Abstract
Most adults with acute lymphoblastic leukemia (ALL) who achieve complete remission (CR) will relapse. We examined the outcome of 609 adults with recurring ALL, all of whom were previously treated on the Medical Research Council (MRC) UKALL12/ECOG2993 study, where the overall survival (OS) of newly diagnosed patients is 38% (95% confidence interval [CI] = 36%-41%) at 5 years. By contrast, OS at 5 years after relapse was 7% (95% CI = 4%-9%). Factors predicting a good outcome after salvage therapy were young age (OS of 12% in patients younger than 20 years vs OS of 3% in patients older than 50 years; 2P < .001) and short duration of first remission (CR1) (OS of 11% in those with a CR1 of more than 2 years versus OS of 5% in those with a CR1 of less than 2 years; 2P < .001). Treatment received in CR1 did not influence outcome after relapse. In a very highly selected subgroup of patients who were able to receive HSCT after relapse, some were long-term survivors. We conclude from a large, unselected series with mature follow-up that most adults with recurring ALL, whatever their prior treatment, cannot be rescued using currently available therapies. Prevention of recurrence is the best strategy for long-term survival in this disease.
Introduction
In contrast to the fate of children with the disease, most adults who develop acute lymphoblastic leukemia (ALL) do not become long-term survivors. Although rates of complete remission (CR) in adult ALL are high—78% to 93% in large, prospective clinical trials—long-term disease-free survival ensues in only approximately 40% of patients.1-8 Salvage therapy can sometimes achieve second remissions for adults with recurring ALL.9-13 In patients who have not already been treated with hematopoietic stem cell transplantation (HSCT), achievement of a second remission is usually followed by high-dose therapy and HSCT wherever donor availability, patient age, performance status, and vital organ function permit. Typically, matched unrelated donors or previously unconsidered higher-risk options such as transplants from haploidentical donors or cord blood transplants will be considered at this stage. Unfortunately, postrelapse strategies rarely result in long-term survival. As a consequence, most studies in ALL now attempt to identify patients at highest risk of relapsing and apply higher-risk therapies at an earlier timepoint. However, the optimal risk-benefit delineation for application of high-risk therapies in first remission is far from clear. In particular, it is unknown what, if any, are the effects of prior therapy on the possibility of salvage after relapse. To address this issue, we analyzed the fate of 609 adult patients who relapsed following treatment on the UKALLXII/ECOG2993 protocol. The length of the study and the number of patients enrolled affords a unique opportunity to examine the fate of a very large, mature series of prospectively enrolled adult patients with ALL who relapsed after a uniform approach to initial therapy.
In examining the data, we specifically asked whether patients who had not already received high-dose chemoradiotherapy followed by HSCT as part of their initial therapy could be salvaged by autologous or allogeneic HSCT after relapse. The analysis provides a clear answer to this question, which is extremely relevant to the therapy of ALL and to the design of future therapeutic trials in adults with ALL.
Furthermore, the data also provide guidance concerning which patients are most likely to benefit from postrelapse therapy as currently conceived. The data allow identification of which patients will fare particularly poorly, for whom alternative and novel therapeutic strategies should be explored at relapse.
Patients, materials, and methods
Study eligibility
Eligible patients were aged 15 to 60 years (15-55 years in the United Kingdom) with newly diagnosed, untreated ALL (FAB L1 or L2 only for ECOG patients) and no prior malignancy. The institutional review boards of each participating center approved the study. All patients gave informed consent.
Initial diagnostic procedures and treatment
Diagnosis of ALL was established by documenting more than 25% marrow lymphoblasts. Confirmation of the diagnosis of ALL by central morphology review was recommended as well as submission of blood or marrow samples for cytogenetic analysis and immunophenotyping. Assessment of Philadelphia chromosome status was by cytogenetic analysis and by molecular methods, where applicable. All patients were treated according to the UKALL12/ECOG E2993 protocol. A simplified schema of original therapy is given in Figure 1. CR was defined as less than 5% bone marrow (BM) blasts with maturation of all other cell lines, peripheral blood neutrophil counts above 1 × 109/L, platelet counts above 100 × 109/L, and no evidence of extramedullary leukemia.
Simplified schema of initial treatment on MRC UKALLX11/ECOG E2993.1 (Fig 1)
Treatment and data collection after relapse
After relapse following initial on-protocol therapy, patients were followed up, but choice of therapy was left to the discretion of physician and patient. Limited data were collected on therapy after relapse, but transplantations, subsequent relapses, and survival or date of death were recorded. Data on achievement of second or subsequent CR were not collected.
Statistical analysis
All patients were centrally registered by telephone at either the Clinical Trial Service Unit (CTSU) in Oxford for MRC patients, or at the ECOG Coordinating Center for ECOG patients. Randomization between autograft and chemotherapy was done on computer at these 2 centers, which were also responsible for the collection of follow-up forms. Data entry and analyses for the whole trial were done at CTSU.
Analyses by prognostic factors and treatment used Kaplan-Meier curves and the log-rank statistic for comparisons. Relative risks are indicated by the observed number of deaths (O) divided by the number that would be expected on the assumption of no difference between groups (E), but using time-to-event analysis to allow for the variable lengths of follow-up. The main analyses were of survival from the date of relapse. Independence of prognostic factors was confirmed by Cox regression analysis, with age and log (white blood cell [WBC] count + 1) included as continuous variables. This trial is ongoing as of July 2006. This report includes all patients who started treatment before November 1, 2003, with follow-up to October 31, 2005.
Results
Patients and patient characteristics
Of 1508 trial patients who were eligible for analysis, 74 patients died in induction and 62 failed to achieve remission. Of the 1372 patients who entered remission, 609 (44%) relapsed at a median of 11 months from the start of treatment. The outcome of these 609 adult patients with ALL in first relapse is the subject of this report. Median follow-up of the 50 survivors is 4 years and 1 month (range, 12 days to 10 years and 11 months).
Of the group, 382 (63%) were male. Most (556 [91%]) patients relapsed within the BM, and this was the sole site of relapse in most (90%) of those patients. A group of 45 (8%) patients relapsed at solely extramedullary sites, while for 8 (1%), the site was unknown. Sites of extramedullary relapse were as follows: central nervous system (CNS), n = 22; skin/soft tissue, n = 8; testes, n = 5; lymph nodes/mediastinum, n = 5; and n = 1 each of ovary, bone, prostate, kidney, and serous effusion. A number of patients (120 [20%]) were Philadelphia chromosome positive (Ph+), which is similar to the percentage (25%) of patients with Ph+ disease registered to the trial as a whole.
Most (81%) patients relapsed within 2 years of diagnosis, although a significant minority (19%) relapsed beyond 2 years after diagnosis. Of the 440 chemotherapy-treated patients, 349 relapsed within 2 years of diagnosis, and 87 relapsed later. Those on chemotherapy who relapsed within 2 years can be considered “relapses on therapy” since the duration of therapy was set to be 18 months from the point of initiation of the consolidation therapy (ie, 23 months).
Risk groups were defined as the following: standard (T-cell ALL with presenting WBC count lower than 100 × 109/L, or non–T-cell ALL with presenting WBC count lower than 30 × 109/L); high (T-cell ALL with WBC count higher than 100 × 109/L, or non–T-cell ALL with WBC count higher than 30 × 109/L); and very high (Ph+ ALL). Nearly half (287, 47%) of patients who relapsed were in the standard risk category, 135 (22%) were in the high-risk category, and 67 (11%) were unclassified. In terms of initial treatment received at diagnosis, 440 (72%) had received chemotherapy alone, and the remainder had been treated with high-dose therapy and BM transplantation (with the exception of 3 patients in which an off-protocol low-intensity conditioning regimen was used). These and other patient characteristics are summarized in Table 1.
Clinical characteristics of 609 adults with recurring ALL
| Characteristic . | No. patients . |
|---|---|
| Age at first diagnosis | |
| 15-19 y | 117 |
| 20-34 y | 236 |
| 35-49 y | 49 |
| Older than 50 y | 74 |
| Sex | |
| Male | 382 |
| Female | 227 |
| WBC count at first diagnosis | |
| Less than 10 × 109/L | 248 |
| 10–49 × 109/L | 178 |
| More than 50 × 109/L | 181 |
| Unknown | 2 |
| Immunophenotype (44 unknown) | |
| B precursor | 409 |
| T-cell | 92 |
| Mature B | 12 |
| Null | 30 |
| Mixed | 22 |
| Unknown | 44 |
| Ph status (56 unknown) | |
| Positive | 120 |
| Negative | 433 |
| Risk group at first diagnosis (67 unknown) | |
| Standard | 287 |
| High | 135 |
| Very high | 120 |
| Unknown | 67 |
| Initial treatment at first diagnosis* | |
| CR1 | |
| Chemotherapy | 440 |
| Sibling allograft | 60 |
| MUD | 17 |
| Autograft | 80 |
| Other | 5† |
| Preremission‡ | |
| Sibling allograft | 6 |
| Autograft | 1 |
| Time from diagnosis to relapse | |
| Less than 1 y | 333 |
| 1-2 y | 160 |
| More than 2 y | 116 |
| Site of relapse, no. patients (8 unknown) | |
| BM alone | 523 |
| BM + CNS | 33 |
| CNS alone | 22 |
| Extramedullary | 23 |
| Unknown | 8 |
| Characteristic . | No. patients . |
|---|---|
| Age at first diagnosis | |
| 15-19 y | 117 |
| 20-34 y | 236 |
| 35-49 y | 49 |
| Older than 50 y | 74 |
| Sex | |
| Male | 382 |
| Female | 227 |
| WBC count at first diagnosis | |
| Less than 10 × 109/L | 248 |
| 10–49 × 109/L | 178 |
| More than 50 × 109/L | 181 |
| Unknown | 2 |
| Immunophenotype (44 unknown) | |
| B precursor | 409 |
| T-cell | 92 |
| Mature B | 12 |
| Null | 30 |
| Mixed | 22 |
| Unknown | 44 |
| Ph status (56 unknown) | |
| Positive | 120 |
| Negative | 433 |
| Risk group at first diagnosis (67 unknown) | |
| Standard | 287 |
| High | 135 |
| Very high | 120 |
| Unknown | 67 |
| Initial treatment at first diagnosis* | |
| CR1 | |
| Chemotherapy | 440 |
| Sibling allograft | 60 |
| MUD | 17 |
| Autograft | 80 |
| Other | 5† |
| Preremission‡ | |
| Sibling allograft | 6 |
| Autograft | 1 |
| Time from diagnosis to relapse | |
| Less than 1 y | 333 |
| 1-2 y | 160 |
| More than 2 y | 116 |
| Site of relapse, no. patients (8 unknown) | |
| BM alone | 523 |
| BM + CNS | 33 |
| CNS alone | 22 |
| Extramedullary | 23 |
| Unknown | 8 |
One BMT on unknown date.
Other indicates one reduced-intensity sibling allogeneic HSCT, one reduced-intensity MUD allogeneic HSCT, one reduced-intensity haploidentical HSCT, one fully conditioned haploidentical HSCT, and one unknown type.
Preremission indicates HSCT performed in absence of achieving CR1.
Outcome after relapse
The median survival after relapse was 24 weeks. Survival at 1 year was 22% (95% CI = 18%-25%), and 7% (95% CI = 4%-9%) at 5 years. Data on achievement of a second remission (CR2) and time to CR2 were not available in sufficient number for analysis. Only 42 of 609 relapsed patients remain alive without further relapse. Median length of follow-up for these patients is 54 months (range, 1-131 months).
Prognostic factors for survival from relapse
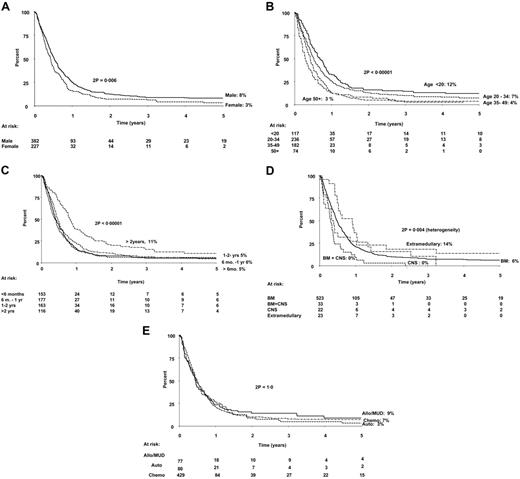
Four factors—age, sex, site of relapse, and time from diagnosis to relapse—were statistically significant as prognostic factors for outcome following relapse. Table 2 details the prognostic factors analyzed and shows the deaths per total number of patients, the relative risk (as observed/expected [O/E]), the P value, 5-year overall survival (OS), and CIs.
Prognostic factors for survival from relapse
| . | No. deaths . | No. patients . | O/E ratio . | 5-y OS, % . | CI . | Log-rank P . |
|---|---|---|---|---|---|---|
| Ph status | >.999 | |||||
| Positive | 397 | 433 | 1.0 | 6 | 4-9 | |
| Negative | 109 | 120 | 1.0 | 9 | 3-14 | |
| Sex | .006 | |||||
| Male | 348 | 382 | .09 | 8 | 5-11 | |
| Female | 211 | 227 | 1.2 | 3 | 0-6 | |
| Age at diagnosis | <.001 (trend) | |||||
| Younger than 20 y | 101 | 117 | 0.7 | 12 | 6-18 | |
| 20-34 y | 212 | 236 | 0.9 | 7 | 4-11 | |
| 35-49 y | 174 | 182 | 1.2 | 4 | 1-7 | |
| 50 y or older | 72 | 74 | 1.5 | 3 | 0-6 | |
| WBC count at diagnosis | .05 (trend) | |||||
| Less than 10 × 109/L | 227 | 248 | 0.9 | 5 | 2-8 | |
| 10-49 × 109/L | 161 | 178 | 1.0 | 9 | 4-13 | |
| More than 50 × 109/L | 169 | 181 | 1.1 | 6 | 3-10 | |
| Immunophenotype | .06 (heterogeneity) | |||||
| Pre-B | 368 | 409 | 0.9 | 8 | 5-10 | |
| T | 87 | 92 | 1.1 | 5 | 0-10 | |
| Mature B | 12 | 12 | 1.2 | 0 | — | |
| Null | 30 | 30 | 1.3 | 0 | — | |
| Mix | 21 | 22 | 1.5 | 5 | 0-13 | |
| Therapy in CR1* | >.999 (heterogeneity) | |||||
| Chemotherapy | 390 | 429 | 1.0 | 7 | 5-10 | |
| Autologous HSCT | 76 | 80 | 1.05 | 3 | 0-8 | |
| Allo-HSCT | 70 | 77 | 1.0 | 9 | 2-16 | |
| Time from diagnosis to relapse | <.001 | |||||
| Less than 2 y | 463 | 493 | 1.0 | 5 | 3-8 | |
| 2 y or longer | 96 | 116 | 0.7 | 11 | 4-17 | |
| Site of relapse | .004 (heterogeneity) | |||||
| BM | 482 | 523 | 1.0 | 6 | 4-8 | |
| BM + CNS | 33 | 33 | 1.7 | 0 | — | |
| CNS | 19 | 22 | 0.9 | 0 | — | |
| Extramedullary | 20 | 23 | 0.7 | 14 | 0-28 |
| . | No. deaths . | No. patients . | O/E ratio . | 5-y OS, % . | CI . | Log-rank P . |
|---|---|---|---|---|---|---|
| Ph status | >.999 | |||||
| Positive | 397 | 433 | 1.0 | 6 | 4-9 | |
| Negative | 109 | 120 | 1.0 | 9 | 3-14 | |
| Sex | .006 | |||||
| Male | 348 | 382 | .09 | 8 | 5-11 | |
| Female | 211 | 227 | 1.2 | 3 | 0-6 | |
| Age at diagnosis | <.001 (trend) | |||||
| Younger than 20 y | 101 | 117 | 0.7 | 12 | 6-18 | |
| 20-34 y | 212 | 236 | 0.9 | 7 | 4-11 | |
| 35-49 y | 174 | 182 | 1.2 | 4 | 1-7 | |
| 50 y or older | 72 | 74 | 1.5 | 3 | 0-6 | |
| WBC count at diagnosis | .05 (trend) | |||||
| Less than 10 × 109/L | 227 | 248 | 0.9 | 5 | 2-8 | |
| 10-49 × 109/L | 161 | 178 | 1.0 | 9 | 4-13 | |
| More than 50 × 109/L | 169 | 181 | 1.1 | 6 | 3-10 | |
| Immunophenotype | .06 (heterogeneity) | |||||
| Pre-B | 368 | 409 | 0.9 | 8 | 5-10 | |
| T | 87 | 92 | 1.1 | 5 | 0-10 | |
| Mature B | 12 | 12 | 1.2 | 0 | — | |
| Null | 30 | 30 | 1.3 | 0 | — | |
| Mix | 21 | 22 | 1.5 | 5 | 0-13 | |
| Therapy in CR1* | >.999 (heterogeneity) | |||||
| Chemotherapy | 390 | 429 | 1.0 | 7 | 5-10 | |
| Autologous HSCT | 76 | 80 | 1.05 | 3 | 0-8 | |
| Allo-HSCT | 70 | 77 | 1.0 | 9 | 2-16 | |
| Time from diagnosis to relapse | <.001 | |||||
| Less than 2 y | 463 | 493 | 1.0 | 5 | 3-8 | |
| 2 y or longer | 96 | 116 | 0.7 | 11 | 4-17 | |
| Site of relapse | .004 (heterogeneity) | |||||
| BM | 482 | 523 | 1.0 | 6 | 4-8 | |
| BM + CNS | 33 | 33 | 1.7 | 0 | — | |
| CNS | 19 | 22 | 0.9 | 0 | — | |
| Extramedullary | 20 | 23 | 0.7 | 14 | 0-28 |
Ph indicates Philadelphia chromosome; pre-B, precursor B; mix, mixed lineage; allo-HSCT, matched or unrelated donor HSCT; and —, not applicable.
Twenty-four patients were excluded: 7 underwent transplantation before remission, 3 with reduced-intensity conditioning regimens, 1 mismatched-related, 1 with unknown-type SCT, 1 with SCT on an unknown date, and 11 who died before 12 weeks and hence could not possibly have reached transplantation.
Stratified analyses showed the independence of these 4 factors. Stepwise Cox regression analyses, with age, sex, relapse (less than 2 years or 2 years or longer), involvement of BM, involvement of CNS, log (WBC + 1), and immunophenotype in the initial model, confirmed this. Other factors analyzed, such as Ph status, WBC at diagnosis, immunophenotype at diagnosis, and, notably, therapy received in first remission (CR1), were not significant prognostic indicators for survival after relapse. The data on analysis of prognostic factors are shown in full in Table 2, with the hazard ratios in Table 3. Relevant survival curves are shown in Figure 2. Inclusion of postrelapse therapy (transplantation or not) as a time-dependent variable in the Cox analyses made little difference to the hazard ratios, although female sex (hazard ratio = 1.21; 95% CI = 0.98-1.50) and BM involvement (hazard ratio = 1.48; 95% CI = 0.95-2.30) were no longer formally significant (both P = .08).
Results of multivariate Cox regression analysis of all relapsed patients
| Variable . | Hazard ratio . | 95% confidence interval . |
|---|---|---|
| Age | 1.02 | 1.01-1.02 |
| Female | 1.27 | 1.06-1.51 |
| Relapse in less than 2 y | 1.63 | 1.30-2.04 |
| BM involvement | 1.51 | 1.06-2.17 |
| CNS involvement | 1.68 | 1.22-2.31 |
| Variable . | Hazard ratio . | 95% confidence interval . |
|---|---|---|
| Age | 1.02 | 1.01-1.02 |
| Female | 1.27 | 1.06-1.51 |
| Relapse in less than 2 y | 1.63 | 1.30-2.04 |
| BM involvement | 1.51 | 1.06-2.17 |
| CNS involvement | 1.68 | 1.22-2.31 |
Probabilities of survival from first relapse: an analysis of prognostic factors. (A) Sex. (B) Age at diagnosis. (C) Time from diagnosis to relapse. (D) Site of relapse. (E) Therapy in CR1. Numbers at risk are shown beneath the graph. Sib Allograft indicates allogeneic HSCT from fully matched sibling donor; MUD allograft, allogeneic HSCT from matched unrelated donor.
Probabilities of survival from first relapse: an analysis of prognostic factors. (A) Sex. (B) Age at diagnosis. (C) Time from diagnosis to relapse. (D) Site of relapse. (E) Therapy in CR1. Numbers at risk are shown beneath the graph. Sib Allograft indicates allogeneic HSCT from fully matched sibling donor; MUD allograft, allogeneic HSCT from matched unrelated donor.
Therapy in relapse: efficacy of transplantation after relapse
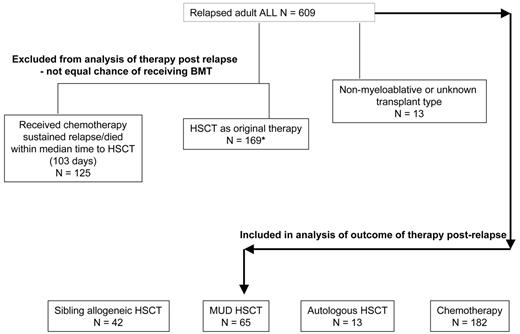
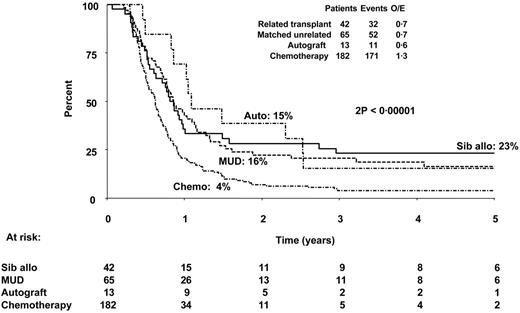
In view of the demonstration that initial therapy received was not prognostic for outcome after relapse, we were interested to examine the prognostic value of treatment received after relapse. In particular, we wished to determine the role of HSCT since this is typically regarded as the only potentially curative therapeutic option. Patients who had already received a transplant as part of their prior therapy (n = 169) were excluded from this analysis, since the possibility of receiving a transplant was not equal with those who had never before received a transplant, and information on second transplantations was not systematically collected. In addition, those who had received a nonmyeloablative or mismatched transplant or a transplant of unknown type after relapse (n = 13) were excluded from comparisons. To adjust for the lead-time bias present in comparisons between transplantation and chemotherapy, 125 patients treated with chemotherapy after relapse who had sustained relapse or died within the median time to transplantation (103 days) were also excluded. A flow chart depicting the patient groups and the analyses performed is given in Figure 3. The data for the patients analyzed show that those treated with HSCT had a superior OS (15% [95% CI = 0%-35%] for autograft [n = 13], 16% [95% CI = 7%-26%] for matched unrelated donor transplantation [n = 65], and 23% [95% CI = 10%-36%] for sibling allograft [n = 42]) to those receiving chemotherapy alone (n = 182) whose OS was only 4% (95% CI = 1%-7%) at 5 years. The differences between the transplantation groups and the chemotherapy group were statistically significant (2P < .001). Figure 4 shows the Kaplan-Meier survival curves illustrating these data. This result was not materially changed when allowance was made (by stratified analysis) for sex, age, time to relapse (less than 2 years and 2 years or more), and type of relapse. Multivariate Cox regression analyses, excluding only those who underwent transplantation before relapse and the few who underwent mini-, mismatched, or cord blood transplantation, including transplantation as a time-dependent variable, confirmed that those who received a transplant had better survival. Comparisons by treatment received, no matter how analyzed, should be treated with caution, since no statistical method can adjust for the unmeasured selection factors involved. However, in this large but selected group of patients with recurring ALL who were able to receive HSCT while in relapse, some were long-term survivors, although the best expected survival was only 23% at 5 years.
Flowchart depicting the patients included and the analyses performed to assess efficacy of HSCT in relapse. *Outcome of these patients who received HSCT as part of original therapy is shown in Figure 2E (N = 157; 12 excluded from analysis due to HSCT prior to CR1 or nonstandard transplant.
Flowchart depicting the patients included and the analyses performed to assess efficacy of HSCT in relapse. *Outcome of these patients who received HSCT as part of original therapy is shown in Figure 2E (N = 157; 12 excluded from analysis due to HSCT prior to CR1 or nonstandard transplant.
Probability of survival according to therapy given in relapse. Those who died within 100 days of relapse and those patients who underwent prior transplantation in CR1 are excluded from this analysis to allow more appropriate comparison of high-dose therapy and HSCT with chemotherapy alone. Sib Allograft indicates allogeneic HSCT from fully matched sibling donor; and MUD allograft, allogeneic HSCT from unrelated donor.
Probability of survival according to therapy given in relapse. Those who died within 100 days of relapse and those patients who underwent prior transplantation in CR1 are excluded from this analysis to allow more appropriate comparison of high-dose therapy and HSCT with chemotherapy alone. Sib Allograft indicates allogeneic HSCT from fully matched sibling donor; and MUD allograft, allogeneic HSCT from unrelated donor.
Discussion
This is the largest series of adults with recurring ALL ever reported. The magnitude of the dataset, homogeneity of initial therapy, and the maturity of follow-up have allowed us to detect some highly statistically significant prognostic factors that are informative both to practicing clinicians and their patients and to those involved in the design of clinical trials in adult ALL. The prognostic factors identified do not depend on response to salvage therapy and therefore could be used to guide a more focused approach to the treatment of recurring ALL by delineating patients in whom a small but important chance for long-term salvage exists from those in whom there is very little realistic opportunity for long-term survival. Our study confirms the findings of smaller/single-center studies with respect to the prognostic value of age and duration of CR1,9,13 and newly identifies female sex and site of relapse as prognostic factors for a poor outcome.
Another important question for this study was the role of initial therapy in determining outcome after relapse. Our data, in contrast to the results of a smaller study of 61 patients with ALL,13 demonstrate that initial therapy of ALL does not affect the outcome after relapse. Patients who relapsed had the same long-term outcome whether or not they received chemotherapy or high-dose therapy and transplantation as part of their initial therapy. Taken together with studies suggesting a survival benefit for allogeneic HSCT in adult ALL, the results presented here suggest that the survival benefits of high-dose chemoradiotherapy in adult ALL are largely in preventing relapse. Once the patient has relapsed, subsequent cure becomes unlikely, whatever the initial therapy.
Despite this, our data do indicate that a small number of the patients who received chemotherapy alone as initial treatment can be rescued and survive for the long term if they receive subsequent high-dose therapy and HSCT at relapse. Only about one quarter (108 of 429) of the patients who initially received chemotherapy were treated with a sibling or unrelated donor HSCT after relapse. Our data do not address whether this was due to lack of attainment of CR2, performance status of the patients, or donor availability. However, it is clear that such patients constitute a very highly selected group with which there is no suitable comparator, either within our study or within other studies. Probably as few as one half of those who relapse will even reach another remission, and many will not reach the point of HSCT for other reasons; hence, those who do undergo HSCT following relapse are already destined to have a better outcome than those who do not. The survival rates are clearly much lower than for those treated on this study with HSCT in CR1: 42% for autograft, 45% for matched unrelated donor allograft, and 52% for sibling allograft, which suggests, unsurprisingly, that HSCT is much more beneficial if used in CR1 rather than in CR2.
Our study has limitations. Since the study did not collect information on which regimens were used to achieve a CR2, nor on the rate of attainment of CR2, we are unable to address which regimens can be recommended in recurring ALL. Nor have we documented to what intensity and with what overall aim our relapsed patients were treated. Therefore, we cannot exclude the fact that a palliative strategy may have been pursued in a number of the patients. There is still no broad agreement on which regimens would be best suited to treat patients with recurring ALL. Smaller series have demonstrated some utility to a number of regimens, but it is clear that results obtained are far from ideal whatever therapy is applied.
Our data, although pertaining to patients with recurring ALL, also contribute to the debate about how patients with de novo disease should be treated. Since the outcome after relapse is so poor, our study underscores the importance of treating adults to maximal benefit “up-front.” There are data from large studies suggesting that allografting in CR1 can result in a better outcome than chemotherapy alone,14,15 although this approach is not yet substantiated for all age/risk groups in adult ALL.
However, since our data demonstrate so conclusively that the prognosis after relapse is dismal for most patients, the substantial risk of transplantation-related mortality as initial therapy is worth taking in patients with high-risk disease. Indeed, there is increasing evidence that the morbidity and mortality of unrelated donor HSCT may differ little from that of sibling donor transplantation in ALL.16 Hence, we draw the conclusion that a strategy to “reserve” HSCT as a postrelapse option is not the best therapeutic approach for most adults. With regard to adolescents and younger adults whose relapse risk is lower, most especially those patients under the age of 20 years, we conclude from the data in our study that such patients—who are already at the lowest risk of relapse—are also among the most likely to be rescued following relapse. As evidence accumulates that the initial therapy of such patients should be with “pediatric” protocols,17-19 our study gives additional weight to the benefit of using “pediatric” strategies: to treat intensively with chemotherapy, but to avoid the short- and long-term toxicity of HSCT. For older adults, risk-adapted therapies, in which the higher-risk therapies are applied as early as possible to those patients destined to have the higher rates of relapse, are now the subject of numerous ongoing studies (eg, the German study GMALL 07/2003, Italian studies GIMEMA ALL0904 and NILG-ALL 09/00, and French study GRAALL 02/2005).
In summary, our data suggest that the vast majority of adult patients with recurring ALL (in contrast to adults with relapsed acute myeloid leukemia20,21 ), whatever their prior therapy, cannot be rescued using currently recognized therapies, even if HSCT is an option. However, in choosing among current available therapies for patients who have relapsed, the best possible results can be obtained in a select subset of patients who are fit and eligible for a high-dose procedure. We suggest that every eligible adult with recurring ALL be included in a prospective study involving novel therapeutic agents.22
Authorship
Author contributions: A.K.F. wrote the paper. All authors contributed to writing the paper and checked the final version. S.M.R. and G.B. controlled and analyzed data. All authors participated in data collection, study design, and coordination. A.H.G. and J.M.R. were study chairs in the United Kingdom and United States, respectively.
A complete list of participants in the MRC UKALLXII/ECOG 2993 trial appears in as a data supplement to the online version of this article.
Correspondence: Adele K. Fielding, Royal Free and University College Medical School, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: a.fielding@medsch.ucl.ac.uk.
Supported by the Leukaemia Research Fund (United Kingdom) (A.K.F.).
R.C. is currently affiliated with Astra Zeneca in the United Kingdom.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Acknowledgments
The authors would like to thank all the doctors in the United Kingdom, United States, Israel, Italy, and New Zealand who participated in the MRC UKALLXII/ECOG 2993 trial.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal