Abstract
In idiopathic thrombocytopenic purpura (ITP), corticosteroids have been widely recognized as the most appropriate first-line treatment, even if the best therapeutic approach is still a matter of debate. Recently, a single high-dose dexamethasone (HD-DXM) course was administered as first-line therapy in adult patients with ITP. In this paper we show the results of 2 prospective pilot studies (monocentric and multicentric, respectively) concerning the use of repeated pulses of HD-DXM in untreated ITP patients. In the monocenter study, 37 patients with severe ITP, age at least 20 years and no more than 65 years, were enrolled. HD-DXM was given in 4-day pulses every 28 days, for 6 cycles. Response rate was 89.2%; relapse-free survival (RFS) was 90% at 15 months; long-term responses, lasting for a median time of 26 months (range 6-77 months) were 25 of 37 (67.6%). In the multicenter study, 95 patients with severe ITP, age at least 2 years and no more than 70 years, were enrolled. HD-DXM was given in 4-day pulses every 14 days, for 4 cycles; 90 patients completed 4 cycles. Response rate (85.6%) was similar in patients classified by age (< 18 years, 36 of 42 = 85.7%; ≥ 18 years, 41 of 48 = 85.4%, P = not significant), with a statistically significant difference between the second and third cycle (75.8% vs 89%, P = .018). RFS at 15 months 81%; long-term responses, lasting for a median time of 8 months (range 4-24 months) were 67 of 90 (74.4%). In both studies, therapy was well tolerated. A schedule of 3 cycles of HD-DXM pulses will be compared with standard prednisone therapy (eg, 1 mg/kg per day) in the next randomized Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) trial.
Introduction
Idiopathic thrombocytopenic purpura (ITP), an autoimmune disease, is characterized by early platelet destruction induced by autoantibodies directed against specific glycoproteins of platelet surface.1,2 Even if up to now corticosteroids have been widely recognized as the most appropriate first-line treatment, the best therapeutic approach to this disorder is still a matter of debate. Indeed, prednisone or prednisolone is administered as front-line therapy to most adult patients with ITP who need to be treated. The starting dose is usually 1 mg/kg per day and this dose is usually continued for 2 to 4 weeks. If a raised platelet count is attained, the dose is gradually tapered over several weeks. With this approach usually an initial response rate of about 50% to 60% is obtained, whereas the long-term remission rate, after therapy discontinuation, ranges between 10% and 20%3-7 or more (25% long-term complete remissions, as reported by Portielje et al8 ).
Furthermore, either standard or high doses of prednisone/prednisolone or high-dose intravenous immunoglobulin (HD-IVIg) are also considered as first-line treatments in children affected by ITP who need therapy. With these approaches a long-term response rate of about 80% is reached.9-11 Nevertheless, a controversy persists whether to give drug therapy to children with ITP with mild or moderate bleeding symptoms, despite a very low platelet count, as a rapid spontaneous remission without any therapy is possible. The published practice guidelines for the treatment of ITP of the American Society of Hematology (ASH)6 and of the British Society of Hematology (BSH),12 both based on expert opinions, give different recommendations. The former guidelines favor treatment based on low platelet count, whereas the latter guidelines prefer a “wait and watch” approach. However, the most part of randomized clinical trials concerning the treatment of children with ITP has considered the rise of platelet count as the unique relevant outcome. In fact, at present, there is a lack of randomized trials focusing also on bleeding, quality of life, adverse effects, and costs.13,14
The administration of pulsed high-dose dexamethasone (HD-DXM) at a dose of 40 mg/d given orally according to a 4-day course repeated each 28 days for 6 consecutive times in adult ITP refractory to several therapy lines, dates back to the mid-1990s. Indeed, in a small cohort of patients a response rate of 100% has been observed,15 but in other studies, the same approach was not so successful.16-19 Andersen's purpose15 was based mainly on some considerations: (1) efficacy of pulsed corticosteroids in reducing immunoglobulin production in clonal B-cell disorders; (2) long half-life and good tolerability of DXM when given in high doses; and (3) very low cost of corticosteroids. Moreover, the efficacy associated with acceptable tolerability of pulsed HD-DXM was previously reported in the treatment of multiple myeloma.20 In a very recent study, HD-DXM was given in a single 4-day course (40 mg/d, orally) in previously untreated adult patients with ITP with very encouraging results: the initial response rate was 85%, the relapse rate 50%, and the sustained response 42%. Moreover, all relapsed patients had a response to a second therapy course, but only a minority of them (18.5%) showed a persistent response after discontinuation of maintenance therapy.21 Although it seems that HD-DXM has a better response rate than conventional prednisone doses, we do not know whether results could be improved by reducing relapse rate, and thus improving persistent responses, as there are no randomized studies.
In this paper we show the results of 2 different prospective pilot studies, a monocenter study and a multicenter study, concerning the use of pulsed HD-DXM in newly diagnosed, untreated patients with ITP.
The aim of the first-mentioned study was to evaluate the feasibility, compliance, and efficacy of the Andersen protocol, proposed for refractory ITP, in untreated, newly diagnosed adult patients. The study was conducted from February 1996 to June 2000. The results were very promising in what concerns efficacy. Nevertheless, we observed an early discontinuation of therapy in more than 48% of patients, either due to medical decision or to low compliance with a long-lasting treatment. On the basis of this preliminary experience, the Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) ITP Working Party planned a multicenter pilot study in which pulsed HD-DXM was given as first-line treatment according to a modified therapy schedule, by reducing the number of courses (from 6 to 4) and by shortening time intervals between them (14 days instead of 28). Adults and children with ITP were enrolled in the second study. The objective was to evaluate efficacy, safety, and patients' compliance.
Patients, materials, and methods
Inclusion criteria
Monocenter pilot study.
Eligible subjects for this study were previously untreated adult patients (age ≥ 20 to ≤ 65 years) with newly diagnosed ITP and a platelet count of no more than 20 × 109/L, or more than 20 × 109/L if bleeding symptoms were present, according to the below reported score (Table 1) Enrollment took place between February 1996 and June 2000 at the Haematology Department of the University La Sapienza of Rome, Hospital Policlinico Umberto I Italy.
Grade and type of bleeding symptom
| Grade . | Bleeding symptom . |
|---|---|
| 0 | Absent |
| 1 | Petechiae |
| 2 | Ecchymoses and/or dripping with moderate loss of blood |
| 3 | Major mucous hemorrhage with copious loss of blood without sequelae |
| 4 | Major mucous and/or parenchymal hemorrhage with copious loss of blood with sequelae and/or life-threatening or death |
| Grade . | Bleeding symptom . |
|---|---|
| 0 | Absent |
| 1 | Petechiae |
| 2 | Ecchymoses and/or dripping with moderate loss of blood |
| 3 | Major mucous hemorrhage with copious loss of blood without sequelae |
| 4 | Major mucous and/or parenchymal hemorrhage with copious loss of blood with sequelae and/or life-threatening or death |
GIMEMA multicenter pilot study.
The eligible population in this study was previously untreated patients (age ≥ 2 to ≤ 70 years) with newly diagnosed ITP and a platelet count of no more than 30 × 109/L, or more than 30 × 109/L if bleeding symptoms were present, according to the reported score (Table 1). Patients were enrolled from June 2001 in 16 “GIMEMA” centers. We chose the age of 18 years old as the cut-off point for segregation between adults and children, because in Italy this is the age limit for referring a patient to an adult center and not to a pediatric ward. Moreover, among the population of patients younger than 18 years, we also chose the age of 10 years old as the segregation point between pre/peripubertal and postpubertal age.
Both our pilot studies did not provide a control group.
In both studies, each enrolled patient or his or her legal guardian signed an informed consent form in accordance with the Declaration of Helsinki. This research was approved by the Council of Biotechnology and the Hematology department of the University of Rome La Sapienza.
Exclusion criteria
In both studies the exclusion criteria were as follows: pregnancy, hypertension, cardiovascular disease, diabetes, liver and kidney function impairment (eg, ALT, AST > 2 times upper normal limit; creatinine > 1.8 mg/dL, respectively), HCV, HIV, HBsAg seropositive status and a recent viral illness or intake of noncorticosteroid anti-inflammatory drugs both occurred within one month before diagnosis. Moreover, the presence of autoimmune hemolytic anemia and connective tissue diseases was also considered as an exclusion criterion. In both studies, patients in whom bone marrow aspirate was not performed were excluded.
Diagnosis of ITP
Diagnosis of ITP was based on commonly adopted criteria: patient's medical history, physical examination, complete blood cell count, and cytomorphologic examination of peripheral-blood smear,6,12 in which no alterations of erythrocytic and leukocytic series should be present. To confirm the diagnosis, bone marrow aspirate was performed on all patients and only those with the presence of a normal or increased number of megakaryocytes, without pathologic alterations of erythroblastic, granuloblastic, and lymphocytic series, were included in the study.
Determination of autoimmunity markers (antinucleus, antimitochondria, anticardiolipin antibodies) and direct antiglobulin test (DAT) was also performed on all enrolled patients.
Bleeding symptoms were classified according to a score system graded from 0 to 4, as reported in Table 1. Bleeding symptoms were considered present if the bleeding score was graded 1 or higher.
Therapy schedules
In the monocenter study the therapy schedule was as follows: DXM was administered intravenously as a 40-mg single daily dose for 4 consecutive days, every 28 days. A total of 6 cycles was planned. If platelet count remained no more than 20 × 109/L between courses or bleeding symptoms related to thrombocytopenia were present, therapy with prednisone at 0.25 mg/kg body weight per day, administered orally, was given. Complete blood cell count was performed on days 5 and 28 of each cycle. Response evaluation was assessed after completion of therapy on day 28 of the latest performed cycle.
In the GIMEMA multicenter pilot study, oral or intravenous DXM was given as a single daily dose of 40 mg for 4 consecutive days, every 14 days for 4 courses. In patients younger than 15 years old the daily dose was 20 mg/m2 (maximum 40 mg/d). Complete blood cell count was performed on the following days: 0-4, 14-18, 28-32, 42-46, and 60. If the platelet count was less than or equal to 30 × 109/L or bleeding symptoms related to thrombocytopenia were present, low-dose DXM (0.035 mg/kg body weight per day, administered orally) was given between courses. Response evaluation was made at day 60 after treatment start.
In both studies a therapy course was defined as the time interval between the first DXM pulse and the fourth one. A therapy cycle was defined as the time interval between the start of a given therapy course and the start of the following course, including the gap between them.
Response evaluation
In both studies the response was evaluated according to the following criteria: complete response (CR), defined as platelet count more than or equal to 150 × 109/L; partial response (PR), defined as platelet count more than or equal to 50 < 150 × 109/L; minimal response (MR), defined as platelet count more than 20 < 50 × 109/L in the monocenter study and more than 30 < 50 × 109/L in the multicenter study; no response (NR), defined as platelet count less than or equal to 20 × 109/L in the monocenter study or less than or equal to 30 × 109/L in the multicenter study, or the persistence of bleeding symptoms related to thrombocytopenia. CR, PR, or MR were stated in the absence of any other treatment for ITP. Persistent complete, partial, and minimal response (pCR, pPR, pMR) were defined as a response lasting at least 2 months after treatment discontinuation.
Adverse events were graded following the CTC-NCI version 2.0.22
Follow-up (FU) was defined as the time elapsing between diagnosis and the last available assessment.
Relapse was defined as a platelet count decrease less than or equal to 20 × 109/L (monocenter study) or less than or equal to 30 × 109/L (multicenter study), or the presence of bleeding symptoms due to thrombocytopenia after the achievement of initial response.
Relapse-free survival (RFS) was defined as the time interval between achievement of response and relapse.
Statistical analysis
Differences in the distributions of variables between groups of patients were analyzed by the χ2 or Fisher exact test.
Relapse-free survival was measured from the date of response achievement to the date of relapse, censoring patients alive without relapse.
The probability of RFS was calculated using the Kaplan-Meier method and the prognostic value of potential factors was assessed using the log-rank test with stratification for risk group.
The multivariate analysis of RFS was done using the Cox proportional hazard model.
All analyses were 2 tailed and were considered statistically significant when P values were less than or equal to 0.05.
All analyses were performed using SAS v.8.02.
Results
Monocenter study
Thirty-seven eligible, previously untreated patients with ITP were enrolled in this study. The main clinical characteristics of the patients are listed in Table 2
Monocenter pilot study: characteristics of enrolled patients
| Characteristic . | Total . |
|---|---|
| Patients, n | 37 |
| Male/female, n | 18/19 |
| Median age, y (range) | 34 (20-58) |
| Median platelet count, × 109/L (range) | 7 (1-22)* |
| Bleeding score | |
| 0 | 7 |
| 1 | 24 |
| 2 | 4 |
| 3 | 2 |
| 4 | 0 |
| Characteristic . | Total . |
|---|---|
| Patients, n | 37 |
| Male/female, n | 18/19 |
| Median age, y (range) | 34 (20-58) |
| Median platelet count, × 109/L (range) | 7 (1-22)* |
| Bleeding score | |
| 0 | 7 |
| 1 | 24 |
| 2 | 4 |
| 3 | 2 |
| 4 | 0 |
There were 2 patients with a platelet count of 22 × 109/L who had a bleeding score of 1.
All patients underwent a mean of 5 therapy cycles (range 3-6); 19 of 37 patients (51.4%) completed 6 cycles. Treatment was stopped before completion in 18 patients (48.6%) due to poor compliance (9 patients: 5 CR, 3 PR, 1 MR), medical decision (4 patients: 2 CR, 2 NR), and adverse events (5 patients: 4 CR, 1 NR).
No patient showed positivity of autoimmune markers or DAT.
Responses were reached in 33 of 37 patients (89.2%): CR in 23 (62.2%), PR in 8 (21.6%); MR in 2 (5.4%), and NR in 4 (10.8%). In the responder patients (CR+PR+MR) median platelet count was 204 × 109/L (range 25-373 × 109/L); in nonresponders the median was 12 × 109/L (range 5-20 × 109/L).
No statistically significant difference of overall response rate was shown by sex (males 16/18, 88.9%; females 17/19, 89.4%: P = not significant).
No statistically significant difference was found between the overall response rate (CR+PR+MR) reached by patients who completed all 6 therapy cycles (18/19, 94.7%) and the rate obtained by patients who completed 3, 4, or 5 cycles, considered altogether (15/18, 83.3%; P = not significant). Comparing the quality of response (CR vs PR+MR), no statistically significant difference was found between the 2 groups: CR, 12 versus 11; PR+MR, 6 versus 4 (P = not significant).
The median FU of 37 evaluable patients was 25 months (range 6-77 months).
Among the 33 responder patients, 8 (24.2%) relapsed after a median time of 19.5 months (range 4-49 months) after response achievement. At relapse, median platelet count was 15 × 109/L (range 8-19 × 109/L).
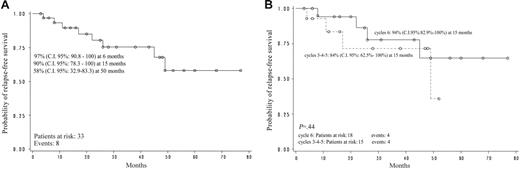
Relapse-free survival of all evaluable responder patients (n = 33) was estimated to be 90% (95% confidence interval [CI]: 78.3-100) at 15 months and 58% (95% CI: 32.9-83.3) at 50 months (Figure 1A). Relapse-free survival according to the cycle number (3, 4, 5 vs 6) at which the response was obtained did not show any statistically significant difference: after 6 cycles, RFS was 94% at 15 months (95% CI: 82.9-100), after 3 to 5 cycles RFS was 84% (95% CI: 62.5-100; P = not significant; Figure 1B).
Monocenter pilot study. (A) Relapse-free survival. (B) Relapse-free survival according to cycle.
Monocenter pilot study. (A) Relapse-free survival. (B) Relapse-free survival according to cycle.
Long-term responses, lasting for a median time of 26 months (range 6-77 months) without relapses and without any therapy, were observed in 25 (75.8%) of 33 responder patients; that is, 25 (67.6%) of 37 of all evaluable patients. In particular, pCR was found in 20 (80%) of 25 patients, pPR in 4 (16%) of 25 patients, and pMR in 1 (4%) of 25 patients. Median platelet count at last control was 220 × 109/L (range 40-281 × 109/L).
Five adverse events were observed in 5 of 37 patients (13.5%): hypertension (CTC-NCI grade 3), anxiety (CTC-NCI grade 2), gastric distress (CTC-NCI grade 2), cataract (CTC-NCI grade 2), and bronchial pneumonia (CTC-NCI grade 2). In these cases treatment with HD-DXM had to be stopped at different time points (after the end of cycles 4, 3, 5, 4, and 5, respectively).
During therapy cycles, no bleeding complications and no salvage or emergency therapies, like platelet transfusions or HD-IVIg, were required.
GIMEMA multicenter pilot study
Ninety-five eligible, previously untreated patients with ITP were enrolled in this study. The main clinical characteristics of these patients are listed in Table 3 Ninety patients (32 males, 58 females) who completed all 4 therapy cycles were evaluable for response at day 60: 4 patients were lost to FU (2 aged < 18 years) after the second therapy cycle and one adult patient stopped treatment early due to gastric distress (CTC-NCI grade 3) after the third course.
GIMEMA multicenter pilot study: characteristics of enrolled patients
| Characteristic . | Total . | At least 2 years of age through 17 years of age* . | At least 18 years of age through 70 years of age . |
|---|---|---|---|
| Patients, n | 95 | 44 | 51 |
| Male/female | 34/61 | 21/23 | 13/38 |
| Median age, y (range) | 20 (2-70) | 5.7 (2-17.9) | 35.1 (19.9-70) |
| Median platelet count, × 109/L (range) | 9 (1-35†) | 8 (1-26) | 10 (1-35†) |
| Bleeding score‡ | |||
| 0 | 4 | 0 | 4 |
| 1 | 42 | 29 | 13 |
| 2 | 21 | 13 | 8 |
| 3 | 1 | 0 | 1 |
| 4 | 0 | 0 | 0 |
| Characteristic . | Total . | At least 2 years of age through 17 years of age* . | At least 18 years of age through 70 years of age . |
|---|---|---|---|
| Patients, n | 95 | 44 | 51 |
| Male/female | 34/61 | 21/23 | 13/38 |
| Median age, y (range) | 20 (2-70) | 5.7 (2-17.9) | 35.1 (19.9-70) |
| Median platelet count, × 109/L (range) | 9 (1-35†) | 8 (1-26) | 10 (1-35†) |
| Bleeding score‡ | |||
| 0 | 4 | 0 | 4 |
| 1 | 42 | 29 | 13 |
| 2 | 21 | 13 | 8 |
| 3 | 1 | 0 | 1 |
| 4 | 0 | 0 | 0 |
This age group was further divided at the 10-years-old mark: 34 patients were ≥ 2 but < 10 years old (16 males, 18 females); 10 patients were ≥ 10 but < 18 years old (5 males, 5 females).
There were 2 adult patients (ages 30 years and 39 years) with platelet counts of 32 × 109/L and 35 × 109/L, and bleeding scores of 2 and 3, respectively.
Bleeding score was available for 68 of 95 patients.
No patient showed positivity of autoimmune markers and or DAT.
Responses were reached in 77 (85.6%) of 90 patients: CR was achieved in 58 (64.5%) patients, PR in 18 (20%) patients, MR in 1 (1.1%) patient; there were 13 (14.4%) nonresponder patients. In the responder patients (CR+PR+MR), the median platelet count was 214 × 109/L (range 35-676 × 109/L), and in nonresponders the median was 14 × 109/L (range 9-25 × 109/L).
No statistically significant difference of overall response rate was shown by sex (males 29/32, 90.6%; females 48/58, 82.8%; P = not significant). The overall response rate was similar in patients classified by age (< 18 years old, 36/42, 85.7%; ≥ 18 years old, 41/48, 85.4%; P = not significant).
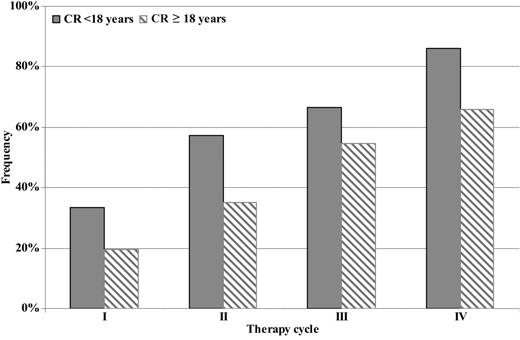
Response evaluation by therapy cycle showed that overall response rates (CR+PR+MR) after the first, second, third, and fourth cycles were as follows: 69.5% (66/95), 75.8% (72/95), 89% (81/91), and 85.6% (77/90), respectively. No statistically significant difference of overall response rates was found between the first and the second cycle (P = not significant), while a statistically significant increase was found between the first and the third cycle (P = .001) and the second and third one (P = .018). After the fourth cycle, no further rise was recorded (P = not significant). Even if the overall response rate in patients under and over 18 years old was similar (85.7% vs 85.4%), a progressive improvement of the quality of response (CR) from the first therapy cycle to the fourth one was observed, in particular in patients younger than 18 years old (Figure 2). Moreover, the quality of response (CR rate vs PR+MR rate) achieved by the 2 mentioned groups was better in terms of statistical significance in patients younger than 18 years old (P = .039) (Table 4). Considering the segregation point of 10 years old in evaluable patients younger than 18 years old (32/42 younger than 10 years old and 10/42 10 years old or older), we noted that total response rates (CR+PR+MR) were 87.5% (28/32) and 80% (8/10), respectively.
GIMEMA multicenter pilot study: CR evaluation and quality of initial response.
Quality of initial response by age
| Age . | Patients . | CR (%) . | PR + MR (%) . | P . |
|---|---|---|---|---|
| 18 y or older | 41 | 27 (66) | 14 (34) | .039* |
| Younger than 18 y | 36 | 31 (86) | 5 (14) | |
| Younger than 10 y | 28 | 23 (82) | 5 (18) | NA† |
| 10 y or older | 8 | 8 (100) | 0 (0) |
| Age . | Patients . | CR (%) . | PR + MR (%) . | P . |
|---|---|---|---|---|
| 18 y or older | 41 | 27 (66) | 14 (34) | .039* |
| Younger than 18 y | 36 | 31 (86) | 5 (14) | |
| Younger than 10 y | 28 | 23 (82) | 5 (18) | NA† |
| 10 y or older | 8 | 8 (100) | 0 (0) |
NA indicates not applicable.
Comparison of first and second rows.
Comparison of third and fourth rows.
In all 90 evaluable patients, considered as a whole and separated by age classes, we evaluated the early rise of platelet count at the fourth day of first therapy cycle; that is, the day after the end of the first therapy course (control at day 4 as the GIMEMA study provided). Moreover, we evaluated, on the same day, how many subjects (adults and children) obtained a rise of platelet levels more than 30 × 109/L (MR), more than or equal to 50 × 109/L (PR), or more than 20 × 109/L (in patients with platelet count ≤ 20 × 109/L at enrollment). The results are shown in Table 5 The median values of platelet counts were similar in adults and children (P = not significant), as was the rate of patients who reached the mentioned values (P = not significant). In particular, 89% of evaluable children reached platelet levels more than 20 × 109/L, 81% reached levels more than 30 × 109/L, and 67% reached levels of at least 50 × 109/L. If we also considered the 2 subgroups of children (ie, those younger than 10 years old and those older than 10 years old), they behaved similarly. The median FU of the 90 evaluable patients was 10 months (range 3-24 months).
GIMEMA multicenter pilot study: platelet count of evaluable patients after the end of the first therapy course
| . | Total . | At least 18 years of age through 70 years of age . | At least 2 years of age through 17 years of age . | P . | ||
|---|---|---|---|---|---|---|
| Total . | Less than 10 . | 10 or greater . | ||||
| Evaluable patients, n | 90 | 48 | 42 | 32 | 10 | NA |
| Median platelet count at fourth day of the first therapy cycle, × 109/L (range) | 92 (2-370) | 87 (2-290) | 99 (7-370) | 98 (7-271) | 110 (28-370) | NS |
| Platelets > 20 × 109/L, no. patients (%) | 68/75* (91) | 35/38* (92) | 33/37* (89) | 25/29* (86) | 8/8* (100) | NS |
| Platelets > 30 × 109/L, no. patients (%) | 76/90 (84) | 42/48 (87) | 34/42 (81) | 26/32 (81) | 8/10 (80) | NS |
| Platelets ≥ 50 × 109/L, no. patients (%) | 66/90 (73) | 38/48 (79) | 28/42 (67) | 21/32 (66) | 7/10 (70) | NS |
| . | Total . | At least 18 years of age through 70 years of age . | At least 2 years of age through 17 years of age . | P . | ||
|---|---|---|---|---|---|---|
| Total . | Less than 10 . | 10 or greater . | ||||
| Evaluable patients, n | 90 | 48 | 42 | 32 | 10 | NA |
| Median platelet count at fourth day of the first therapy cycle, × 109/L (range) | 92 (2-370) | 87 (2-290) | 99 (7-370) | 98 (7-271) | 110 (28-370) | NS |
| Platelets > 20 × 109/L, no. patients (%) | 68/75* (91) | 35/38* (92) | 33/37* (89) | 25/29* (86) | 8/8* (100) | NS |
| Platelets > 30 × 109/L, no. patients (%) | 76/90 (84) | 42/48 (87) | 34/42 (81) | 26/32 (81) | 8/10 (80) | NS |
| Platelets ≥ 50 × 109/L, no. patients (%) | 66/90 (73) | 38/48 (79) | 28/42 (67) | 21/32 (66) | 7/10 (70) | NS |
NA indicates not applicable; NS, not significant.
Patients with platelet count ≤ 20 × 109/L at enrollment.
After a median time of 6.5 months (range 3-10 months) from response achievement, 10 of 77 (13%) responder patients relapsed. Median platelet count at relapse was 21 × 109/L (range 3-29 × 109/L). The relapse rate was lower in patients younger than 18 years old (1/36; 2.7%) when compared with that of patients older than 18 years old (9/41; 22%) (P = .01).
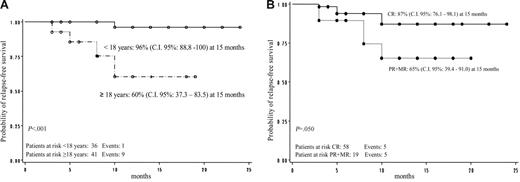
Overall RFS was 81% (95% CI: 70.6-92.3 at 15 months). No difference regarding RFS was shown according to sex in responder patients (P = not significant). Relapse-free survival according to age was 96% (95% CI: 88.8-100.0 at 15 months) for patients younger than 18 years old and 60% (95% CI: 37.3-83.5 at 15 months) for patients 18 years old or older; this difference was statistically significant (P < .001; Figure 3A). Relapse-free survival according to the quality of initial response was 87% (95% CI: 76.1-98.1 at 15 months) for patients who achieved CR and 65% (95% CI: 39.4-91.0 at 15 months) for patients who reached PR or MR (P = .05; Figure 3B).
GIMEMA multicenter pilot study. (A) Relapse-free survival by age. (B) Relapse-free survival according to quality of initial response.
GIMEMA multicenter pilot study. (A) Relapse-free survival by age. (B) Relapse-free survival according to quality of initial response.
Nevertheless, due to the association between the 2 factors (age and quality of initial response; P = .003), only age remains significant in a multivariate Cox model (Table 6) In other words, in the same age class the quality of initial response does not influence the outcome.
GIMEMA multicenter pilot study: multivariate analysis for relapse-free survival
| . | P . | Hazard ratio . | Lower confidence limit 95% . | Upper confidence limit 95% . |
|---|---|---|---|---|
| Type of response* | .2507 | 0.480 | 0.137 | 1.680 |
| Age < 18 y versus ≥ 18 y | .0182 | 12.592 | 1.537 | 103.159 |
| . | P . | Hazard ratio . | Lower confidence limit 95% . | Upper confidence limit 95% . |
|---|---|---|---|---|
| Type of response* | .2507 | 0.480 | 0.137 | 1.680 |
| Age < 18 y versus ≥ 18 y | .0182 | 12.592 | 1.537 | 103.159 |
Complete versus partial + minimal.
Long-term responses, lasting for a median time of 8 months (range 4-24 months) without relapses and without any therapy, were observed in 67 of 77 responder patients (87%); that is, 67 of all 90 (74.4%) evaluable patients. In particular, pCR was achieved in 53 of 67 (79.1%) patients, pPR in 11 of 67 (16.4%) patients, and pMR in 3 of 67 (4.5%) patients. The overall persistent response rate in patients younger than 18 years old (35/36) was 97.2% and in those 18 years old or older (32/41) it was 78% (P = .015).
Treatment with pulsed HD-DXM was well tolerated. Adverse events were recorded in 2 of 95 enrolled patients (2.1%), both of whom are adults. Transitory hypertension was found in one patient, and gastric distress in another patient who was withdrawn from treatment after the third therapy course. Both events were grade 3 (CTC-NCI 2.0). No patient younger than 18 years old discontinued the treatment for adverse events related to therapy.
During therapy cycles, no bleeding complications were recorded and no salvage or emergency therapies, such as platelet transfusions or HD-IVIg, were required. Nonresponder patients (29 after first cycle, 23 after second cycle, 10 after third cycle) received low-dose DXM between courses.
Discussion
The rationale of the initial therapeutic approach of ITP is either to overcome bleeding risk or to achieve a stable response, possibly without any further treatment, and thus, avoid long-term side effects such as infections or metabolic alterations.
It is well known that, up to now, prednisone or prednisolone is considered the most largely used therapeutic approach as first-line treatment for ITP patients, especially adults, even if consistent data regarding the best dosage are missing. With prednisone or prednisolone given at a standard daily dose of around 1 mg/kg for 2 to 4 weeks, an initial response rate of 50% to 60% is generally obtained, but the long-term response rate without any therapy is very low (10%-25%).3,5-7
Moving from the Andersen experience,15 concerning the use of pulsed HD-DXM given in resistant/refractory ITP with very satisfactory results, we planned a first pilot monocenter study with the aim of evaluating efficacy, safety, and tolerability of this treatment as first-line therapy in previously untreated adult ITP patients, according to a 6-cycle therapy schedule. The initial response rate was very encouraging (about 90%) and long-term response was about 68% (25/37, with 20 CR) in all evaluable patients (median FU 26 months). Notwithstanding the number of therapy cycles administered (6 or less), there was no statistically significant difference on the initial response rate and on RFS at 15 months. Nevertheless, a shorter therapy schedule could be advisable, especially if we consider that 9 patients discontinued treatment before completion of therapy due to poor compliance. On the basis of these encouraging results, the GIMEMA ITP Working Party planned a multicenter pilot study with the aim of obtaining a better compliance and feasibility, together with a satisfactory efficacy, modifying the first study therapy schedule by reducing the number of therapy courses (4 instead of 6) and the interval between them (14 days instead of 28 days).
The GIMEMA multicenter pilot study showed that the pulses of HD-DXM, given at shorter time intervals, were feasible and well tolerated by adults and children with ITP. Patients' compliance was good. Efficacy was proved by a high initial response rate (about 86%) and a low overall relapse rate (13%), with an RFS of 81% at 15 months. The greatest overall initial response rate was reached after completion of the third therapy cycle, without any further significant increase after the fourth cycle, even if the quality of response (CR versus PR+MR rate) tends to improve through cycles, until the fourth one (Figure 2).
The GIMEMA multicenter pilot study has been planned to enroll adults and children with ITP. Despite the fact that the initial overall response rate was similar in patients younger and older than 18 years old (85.7% vs 85.4%), children's outcomes during the FU were better than those of the adults. In fact, the patients younger than 18 years old showed a lower relapse rate than older patients (2.7% vs 22%; P = .01) and a higher sustained response rate in time (Figure 3A). Although the initial response rate and long-term outcome of children are similar to what was reported in previously published series,9-11 we did not experience any adverse events related to bleeding during the early period of treatment, perhaps because of the rapid achievement of a safe platelet count just at the end of the first therapy course in a large part of patients. It is noteworthy that this is true for children belonging to both age subgroups (ie, younger and older than 10 years), although there was a discrepancy as it concerned the number of evaluable patients (32 vs 10; Table 5). The prevention of severe bleeding, indeed, is one of the major aims of ITP treatment in children.
The quality of the initial response rate influenced the long-term response. In fact, patients who obtained a CR showed a better outcome with a higher RFS rate than patients who reached PR or MR (87% vs 65%; Figure 3B). In any case, on the multivariate analysis the only independent factor influencing the outcome is age (Table 6).
As for adult patients, comparing the results obtained in both studies to those reported in the literature with conventional treatment, a better initial response rate and an increased long-term response rate (about 67% versus 10%-25%) were observed.3-8
In the multicenter study we observed better patient compliance, probably due to a shorter duration of the therapy schedule without relevant side effects. Moreover, no bleeding complications or emergency therapies were recorded during therapy cycles in adults or children. In fact, according to the GIMEMA protocol, nonresponder patients received low-dose DXM between therapy courses.
The results obtained by the GIMEMA multicenter pilot study in adults are comparable to those recently reported by Cheng et al21 for the initial response rate (85% vs 85%). However, the relapse rate of 22% observed in the GIMEMA study is very different from that observed by Cheng et al, who reported a 50% relapse rate within 6 months from response achievement. We have obtained a persistent response rate of 66.7% (32/48 evaluable adult patients) without any therapy, whereas in the Cheng et al study a persistent response rate of 42% was reported.21 Furthermore, our data seem to be similar to those of Borst et al,23 who more recently reported a long-term response of 59%. However, they observed an adverse event rate of 22%, and in our 2 studies adverse event rates were 13.5% and 2.1%, respectively.
Therefore, our results confirm that HD-DXM can be proposed as a first-line treatment for both adults and children with ITP. Moreover, repeated cycles of therapy seem to be more efficient than only one cycle, as proposed by Cheng et al,21 to achieve long-term response, especially in adults. In fact, in the GIMEMA experience a persistent response after 4 therapy cycles is achieved in about 67% of adult patients, as compared with the 42% response obtained by Cheng et al with only one cycle.
Furthermore, because there was no statistically significant difference in the initial overall response rate between the third and fourth cycles, using only 3 therapy cycles could be more appropriate to reach a better patient safety and tolerability level, and to maintain efficacy. In conclusion, randomized clinical trials are necessary to confirm these issues, and thus, pulses of HD-DXM given over 3 cycles will be proposed in the next randomized GIMEMA clinical trial, versus conventional prednisone treatment given at a daily dose of 1 mg/kg for 4 weeks, for previously untreated adult patients with ITP.
Authorship
Conflict of interest disclosure: The authors declare no competing financial interests.
A complete list of the individuals comprising the GIMEMA Thrombocytopenia Working Party can be found in Document S1, available on the Blood website; click on the “Supplemental Document” link at the top of the online article.
Correspondence: Maria Gabriella Mazzucconi, Dipartimento di Biotecnologie Cellulari ed Ematologia, Università degli Studi di Roma La Sapienza, Via Benevento 6, 00161 Roma, Italy; e-mail: mazzucconi@bce.uniroma1.it.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We wish to thank the following GIMEMA members: L. Camba (Milano), R. Mozzana (Gallarate), D. Cultrera (Catania), E. Elli (Monza), M. Morselli (Modena), N. Filardi (Potenza), S. Galimberti (Pisa), and M. Tambone-Reyes (Cefalù).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal