Abstract
The thymus provides a specialized site for the production of T cells capable of recognizing foreign antigens in the context of self–major histocompatibility complex (MHC) molecules. During development, the thymus arises from an epithelial rudiment containing bipotent progenitors that differentiate into distinct cortical and medullary epithelial cells to regulate the maturation and selection of self-tolerant CD4+ and CD8+ T cells. In addition to their differentiation, thymic epithelial cells undergo cellular expansion to ensure that sufficient intrathymic cellular niches are available to support the large number of immature thymocytes required to form a self-tolerant T-cell pool. Thus, intrathymic T-cell production is intimately linked to the formation and availability of niches within thymic microenvironments. Here, we show the increase in intrathymic niches caused by the proliferation of the epithelium in the developing thymus is temporally regulated, and correlates with the presence of a population of fetal thymic mesenchyme defined by platelet-derived growth factor receptor α (PDGFRα) expression. Depletion of PDGFRα+ mesenchyme from embryonic thymi prior to their transplantation to ectopic sites results in the formation of functional yet hypoplastic thymic tissue. In summary, we highlight a specialized role for PDGFRα+ fetal mesenchyme in the thymus by determining availability of thymic niches through the regulation of thymic epithelial proliferation.
Introduction
The production of a functionally competent peripheral T-cell pool with a diverse T-cell receptor (TCR) repertoire is essential in mounting an effective immune response to invading pathogens. Most, if not all, peripheral CD4+ and CD8+ T cells bearing the αβ form of the TCR complex are generated in the thymus from migrant progenitors that arise in the fetal liver and bone marrow.1,2 Although recent evidence shows that these progenitors may show some degree of commitment to the T-cell lineage prior to thymus colonization,3-5 entry into the thymus and contact with the specialized microenvironment provided by thymic stromal cells is required to induce further development along the T lineage. This development involves a stepwise program of differentiation, with early development characterized by progression through the double-negative (DN) 1 to 4 stages that are defined by CD44 and CD25 expression.6-8 During these early phases, DN T-cell precursors undergo commitment to the αβ or γδ lineage, and in the case of the former, signaling through the pre-TCR complex is required for maturation beyond the DN3 stage and progression to the CD4+CD8+ stage, where the αβ TCR is first expressed.9 As somatic recombination of TCRα and TCRβ chain genes occurs randomly, CD4+CD8+ thymocytes are then screened on the basis of their αβ TCR specificity by positive and negative events before further maturation into the CD4+ and CD8+ lineages.10 Ultimately, these developmental processes result in the formation of self-tolerant major histocompatibility complex (MHC) class I–restricted CD8+ and MHC class II–restricted CD4+ T cells that leave the thymic medulla and contribute to the peripheral T-cell pool.11,12
There is now accumulating evidence that the epithelial compartment of the thymic stroma plays a crucial role in supporting these developmental processes. This includes the expression of Notch ligands13,14 that interact with Notch on DN thymocytes to provide essential maturation signals15-17 as well as the production of secreted factors, including Wnts,18 interleukin-7 (IL-7),19 and stem cell factor (SCF),20 that also play key roles during thymocyte development. Moreover, thymic epithelial cells are heterogeneous, consisting of subcapsular, cortical, and medullary subsets that are likely to provide specialized microenvironments to support particular stages of T-cell development.1,21 For example, cortical epithelial cells a play key role in positive selection; the availability of cortical niches have been shown to be a limiting factor in this process.22,23 On the other hand, the medullary epithelium and dendritic cells play specialized roles in negative selection24,25 and may influence the development of regulatory T cells.26 In this context, developmental abnormalities affecting different thymic epithelial cell compartments have been shown to result in T-cell disorders such as autoimmunity27,28 and T-cell immunodeficiency,29,30 emphasizing the crucial role of thymic epithelial cell populations in the development of an extensive peripheral T-cell repertoire that is nonreactive to self antigen.
In this study, we have investigated the mechanisms regulating the development of thymic epithelial cells leading to the formation of functionally competent thymic microenvironments. We show that during normal development, thymus growth involves a temporally regulated phase of thymic epithelial proliferation, resulting in an increase in the number of stromal-cell niches able to support thymocyte development. In addition, we have defined a population of nonepithelial platelet-derived growth factor receptor α–positive (PDGFRα+) fetal mesenchymal cells and shown that the presence and developmental kinetics of this specialized subset of stromal cells correlates with the pattern of epithelial proliferation, consistent with an important role for these cells as a source of signals regulating normal thymus growth. We also present in vivo functional evidence that, in the absence of such mesenchyme, the differentiation of thymic epithelial subsets from bipotent progenitors recently shown to be present in the embryonic day (E) 12 thymus rudiment31 is initiated normally, but the thymus remains hypoplastic. Such hypoplastic thymi support a normal pattern of T-cell development but with greatly reduced overall numbers of thymocytes, reflecting the limited availability of epithelial support or niches for thymocyte development and selection. In summary, our data define a crucial role for thymic mesenchyme in the determining thymus size and capacity for T-cell production by regulating the extent of the epithelial microenvironments essential for the development of T-cell progenitors into functional T cells.
Materials and methods
Mice
BALB/c (H-2d) mice were bred and maintained under specific pathogen–free (SPF) conditions in the Biomedical Services Unit at the University of Birmingham. The day of vaginal plug detection was designated as day 0.
Antibodies, immunoconjugates, and flow cytometry
The following were used for flow cytometric analysis: anti–CD4-PE (RM4-5), anti-CD8–fluorescein isothiocyanate (FITC) (53-6.7), biotin-conjugated anti-PDGFRα (APA5), and biotin-conjugated anti-Ly51 (clone 6C3). All were supplied by eBioscience (San Diego, CA) and were followed by streptavadin PE (BD Pharmingen, San Diego, CA) and anti-CD45–FITC (30-F11; BD Pharmingen). APC-conjugated anti-EpCAM1 (epithelial cell adhesion molecule, clone G8.8; kind gift from Dr Moon Kim, Inha University, Incheon, Korea) and FITC-conjugated anti–pan-cyokeratin (clone C-11) was supplied by Sigma (St Louis, MO). Flow cytometric analysis was performed using a dual-laser LSR machine (Becton Dickinson, San Jose, CA), with forward/side scatter gates set to exclude nonviable cells.
Analysis of thymic epithelial proliferation
Thymus lobes were dissected from mice of the indicated ages and disaggregated using 0.25% trypsin (Sigma). Suspensions of cells from neonatal thymi were stained with APC-conjugated anti-CD45 to aid in the identification of CD45− stroma. Cells were permeabilized using the eBioscience Fixation and Permeabilization kit. FITC-conjugated anti–pan-cytokeratin and PE-conjugated anti-Ki67 (clone B56; BD Pharmingen) were used to detect thymic epithelial proliferation.
Immunohistochemistry
Frozen sections of thymic grafts, recovered from under the kidney capsule after 3 weeks, were prepared as described.32 Tissue sections of 5 μm thickness were fixed in ice-cold acetone, and stained with either anti–cytokeratin 5 (Covance, Berkeley, CA) and anti–cytokeratin 8 (clone LE41; kind gift from B. Lane, University of Dundee, Scotland) or the panmesenchyme marker ERTR7 (kind gift from W. van Ewijk, Leiden University Medical Centre, the Netherlands). Primary antibodies were detected using anti–rabbit Alexa Fluor 350 (Molecular Probes, Eugene, OR), anti–mouse FITC (Caltag, Burlingham, CA) or anti–rat FITC (Southern Biotech, Birmingham, AL), respectively. Sections were mounted in antifade glycerol solution (Citifluor, Canterbury, United Kingdom). Sections were viewed using a Zeiss Axioplan microscope (Welwyn Garden City, United Kingdom). Tissue sections were analyzed using an Axioplan 2 microscope (Zeiss, Jena, Germany) fitted with Zeiss Plan Neofluar objectives (20×/0.50 and 40×/1.3). Images were captured using a Hamamatsu Orca-ER camera (Welwyn Garden City, United Kingdom), and analyzed using SmartCapture X software version 2.5.9 (Digital Scientific, Cambridge, United Kingdom).
Analysis of the cellular compartments of the E12 thymus
E12 thymic lobes with surrounding mesenchyme still attached were dissected with the aid of a stereo-dissecting microscope and no. 5 Watchmakers forceps (Taab, Aldermaston, United Kingdom). Freshly isolated lobes were disaggregated by incubation in 0.25% trypsin and then labeled with antibodies to EpCAM1, PDGFRα, and CD45, as described.5
Preparation of mesenchyme-free thymic epithelial rudiments for transplantation
To remove the surrounding perithymic mesenchyme from the inner epithelial core, E12 thymus lobes were incubated at 37°C for 20 minutes in 2.5 mg/mL collagenase D (R&D Systems, Minneapolis, MN) in Ca2+/Mg2+-free PBS. Under direct visual observation using a dissecting microscope, lobes were then drawn into a fine, mouth-controlled glass pipette to separate surrounding mesenchyme from the inner epithelial rudiment. To control for the possibility that removal of perithymic mesenchyme damages the inner epithelial core, stripped epithelial rudiments were reassociated overnight with E12 perithymic mesenchyme, and then grafted under the kidney capsule as described below, under “Kidney capsule transplantation.” In some experiments, mesenchyme-stripped and intact E12 thymus lobes were transferred into fresh solutions of 0.25% trypsin in 0.02% EDTA to produce a single-cell suspension for flow cytometric analysis.
Kidney capsule transplantation
Whole E12 thymus lobes, or lobes with perithymic mesenchyme removed, were placed under the renal capsules of 4- to 6-week-old syngeneic mice, as described.33 After 3 weeks, grafts were harvested and analyzed for thymocyte cellularity and T-cell development, or by immunohistochemistry, as appropriate.
Detection of host-derived mesenchyme in thymus grafts
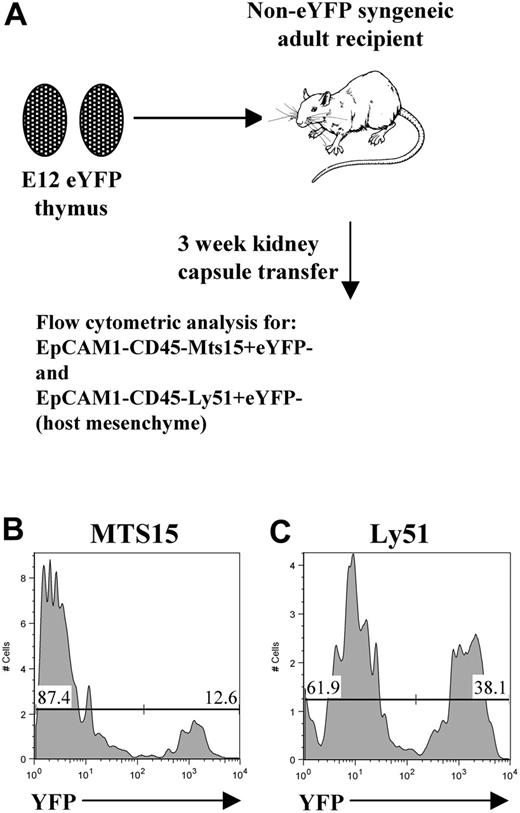
To identify the presence of host-derived mesenchyme cells in grafted thymus tissue, E12 thymus lobes were isolated from enhanced yellow fluorescent protein (eYFP) transgenic mice as described31 and transplanted under the kidney capsules of wild-type (WT) syngeneic mice. After 3 weeks, grafts were harvested and digested with trypsin, and stained with anti-CD45 and anti-EpCAM1 together with Mts15 (a kind gift from R. Boyd and D. Gray, Monash University, Melbourne, Australia) or anti-Ly51, antibodies that react with mesenchyme cells,33,34 allowing the identification of host-derived (eYFP−CD45−EpCAM1−Mts15+ and eYFP−CD45−EpCAM1−Ly51+) mesenchyme.
Semiquantitative reverse transcriptase–PCR analysis
PDGFRα+ thymic mesenchyme was prepared from disaggregated E12 thymus lobes, as described above, under “Preparation of mesenchyme-free thymic epithelial rudiments for transplantation.” For adult kidney capsule–derived mesenchyme, the mesenchymal capsule was carefully peeled away from excised 4- to 6-week-old adult kidneys, and small pieces were placed in 6-well tissue-culture plates. After 48 hours, nonadherent cells were removed, and remaining adherent cells were cultured in DMEM containing 10% FCS. To obtain high-purity cDNA, mRNA was purified from cells using μMacs One-step cDNA kit (Miltenyi Biotech, Auburn, CA). β-actin was used as the housekeeping gene for sample normalization, prior to amplifying the target genes of interest. Reactions were conducted in a Peltier Thermal Cycler PTC-200 (MJ Research, Genetic Research Instrumentation, Braintree, Essex, United Kingdom), as described5 where during cycling, 3 10-μL samples were removed from each reaction in cycling intervals; the range of cycle numbers depended on the gene. Polymerase chain reaction (PCR) products were analyzed by ethidium bromide gel electrophoresis and identified by fragment size. Densitometrical analysis was determined using Syngene Gel Documentation Gene Tools software (Cambridge, United Kingdom). Graphs show ratios of mRNA for the genes of interest relative to β-actin. Error bars show SEM of the ratios.
Primer sequences and amplicon sizes were as follows: β-actin, forward 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ and reverse 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′ (349 bp); FGF-7, forward 5′-CTC TAC AGG TCA TGC TTC CAC C-3′ and reverse 5′-ACA GAA CAG TCT TCT CAC CCT -3′ (174 bp); FGF-10, forward 5′-CAG CGG GAC CAA GAA TGA AG -3′ and reverse 5′-TGA CGG CAA CAA CTC CGA TTT -3′ (77 bp); IGF-1, forward 5′-CAG GCT ATG GCT CCA GCA TTC GG-3′ and reverse 5′-CAG ATC ACA GCT CCG GAA GC-3′ (77 bp); IGF-2, forward 5′-GAG CTT GTT GAC ACG CTTCAG TTT GTC -3′ and reverse 5′-ACG TTT GGC CTC TCT GAA CTC TTT GAG -3′ (357 bp); and IGF-1R, forward 5′-GAC ATC CGC AAC GAC TAT CAG-3′ and reverse 5′-GTA GTT ATT GGA CAC CGC ATC-3′ (393 bp).
Results
Temporal regulation of thymic epithelial proliferation
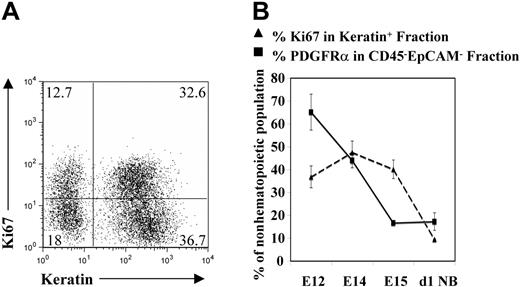
The early fetal thymus consists of an endodermally derived epithelial outbudding of the third pharyngeal pouch that is encapsulated by condensing neural crest–derived mesenchyme.5,35 At around E12 of gestation, when the thymic rudiment is undergoing the first wave of progenitor colonization, cortical and medullary microenvironments distinctive of the postnatal thymus are yet to form.5,35,36 Epithelial cells within this early rudiment are still immature and include bipotent progenitors for cortical and medullary epithelial subsets.31 To investigate the events leading to the formation of the definitive thymic epithelial microenvironment, we first examined the proliferative status of cells within the epithelial component of the thymus at successive stages of ontogeny. Flow cytometric analysis was carried out on disaggregated thymus cell suspensions using colabeling with the panepithelial marker cytokeratin and the proliferation marker Ki6737 to allow quantitative analysis of proliferation specifically within the epithelial component of the thymus (Figure 1A). Using this approach, the proportion of epithelial cells in cell cycle was found to vary during thymus ontogeny, with peak proliferation of cytokeratin+ cells observed at E14, followed by a gradual decrease until only a small proportion of epithelial cells were Ki67+ in the neonatal thymus (Figure 1B). Thus, while the early fetal thymus contains a high proportion of proliferating epithelial cells, this decreases with increasing developmental age.
Thymic epithelial cells undergo a temporally regulated phase of cellular proliferation. Freshly dissected thymus lobes were disaggregated and analyzed by flow cytometry to detect expression of cytokeratin and the proliferation marker Ki67. (A) A typical example of an E14 thymus preparation, with double-positive cytokeratin+Ki67+ cells representing proliferating thymic epithelial cells. Figures in quadrants represent the percentage of the analyzed population. (B) The percentages of cytokeratin+Ki67+ cells present in the thymi of the indicated ages, together with the percentage of PDGFRα+ mesenchyme within the nonhemopoeitic nonepithelial (CD45−EpCAM1−) thymic fraction. Results are averaged from at least 3 independent experiments, and are presented with standard deviations (error bars).
Thymic epithelial cells undergo a temporally regulated phase of cellular proliferation. Freshly dissected thymus lobes were disaggregated and analyzed by flow cytometry to detect expression of cytokeratin and the proliferation marker Ki67. (A) A typical example of an E14 thymus preparation, with double-positive cytokeratin+Ki67+ cells representing proliferating thymic epithelial cells. Figures in quadrants represent the percentage of the analyzed population. (B) The percentages of cytokeratin+Ki67+ cells present in the thymi of the indicated ages, together with the percentage of PDGFRα+ mesenchyme within the nonhemopoeitic nonepithelial (CD45−EpCAM1−) thymic fraction. Results are averaged from at least 3 independent experiments, and are presented with standard deviations (error bars).
Declining thymic epithelial proliferation correlates with a loss of thymic PDGFRα+ mesenchyme
To investigate possible mechanisms regulating this temporal pattern of epithelial cell proliferation in the developing thymus, we next analyzed the presence of other cell types that could influence this process. Flow cytometric analysis of disaggregated preparations of E12 thymic rudiments shows that few CD45+ progenitors are present in the thymus at this early stage (Figure 2A), consistent with our previous observations arguing against a role for hemopoietic cells in providing signals regulating initial epithelial proliferation in the fetal thymus.38,39 Further analysis of the E12 thymus found it to consist predominantly of 2 CD45− stromal cell types: cytokeratin+ epithelial cells, and cytokeratin− cells, with most of the latter staining positive for expression of the neural crest–derived mesenchymal marker PDGFRα (Figure 2B). Notably, ontogenetic analysis of this PDGFRα+ population of mesenchyme in the thymus revealed a temporal reduction in its contribution to the nonhematopoietic nonepithelial component of the thymus (Figure 1B), which correlated closely with the reduction in thymic epithelial proliferation described in the previous section. While the above data show that PDGFRα can be used as a useful marker to identify mesenchymal cells, it is not clear whether PDGFRα expression is functionally significant, although it is interesting to note that patched mice, which carry a natural mutation in pdgfra, have a smaller thymus compared with that of littermate controls.40 Whatever the case, the relationship described here between the presence of PDGFRα+ mesenchyme and thymic epithelial proliferation is strongly suggestive of a role for these cells in the development of thymic epithelial microenvironments to provide increased numbers of niches for developing thymocytes.
The E12 thymus anlagen has 3 distinct cellular compartments. E12 thymus lobes were disaggregated and analyzed by flow cytometry for expression of the panhemopoietic marker CD45 (A), and the panepithelial marker cytokeratin together with the mesenchyme marker PDGFRα (B). Note that most cells at this stage are CD45− stromal cells, consisting of 2 dominant cytokeratin+PDGFRα− epithelial and cytokeratin−PDGFRα+ mesenchymal subsets. Data shown are representative of 4 separate experiments. Figures in quadrants are representative of the percentage of the analyzed population.
The E12 thymus anlagen has 3 distinct cellular compartments. E12 thymus lobes were disaggregated and analyzed by flow cytometry for expression of the panhemopoietic marker CD45 (A), and the panepithelial marker cytokeratin together with the mesenchyme marker PDGFRα (B). Note that most cells at this stage are CD45− stromal cells, consisting of 2 dominant cytokeratin+PDGFRα− epithelial and cytokeratin−PDGFRα+ mesenchymal subsets. Data shown are representative of 4 separate experiments. Figures in quadrants are representative of the percentage of the analyzed population.
PDGFRα+ thymic mesenchyme regulates growth but not differentiation of thymic epithelial microenvironments
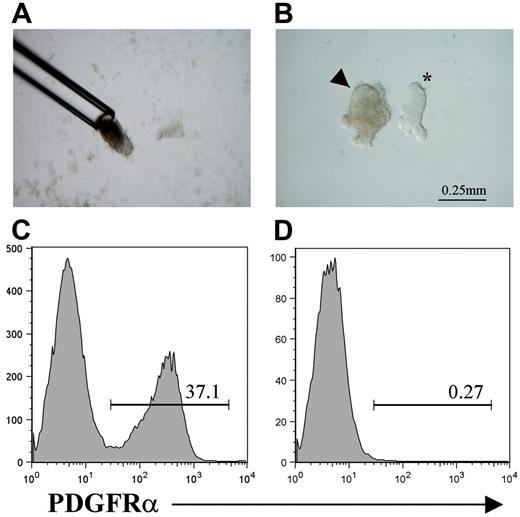
To test the hypothesis that PDGFRα+ fetal thymus mesenchyme plays a specific role in regulating thymic epithelial cell development in vivo, we prepared mesenchyme-free thymus rudiments by removing the surrounding perithymic mesenchyme from isolated E12 thymus lobes. Thus, E12 thymus lobes were treated briefly with collagenase to loosen the surrounding mesenchyme, which was then completely removed by drawing the lobes up into a mouth-controlled fine capillary pipette (Figure 3A). Such an approach results in the separation of surrounding mesenchyme, leaving an intact core of thymic epithelium (Figure 3B). Importantly, when epithelial cores prepared in this way were further disaggregated to form a single-cell suspension, in contrast to whole-thymus preparations (Figure 3C), they were found to be devoid of PDGFRα+ mesenchyme (Figure 3D), indicating the efficiency of the separation to isolate mesenchyme-free thymic epithelial rudiments.
Preparation of mesenchyme-free thymic epithelial rudiments. To separate the mesenchymal and epithelial components of the E12 thymus, lobes were incubated briefly in collagenase and then drawn up into mouth-controlled glass pipette (A), which shears surrounding mesenchyme, resulting in a smooth epithelial core (B) (arrowhead). *An unmanipulated E12 thymus lobe with surrounding mesenchyme still attached is shown for comparison (B). Compared with cell suspensions from whole E12 thymus lobes (C), epithelial rudiments prepared in this way are devoid of cells expressing the mesenchyme marker PDGFRα (D). Data shown are typical of 3 separate experiments.
Preparation of mesenchyme-free thymic epithelial rudiments. To separate the mesenchymal and epithelial components of the E12 thymus, lobes were incubated briefly in collagenase and then drawn up into mouth-controlled glass pipette (A), which shears surrounding mesenchyme, resulting in a smooth epithelial core (B) (arrowhead). *An unmanipulated E12 thymus lobe with surrounding mesenchyme still attached is shown for comparison (B). Compared with cell suspensions from whole E12 thymus lobes (C), epithelial rudiments prepared in this way are devoid of cells expressing the mesenchyme marker PDGFRα (D). Data shown are typical of 3 separate experiments.
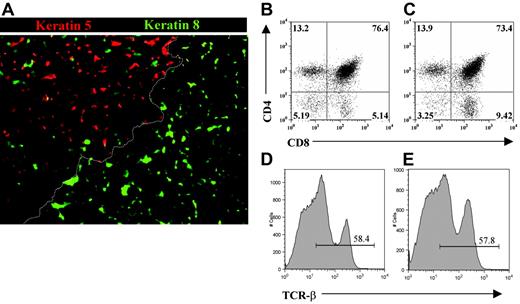
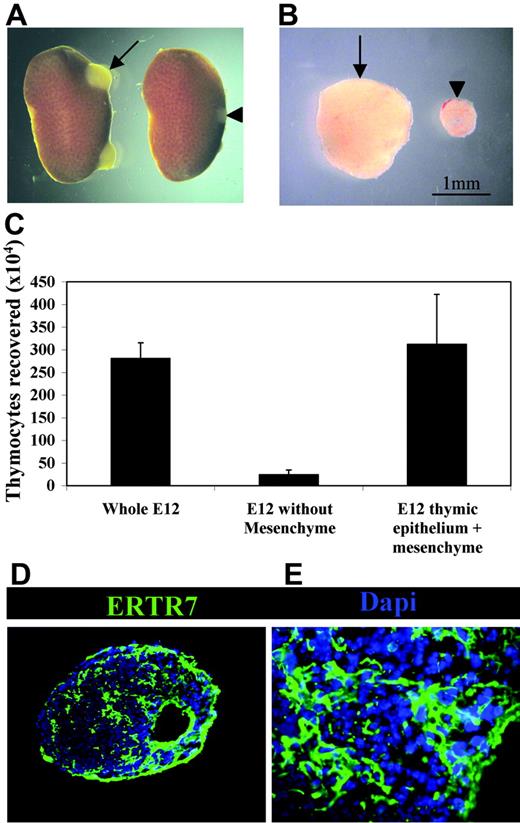
To directly investigate the involvement of PDGFRα+ fetal thymic mesenchyme in thymus development and growth under in vivo conditions, we grafted both intact and mesenchyme-free E12 thymus lobes under the kidney capsules of adult syngeneic recipients. At this developmental stage, epithelial cells are immature and largely of a keratin 5+8+ double-positive phenotype.38,41 When grafts were harvested after 3 weeks, as in unmanipulated grafts (not shown), epithelial cells within mesenchyme-stripped thymi were found to have differentiated into distinct keratin 5−8+ cortical and keratin 5+8− medullary subsets together with the appearance of organized cortical and medullary areas (Figure 4A). Moreover, both intact and mesenchyme-stripped grafted thymi were able to support the maturation of T-cell precursors into CD4+CD8+ and more mature CD4+CD8− and CD4−CD8+ subsets expressing the αβ TCR complex (Figure 4B-E). Thus, the ability to form organized cortical and medullary microenvironments that support the development of T-cell precursors indicates that the presence of fetal PDGFRα+ mesenchyme cells are not required for differentiation of immature thymic epithelial cells. Critically, however, mesenchyme-depleted thymic epithelial rudiments were hypoplastic with a marked absence of growth compared with lobes containing fetal mesenchyme (Figure 5A-B), with hypoplasia being associated with a profound reduction in total thymocyte numbers (Figure 5C). The observed lack of growth of mesenchyme-stripped thymic rudiments was not due to technical issues caused by the enzymatic separation procedure, as stripped lobes reassociated with fetal mesenchyme grew effectively in vivo and upon harvesting were found to have a thymocyte cellularity comparable with that of unmanipulated grafts (Figure 5C). Furthermore, this lack of thymus growth was not due to a lack of mesenchyme per se within the grafted tissue, as immunohistochemical analysis using the panfibroblast marker ERTR7 showed that mesenchymal cells were readily detectable within stripped thymus lobes (Figure 5D-E). To provide direct evidence for the presence of host-derived mesenchyme in thymus grafts, we transplanted eYFP transgenic E12 thymus lobes under the kidney capsules of WT adult mice. When used in association with anti-CD45 and anti-EpCAM1 to exclude haemopoeitic and epithelial cells, analysis with 2 antibodies shown to react with mesenchyme cells (MTS1533 and Ly5134 ) showed the presence of host-derived (eYFP−) mesenchyme within the graft (Figure 6). Collectively, these findings suggest that there is a specific requirement for PDGFRα+ fetal thymic mesenchyme in the expansion of fetal thymic epithelial cells in order to provide sufficient stromal niches for increasing numbers of developing thymocytes.
Thymic epithelial progenitors generate functional and organized microenvironments in the absence of PDGFRα-expressing fetal mesenchyme. Mesenchyme-free E12 thymus rudiments, placed under the kidney capsule for 3 weeks, were analyzed by immunohistochemistry for expression of the cortical epithelial marker cytokeratin 8 and the medullary epithelial marker cytokeratin 5 (A). Note that distinct cytokeratin 5−8+ cortical and K5+8− medullary areas are present and are separated by a cortico-medullary junction (dotted line). Thymocytes harvested from whole (B,D) or mesenchyme-stripped (C,E) E12 thymus grafts were analyzed by flow cytometry for expression of CD4 and CD8, and the αβ T-cell receptor. Results are representative of least 3 independent experiments. Figures in quadrants represent the percentage of the analyzed population.
Thymic epithelial progenitors generate functional and organized microenvironments in the absence of PDGFRα-expressing fetal mesenchyme. Mesenchyme-free E12 thymus rudiments, placed under the kidney capsule for 3 weeks, were analyzed by immunohistochemistry for expression of the cortical epithelial marker cytokeratin 8 and the medullary epithelial marker cytokeratin 5 (A). Note that distinct cytokeratin 5−8+ cortical and K5+8− medullary areas are present and are separated by a cortico-medullary junction (dotted line). Thymocytes harvested from whole (B,D) or mesenchyme-stripped (C,E) E12 thymus grafts were analyzed by flow cytometry for expression of CD4 and CD8, and the αβ T-cell receptor. Results are representative of least 3 independent experiments. Figures in quadrants represent the percentage of the analyzed population.
PDGFRα-expressing fetal mesenchyme regulates thymus growth and the availability of intrathymic niches. (A) Kidneys excised from mice that 3 weeks earlier received either whole unmanipulated (arrow) or mesenchyme-stripped (arrowhead) E12 thymus grafts under the renal capsule. Note the increased growth achieved in unmanipulated versus stripped thymus grafts (B). Importantly, grafts formed from epithelial cores reassociated with mesenchyme prior to transplantation were found to grow and contain similar thymocyte numbers in a manner comparable with unmanipulated thymus lobes. Grafts of unmanipulated of mesenchyme-stripped thymus lobes were analyzed for either thymocyte cellularity (C) or by immunohistochemistry using ERTR7 to identify host-derived mesenchyme that had invaginated the graft (D-E). Data shown are typical of 3 separate experiments. Results are averaged from at least 3 independent experiments, and are presented with standard deviations (error bars).
PDGFRα-expressing fetal mesenchyme regulates thymus growth and the availability of intrathymic niches. (A) Kidneys excised from mice that 3 weeks earlier received either whole unmanipulated (arrow) or mesenchyme-stripped (arrowhead) E12 thymus grafts under the renal capsule. Note the increased growth achieved in unmanipulated versus stripped thymus grafts (B). Importantly, grafts formed from epithelial cores reassociated with mesenchyme prior to transplantation were found to grow and contain similar thymocyte numbers in a manner comparable with unmanipulated thymus lobes. Grafts of unmanipulated of mesenchyme-stripped thymus lobes were analyzed for either thymocyte cellularity (C) or by immunohistochemistry using ERTR7 to identify host-derived mesenchyme that had invaginated the graft (D-E). Data shown are typical of 3 separate experiments. Results are averaged from at least 3 independent experiments, and are presented with standard deviations (error bars).
E12 thymus grafts contain host-derived mesenchyme. To determine the origin of mesenchymal cells present in thymus grafts, E12 thymus lobes from eYFP transgenic mice were transplanted into non-eYFP WT adult hosts (A). Grafts were harvested after 3 weeks, and mesenchyme cells were identified on the basis of an EpCAM1−CD45− phenotype to exclude all hemopoietic and epithelial cells, together with the markers Ly51 and MTS15. (B-C) Host-derived eYFP−MTS15+ and eYFP−Ly51+ mesenchyme cells are readily detectable in the thymus grafts. Data shown are representative of at least 3 separate experiments.
E12 thymus grafts contain host-derived mesenchyme. To determine the origin of mesenchymal cells present in thymus grafts, E12 thymus lobes from eYFP transgenic mice were transplanted into non-eYFP WT adult hosts (A). Grafts were harvested after 3 weeks, and mesenchyme cells were identified on the basis of an EpCAM1−CD45− phenotype to exclude all hemopoietic and epithelial cells, together with the markers Ly51 and MTS15. (B-C) Host-derived eYFP−MTS15+ and eYFP−Ly51+ mesenchyme cells are readily detectable in the thymus grafts. Data shown are representative of at least 3 separate experiments.
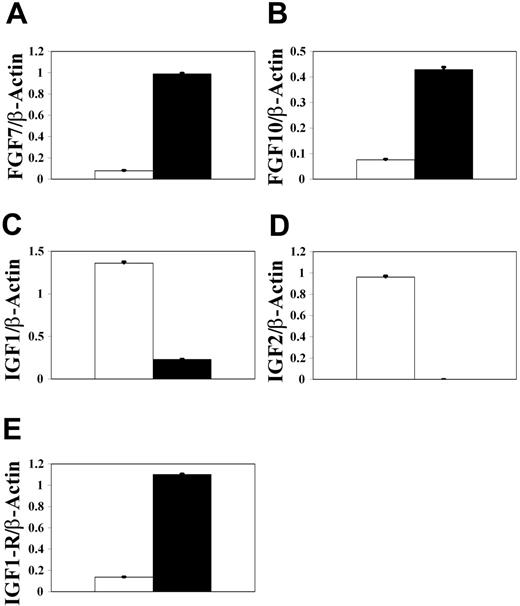
The presence of adult host-derived mesenchyme in thymus grafts suggests that these cells are unable to provide the appropriate signals that regulate proliferation of E12 thymic epithelial progenitors, making them distinct from fetal PDGFRα+ mesenchyme cells. To try to identify possible regulators of epithelial progenitor proliferation, we used reverse transcriptase (RT)–PCR analysis to analyze gene expression in adult kidney-derived mesenchyme and E12 PDGFRα+ thymic mesenchyme. Interestingly, FGF7 and FGF10 mRNAs were detectable in kidney capsule mesenchyme, but not in E12 PDGFRα+ thymic mesenchyme (Figure 7), suggesting that the lack of growth of E12 thymic epithelium is not due to the lack of availability of FGF7 and FGF10. Such an observation is significant, as we have shown previously that mesenchyme cells present in the E14 thymus provide fibroblast growth factors (FGFs) that regulate epithelial cell proliferation at this later stage. The lack of detectable expression of FGF7 and FGF10 by E12 mesenchyme suggests that a different mechanism regulates the proliferation of bipotent epithelial progenitors present in the E12 thymus.31 Indeed, when we next analyzed expression of IGF1 and IGF2, factors known to be mitogenic for epithelial cells in several tissues,42,43 we found them to be readily detectable in E12 PDGFRα+ thymic mesenchyme but not in kidney capsule mesenchyme. Moreover, IGF1 and IGF2 expression by E12 thymic mesenchyme was found to correlate with expression of the IGF1 receptor by E12 thymic epithelial cells, suggesting a role for IGF-IGF1R in early thymus growth.
PDGFRα+ E12 thymic mesenchyme but not kidney capsule mesenchyme expresses insulinlike growth factors, which correlates with IGF1R expression by thymic epithelium. Semiquantitative RT-PCR was used to compare expression of FGF7 (A), FGF10 (B), IGF1 (C), and IGF2 (D) in E12 PDGFRα+ thymic (▪) and adult kidney capsule mesenchyme (□). In addition, IGF1-receptor expression (E) was analyzed in thymic epithelium (▪) and PDGFRα+ mesenchyme (▪) from E12 thymus lobes. Note that expression of IGF1 and IGF2 mRNA by fetal thymic mesenchyme correlates with IGF1R expression by thymic epithelium. Data shown are representative of 3 separate experiments and are expressed as mean ± SEM.
PDGFRα+ E12 thymic mesenchyme but not kidney capsule mesenchyme expresses insulinlike growth factors, which correlates with IGF1R expression by thymic epithelium. Semiquantitative RT-PCR was used to compare expression of FGF7 (A), FGF10 (B), IGF1 (C), and IGF2 (D) in E12 PDGFRα+ thymic (▪) and adult kidney capsule mesenchyme (□). In addition, IGF1-receptor expression (E) was analyzed in thymic epithelium (▪) and PDGFRα+ mesenchyme (▪) from E12 thymus lobes. Note that expression of IGF1 and IGF2 mRNA by fetal thymic mesenchyme correlates with IGF1R expression by thymic epithelium. Data shown are representative of 3 separate experiments and are expressed as mean ± SEM.
Discussion
Thymic cortical and medullary epithelial cells provide specialized microenvironments to support the stepwise maturation of T-cell precursors that colonize the thymus from the blood. In this study, we have investigated the mechanisms that regulate thymus growth and the availability of intrathymic epithelial niches that support normal numbers of developing T-cell precursors. We find that the epithelial compartment in the early fetal thymus contains a high proportion of proliferating cells, a finding that links initial thymus growth with an increase in thymic epithelial numbers. This phase of epithelial proliferation in the early thymus, which correlates well with a rapid increase in total thymocyte numbers at these stages (Penit and Vasseur44 and data not shown), is likely to serve to increase the availability of intrathymic stromal niches required to support the large numbers of immature thymocytes generated by expansion of the first waves of thymus-colonizing T-cell precursors. In line with this possibility, we show that although thymic epithelial differentiation and organization into functional cortical and medullary microenvironments occur normally in the absence of thymus growth, the thymus remains small and does not contain sufficient intrathymic niches to support normal numbers of immature thymocytes. This finding has important implications for our understanding of the mechanisms regulating initial thymus development, and potentially thymus regeneration, as it suggests that the mechanisms regulating proliferation and differentiation of epithelial progenitors are distinct.
By analyzing cellular compartments of the early embryonic thymus, we identified a correlation between declining epithelial cell proliferation and the presence of PDGFRα+ mesenchyme cells. This suggests that the latter play an important role in providing proliferative signals to the thymic epithelium, a possibility supported by our finding that removal of PDGFRα+ mesenchyme prevented normal thymus growth in vivo. In addition, the presence in hypoplastic thymus grafts of adult host kidney capsule–derived mesenchyme, shown directly using transplants of eYFP fetal donor thymus grafts, suggests that fetal thymic PDGFRα+ mesenchyme are specialized in their ability to regulate thymus growth. While the nature of this specialization is not fully understood, we have previously shown using an in vitro approach that at E14 of gestation, fetal thymic mesenchyme produces growth factors such as FGF7 and FGF10 that can induce proliferation of FGFR2iiib-expressing thymic epithelial cells,38 a finding that correlates with reduced thymus size in FGFR2iiib-deficient mice.45 However, the question of whether FGFs play a role in the expansion of recently described bipotent epithelial progenitors that are present in the E12 thymus lobes used in this study has not been addressed. Indeed, semiquantitative PCR analysis shows that E12 PDGFRα+ thymic mesenchyme lacks detectable FGF7 and FGF10 expression, while kidney capsule mesenchyme expresses readily detectable levels of FGF7 and FGF10, which argues against the notion that E12 thymic epithelial grafts fail to growth because of a lack of local FGFs. Taken together with our earlier study,38 these observations suggest that while FGFs regulate proliferation of epithelial cells at later stages of development, a distinct mechanism regulates proliferation of bipotent epithelial progenitors in the E12 thymus. Indeed, in further analysis, we found that IGF1 and IGF2 mRNA detected in E12 PDGFRα+ fetal thymic mesenchyme, but not in adult kidney capsule mesenchyme, findings that correlate with the expression of IGF1-receptor mRNA by E12 thymic epithelium. Collectively, these observations suggest that IGF-IGF receptor interactions may play a role in the growth of the E12 thymus. Such a hypothesis is supported by the demonstration that IGFs play a role in the proliferation of epithelial cells in other tissues, such as the mammary gland,42,43 as well as in increased thymus size reported in transgenic mice overexpressing IGF2.46
Finally, our finding that there is a decline in PDGFRα+ thymic mesenchyme during thymus development fits well with other studies showing that the neural crest contribution to the thymus, which initially acts as an important source of perithymic mesenchyme in the early thymus, is difficult to detect by the time the neonatal period is reached.47-49 Collectively, these findings support the notion that neural crest–derived mesenchyme represents a transient thymic cell type whose presence in the thymus is temporally regulated and that determines the initial phase of embryonic thymus growth. Although a small population of PDGFRα+ mesenchyme is still present in the neonatal and adult thymus (Figure 1; W.E.J., unpublished observations, November 2005), it remains to be established whether these cells play any role in regulating epithelial cell proliferation in the postnatal period. In this context, strategies to stimulate re-expansion or functional reactivation of this population could promote thymus recovery following ablative therapy or age/disease-related involution. Alternatively, intrathymic transplantation of PDGFRα+ mesenchyme and/or identification of the products mediating the effects of these cells on epithelial proliferation may also provide strategies to restore thymic growth and thereby T-cell output.
Authorship
Author contributions: W.E.J., S.W.R., and S.M.P. designed and performed the research; E.J.J. and G.A. designed the research and wrote the paper.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Graham Anderson, MRC Centre for Immune Regulation, Institute for Biomedical Research, Birmingham University Medical School, Birmingham B15 2TT, United Kingdom; e-mail: g.anderson@bham.ac.uk.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Supported by the EU FP6 Thymaide Project, and an MRC Programme Grant to E.J.J. and G.A.
Acknowledgments
We are grateful to Drs M. Kim and A. Farr for provision of the anti-EpCAM1 antibody, and Terry Jones for expert technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal