the chemotherapy patients had to live as long as, or 4 or more weeks longer than, the time from CR1 to transplantation date in their transplant pair-mates. Six more chemotherapy matches were found for the survival analysis than for the RFS analysis because the 6 patients had an RFS that was shorter than the interval from CR1 date to transplantation date for their prospective pair-mate, but a survival time that was longer than this interval. Twenty-nine of the 49 matches favored transplantation and 9 favored chemotherapy, with 11 ties. The probability that the observed and expected beta distributions were different corresponding to the probability that transplantation was superior was 0.994; the P value was .02. The 2 patients who underwent RIC-HSCT for whom matches could not be found each remain alive at slightly less than 3 years from transplantation date.
Survival according to consult, donor, and transplant status. Results were similar if the 13 patients with no, unavailable, or untyped siblings and the 14 with no sibling donors are considered separately.
Survival according to consult, donor, and transplant status. Results were similar if the 13 patients with no, unavailable, or untyped siblings and the 14 with no sibling donors are considered separately.
Discussion
To date, the great majority of studies of RIC-HSCT in AML have been single-arm phase 2 studies.9-15 These have clearly demonstrated that the procedure is feasible, and may produce long-term RFS, and survival, in selected patients. While much attention has focused on comparison of outcomes after reduced-intensity and conventional myeloablative conditioning, with most but not all studies demonstrating a benefit for RIC,16-18 less attention has been given to comparison of RIC-HSCT with chemotherapy,20 an issue of obvious importance to patients. Furthermore, the applicability of RIC-HSCT to older patents with AML has not been prospectively explored. This paper addresses these issues.
Our data suggest a high likelihood (probability > 99%) that, after accounting for age, cytogenetics, AHD status, and “lead-time bias,”21 RFS and survival are longer in older patients if they undergo RIC-HSCT rather than chemotherapy in CR1. This suggestion can be criticized on several grounds. For example, the results might have been different had different chemotherapies been used or more than a median of 3 cycles of chemotherapy (one induction, 2 after remission) been administered. However, Goldstone et al22 did not find that 6 total courses were more beneficial than 3 in patients 56 years and older, and physicians presumably had reasons that justified their decision to give some patients fewer than 3 courses. While we could have used as chemotherapy controls only patients who received at least 3 total courses, this would introduce various biases, expressly contradicting the concept of “intention-to-treat.” Our matching method can be criticized because it equates all “non −5/−7” cytogenetic abnormalities and disregards an AHD's length. More generally, we have stressed the significance of unknown covariates, which here might have acted to favor RIC-HSCT, and the attendant need for randomization.23 However physicians and patients are unlikely to participate in a trial randomizing patients with a donor between RIC-HSCT and chemotherapy in CR1.
The relevance of a “successful” treatment is clearly less if the population that benefited is dissimilar from most patients with the disease in question. The median age of the 14 patients undergoing RIC-HSCT was only 57 years (with only 4 patients aged 60-64 years and 2 > 65 years), and these 14 were indeed younger than the other 3 groups depicted in Table 1. Furthermore, only 2 of the 14 had the particularly unfavorable −5/−7 abnormality, and again the frequency of −5/−7 was lowest in the group that underwent transplantation (Table 1). Hence, the 14 appear less than fully representative of “older” patients with AML in CR1. However, the relevance of RIC-HSCT to older patients in CR1 is most called into question by the fact that only 14% of our patients in CR1 (and only 5% of all 259 patients) underwent RIC-HSCT. It is thus of obvious importance to ascertain why the procedure was done so infrequently. Although the criteria that define a “transplant candidate” may not be entirely objective, it appears that at least 29 (34%) of the 85 patients who did not undergo RIC-HSCT were in fact not transplant candidates because they were “too ill” (15 patients, Table 2), uninterested in the procedure (5 patients), without available matched sibling and unrelated donors (7 patients), or refused/were unable to have an unrelated donor search (2 patients). Of the remaining 56 patients who did not undergo transplantation who might thus be considered potential transplant candidates, 12 had an identified but unused donor, 23 had an unclear sibling status (the 35 in Table 2 minus the 12 with an unused donor), and 21 had no available sibling donor (the 30 [11 + 7 + 12] in Table 2 minus the 9 in whom an unrelated donor search was unsuccessful or could not be performed). Based on results in patients seen by the Transplant Service and presented in “Donor availability and transplantation rate,” 10 of the 23 might have been expected to have a sibling donor (assuming 76% of the 23 would have available siblings with 55% of the patients with siblings having an HLA match). If each of the 13 without a sibling donor together with the 21 noted 2 sentences above who were known to have no available sibling donors and did not have an unrelated donor search were to have undergone such a search, an additional 34 unrelated donor searches would have been performed. Assuming 3 months are needed to complete such a search, which would be successful in 75% of cases, and noting that 30% of the 99 patients who entered CR1 relapsed within 3 months suggest that an unrelated donor would have been found in time for a transplantation in CR1 for 18 (34 × .75 × .70) of the 34 patients noted in the preceding sentence. Thus, up to 40 donors (10 hypothetical sibling donors and 18 hypothetical unrelated donors together with the 12 known donors who were not used) might have been expected to have been found for the 56 patients who were not definitely known to be candidates who would not undergo transplantation. Hence, 40 (47%) of the 85 patients who did not undergo RIC-HSCT might be considered “potential transplant candidates.” This figure is almost certainly an overestimate, reflecting an idealized scenario, since it is reasonable to assume that, although explanations may be elusive, patients were not seen by the Transplant Service for valid or unpreventable reasons, the latter, for example, including the possibility that support personnel did not call in the consult. Similarly, transplant physicians almost certainly had valid reasons to not proceed with unrelated donor searches, and to not perform transplantation in some patients with known donors, although some of the failure to conduct unrelated donor searches likely reflected the novelty of unrelated donor transplantation during much of the time (2001-2003) during which our protocol was operative.
If RIC-HSCT is plausibly more effective than chemotherapy in older patients with abnormal cytogenetics, but if, as suggested in the preceding paragraph, at most 50% of patients entering CR1 are candidates, methods to extend the procedure's applicability are needed. Perhaps the most effective method would be to use RIC-HSCT as initial therapy. Such a strategy would prevent patients from being disqualified because they became too ill during chemotherapy induction (as occurred in 10 of the 15 not referred to the Transplant Service because of “illness”). Of more importance, it would have allowed RIC-HSCT to be performed in at least some of the 61% of our patients who never entered CR1, and thus were ineligible for our protocol. Indeed Schmid et al24 have used RIC-HSCT following conditioning with fludarabine, ara-C, and AMSA in patients with refractory, active AML and noted this regimen's “high activity rate.” A problem with undertaking a similar approach in untreated patients is the need for time to identify donors, obtain insurance clearance, and others. It is plausible that initial therapy can be safely delayed in many older patients. Thus, we have recently reported that, subject to various potential selection biases, CR and survival rates in patients 65 years or older with untreated AML and white blood cell (WBC) counts less than 50 000/μL were unaffected by time from M. D. Anderson presentation (itself, some time after AML is diagnosed by a primary physician) to initiation of therapy, with 53 patients delayed at least 1 month.25 In practice, a more realistic approach might be to use “low-intensity” therapies (eg, 5-azacitidine, decitabine, lenalidomide) during the time RIC-HSCT is being organized, with a view to offer the procedure in the event that these therapies are unsuccessful.
Authorship
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Correspondence: Elihu Estey, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: ehestey@mdanderson.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal