Abstract
We have reported that anti-idiotype antibody response and FcγRIIIa 158 valine/valine (V/V) genotype both correlate with better outcome in a group of 136 follicular lymphoma patients receiving idiotype vaccination after induction chemotherapy. Here, we examined whether this correlation is related in any way to the chemotherapy response. In patients with complete response (CR), the 5-year progression-free survival (PFS) was 69% for patients with antibody response and/or V/V genotype, while the PFS was only 40% for patients with neither; the median time to progression (TTP) was 10.47 versus 3.46 years (P = .012). In patients with partial response (PR), the 5-year PFS was 57% for patients with antibody response and/or V/V genotype, and 17% for patients with neither; the median TTP was not reached versus 1.31 years (P = .001). This study further confirms the strong association of clinical outcome with antibody response and with the functionally more active form of the Fc receptor in patients receiving idiotype vaccination regardless of their response to induction chemotherapy.
Introduction
Active immunotherapies that generate long-lasting immune responses against tumor antigen(s) by the patient may be an appealing strategy to treat non-Hodgkin lymphoma. One approach being tested is vaccination against the unique sequences of immunoglobulin (idiotype, Id) expressed by each patient's tumor.1 The goal of vaccination is to generate anti-Id immune responses, which may actively eliminate tumor cells and lead to better outcome.2 Lymphoma patients vaccinated with Id protein make humoral and cellular anti-Id immune responses to a variable degree, depending upon the vaccine regimen.3-5 In one study, anti-Id cellular immune responses are believed to induce molecular remission in vaccinated patients.6 In contrast, we have recently shown that anti-Id humoral immune responses and FcγRIIIa 158 V/V genotype are associated with better clinical outcome in a larger group of lymphoma patients.7 In these studies, patients received Id vaccines when they were in remission after induction chemotherapy. Therefore, the response to induction chemotherapy could have had an impact on their outcome. In this report, we determined the influence of chemotherapy response on the clinical outcome and whether the predictive value for better outcome of antibody response and V/V genotype applied to patients who had different chemotherapy responses.
Patients, materials, and methods
Idiotype vaccination studies
This retrospective study included 136 patients who received idiotype vaccination using different study protocols between 1988 and 2000. The patient characteristics are summarized in Table S1, which is available on the Blood website (see the Supplemental Materials link at the top of the online article). To be included in vaccine trials, all patients were required to receive induction chemotherapy to achieve at least a partial response before vaccination. The responses were scored according to the criteria of Cheson et al.8 The follicular lymphoma international prognostic index (FLIPI) score at the time of induction chemotherapy was available on 127 patients to determine their risk group as described.9
Vaccinations were initiated at least 2 months after completion of chemotherapy. During the vaccination, 86 patients received chemical adjuvant, 18 patients received GM-CSF, and 32 patients had Id protein-pulsed dendritic cells.1,5,10,11 The vaccination was usually composed of 4 to 5 monthly injections according to individual protocols. Postvaccination follow-up was conducted every 3 to 4 months for the first 2 to 3 years, and then semiannually or annually. The median follow-up after induction chemotherapy was 8.26 years for the entire group. All vaccination studies were conducted according to institutional review board–approved protocols, and informed consent was obtained from all patients.
Immune response assessments
A specific humoral anti-Id immune response was determined by prespecified criteria when a 4-fold increase in anti-Id antibody titer was found after vaccination compared with before vaccination and with the irrelevant Id proteins using enzyme-linked immunosorbent assay.5,11 Anti-Id cellular immune response was determined by T-cell proliferation assays by culturing peripheral blood mononuclear cells (PBMCs) in media alone or with tumor Id, or irrelevant Id proteins.1,11,12 Prespecified criteria required that incorporation of [3H]-thymidine more than twice the background (media alone) was observed on 2 or more occasions to be considered positive. Immune responses were measured before vaccination, 2 weeks following each vaccination, and 3 and 6 months after last injection. In general, anti-Id immune responses were detected after 3 to 4 vaccinations and peaked after 4 to 5 vaccinations. The anti-Id antibodies were sustained for several months. The cellular immune responses declined quickly after last vaccine and were undetected after 3 months in most of the cases.
Analysis of FcγRIIIa and FcγRIIa polymorphisms
Genomic DNA was prepared from tumor cells or PBMCs using a QIAGEN DNA extraction kit (QIAGEN, Valencia, CA) or from the serum as described.12 The FcγRIIIa 158 V/F and FcγRIIa 131 H/R genotypes were determined using TaqMan technology on an ABI Prism 7900HT Sequence Detector System (Applied Biosystems, Foster City, CA) with FcγRIIIa- and FcγRIIa-specific primer pairs and allele-specific probes.7
Statistical analysis
The median time to progression (TTP) and difference in the progression-free survival (PFS) were determined using the Kaplan-Meier estimation and log-rank statistic (PRISM for Macintosh; GraphPad Software, San Diego, CA). A multivariate analysis using Cox proportional hazard model was performed to identify independent prognostic variables influencing the PFS (StatView 5.0.1; SAS, Cary, NC).
Results and discussion
Responses to induction chemotherapy and clinical outcome
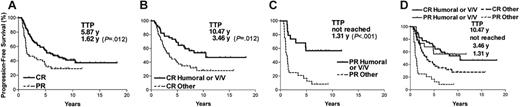
The anti-Id immune response has been shown to correlate with better clinical outcome in follicular lymphoma patients who received a custom Id vaccine.5,10 However, these patients all received induction chemotherapy of different regimens to reduce tumor burdens and all were in remission at the time of vaccination (Table S2). We first analyzed the influence of chemotherapy response on clinical outcome of a group of 136 consecutive vaccinated patients from our previous report, for whom even longer follow-up is now available. Overall, 93 (68%) patients achieved complete response (CR) and 43 (32%) patients achieved PR after chemotherapy. The development of anti-Id humoral or cellular immune response and the FcγRIIIa and IIa genotype distribution did not differ between CR and PR patients (Table S1). As expected, the CR patients had longer TTP after the last chemotherapy. The estimated 5-year PFS was 54% for CR and 34% for PR patients, with median TTP estimate at 5.87 and 1.62 years, respectively (P = .012) (Figure 1A). Certain chemotherapy reagents such as fludarabine have been to shown to suppress T-cell function13 and might have a negative effect on idiotype vaccination. However, in our study, use of fludarabine or adriamycin had no effect on the clinical outcome (Table 1).
Kaplan-Meier estimates of progression-free survival by response to induction chemotherapy, and humoral immune response and V/V genotype. Progression-free survival curves were plotted by response to induction chemotherapy (A), or by humoral anti-Id immune responses and/or FcγRIIIa 158 V/V genotype in CR (B), PR (C), and all (D) patients. CR represents patients with complete response or complete response unconfirmed. PR represents patients with partial response. Other represents patients without either anti-Id antibodies or FcγRIIIa 158 V/V genotype. TTP indicates median time to progression.
Kaplan-Meier estimates of progression-free survival by response to induction chemotherapy, and humoral immune response and V/V genotype. Progression-free survival curves were plotted by response to induction chemotherapy (A), or by humoral anti-Id immune responses and/or FcγRIIIa 158 V/V genotype in CR (B), PR (C), and all (D) patients. CR represents patients with complete response or complete response unconfirmed. PR represents patients with partial response. Other represents patients without either anti-Id antibodies or FcγRIIIa 158 V/V genotype. TTP indicates median time to progression.
Prognostic factors for freedom from progression: Cox proportional hazard model
| . | Relative benefit* . | CI . | P† . |
|---|---|---|---|
| 158 V/V | 4.41 | 1.90-10.25 | <.001 |
| 131 H/H | 0.68 | 0.41-1.13 | .139 |
| Humoral immune response | 2.64 | 1.57-4.42 | <.001 |
| Cellular immune response | 0.61 | 0.35-1.05 | .075 |
| CR | 2.23 | 1.36-3.64 | .001 |
| Use of adriamycin | 0.67 | 0.37-1.21 | .183 |
| Use of fludarabine | 1.27 | 0.72-2.26 | .416 |
| Stage IV | 0.69 | 0.40-1.18 | .177 |
| Clinical symptoms‡ | 1.34 | 0.63-2.86 | .453 |
| Age | 0.98 | 0.96-1.01 | .182 |
| Time from diagnosis to induction chemotherapy | 1.00 | 1.00-1.01 | .051 |
| GM-CSF adjuvant | 0.67 | 0.33-1.35 | .259 |
| High/intermediate FLIPI§ | 0.45 | 0.19-1.02 | .060 |
| . | Relative benefit* . | CI . | P† . |
|---|---|---|---|
| 158 V/V | 4.41 | 1.90-10.25 | <.001 |
| 131 H/H | 0.68 | 0.41-1.13 | .139 |
| Humoral immune response | 2.64 | 1.57-4.42 | <.001 |
| Cellular immune response | 0.61 | 0.35-1.05 | .075 |
| CR | 2.23 | 1.36-3.64 | .001 |
| Use of adriamycin | 0.67 | 0.37-1.21 | .183 |
| Use of fludarabine | 1.27 | 0.72-2.26 | .416 |
| Stage IV | 0.69 | 0.40-1.18 | .177 |
| Clinical symptoms‡ | 1.34 | 0.63-2.86 | .453 |
| Age | 0.98 | 0.96-1.01 | .182 |
| Time from diagnosis to induction chemotherapy | 1.00 | 1.00-1.01 | .051 |
| GM-CSF adjuvant | 0.67 | 0.33-1.35 | .259 |
| High/intermediate FLIPI§ | 0.45 | 0.19-1.02 | .060 |
CI indicates 95% confidence interval.
Relative benefit indicates longer freedom from progression from last chemotherapy.
All P values are 2 sided and considered to be statistically significant for P < .05.
Clinical symptoms are fever, night sweats, and weight loss.
Analyzed on 127 patients with available FLIPI score.
The clinical impact of anti-Id Abs and V/V genotype in different chemotherapy response groups
We have previously shown that anti-Id antibody response and/or V/V genotype correlated with longer TTP.7 This result suggested the importance of vaccine-induced antitumor antibodies and that these antibodies mediate tumor killing more efficiently with effector cells bearing the FcγRIIIa of V/V genotype. Here, we analyzed the correlation of anti-Id Abs and V/V genotype together with clinical outcome in different chemotherapy response subgroups. The TTP of CR patients with anti-Id Abs and/or V/V genotype (total of 45 patients) was longer than CR patients with neither (10.47 vs 3.46 years, P = .012), with estimated 5-year PFS of 69% and 40%, respectively (Figure 1B). Similarly, PR patients with anti-Id Abs and/or V/V genotype (total of 19 patients) also had a longer TTP than patients with neither (not reached vs 1.31 years, P < .001), with 5-year PFS of 57% and 17%, respectively (Figure 1C). The observations in both animal models and early clinical studies suggest that vaccines may be more effective in the setting of minimal residual disease.14 Therefore, it was predicted that clinical impact of Id vaccination would have been more pronounced in CR patients.6 In contrast to the prediction, prolonged TTP associated with anti-Id Abs and/or V/V genotype was clearly shown in both CR and PR patients (Figure 1B, 1C). In fact, the prolonged TTP was even more profound in PR patients. The PR patients who had anti-Id Abs and/or V/V genotype had an identical survival curve compared with CR patients with anti-Id Abs and/or V/V genotype (TTP: not reached vs 10.47 years, P = .899). In contrast, for those who had neither, PR did significantly worse than CR patients (TTP: 1.31 vs 3.46 years, P = .004) (Figure 1D). This suggested that antibody response may be able to control the gross residual disease and delay tumor progression in PR patients. It is the first time that a potential benefit of idiotype vaccination has been implicated in a large number of patients with gross disease. In contrast, the anti-Id cellular immune response had no impact on TTP in either CR or PR patients (data not shown). In fact, patients with cellular immune response showed a trend toward a worse outcome (Table 1). The biologic explanation for this observation is not clear. One possibility is that our assay measured CD4+ T-cell response but did not measure the potentially antitumor cytotoxic CD8+ T cells.
The FLIPI scores were available on 127 patients. Patients of different risk groups did not differ in their chemotherapy response or in their anti-Id immune responses (Table S3). However, low-risk patients had longer TTP than intermediate/high-risk patients (Figure S1A). Additionally, in the intermediate/high-risk patients, anti-Id Abs and/or V/V genotype was associated with a longer TTP (10.47 vs 2.18 years, P < .001), while no difference was found within the low-risk patients (Figure S1B-C). This result further confirmed the association of better outcome with anti-Id Abs and/or V/V genotype in patients with unfavorable clinical features. In a multivariate analysis, CR along with anti-Id Abs and FcγRIIIa 158 V/V genotype emerged as 3 independent positive predictors for longer PFS, whereas none of the others had an impact (Table 1).
Our data support a model in which antitumor antibody-dependent cell-mediated cytotoxicity (ADCC) plays an important role in the antitumor effect of Id vaccination in both CR and PR patients. Therefore, in contrast to previous assumptions, idiotype vaccination should not be limited to patients with minimal residual disease. While some patients may have gross diseases after induction, the vaccine-induced antibody response may be able to control the disease and delay tumor progression and the need for subsequent treatment. None of the patients in this report received rituximab during their prevaccination cytoreduction. The clinical effect of Id vaccination may differ in rituximab-treated patients.15
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Kai Weng, Division of Oncology CCSR 1105, 269 Campus Dr, Stanford University School of Medicine, Stanford, CA 94305-5306; e-mail: wkweng@stanford.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work was supported by grants CA34233 and CA33399 from the US Public Health Service–National Institutes of Health (NIH). W.K.W. is recipient of an NIH-NCI K08 Award CA111827. R.L. is an American Cancer Society Clinical Research Professor.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal