Abstract
Children with acute myelogenous leukemia (AML) have a high risk of infectious complications that might be reduced by prophylactic granulocyte colony-stimulating factor (G-CSF). However, G-CSF could induce AML blast proliferation. The prospective randomized trial AML-BFM 98 investigated the impact of G-CSF on hematopoetic recovery and infectious complications (primary endpoints) and on outcome (secondary endpoint) in children (aged 0-18 years) with de novo AML. Patients with more than 5% blasts in day-15 bone marrow or with FAB M3 were not included. Between 1998 and 2003, 161 children with AML were randomized to receive G-CSF after inductions 1 and 2, whereas 156 patients were assigned to the control group. Time of neutropenia after inductions 1 and 2 was significantly shorter in the G-CSF group (23 vs 18 days and 16 vs 11 days; P = .02 and = .001, respectively). G-CSF did not decrease the incidence of febrile neutropenia (72 and 36 patients vs 78 and 37 patients, respectively), microbiologically documented infections (27 and 25 patients vs 36 and 19 patients, respectively) and infection-associated mortality (5 vs 2 patients). Both groups had similar 5-year event-free survival (EFS; 59% ± 4% vs 58% ± 4%). Since G-CSF does not influence the risk of infectious complications or outcome in children undergoing therapy for AML, one cannot advocate the routine use of G-CSF in this patient group.
Introduction
Therapy for acute myelogenous leukemia (AML) is one of the most intensive treatment modalities in pediatric oncology. It has been shown that most patients experience one or more infectious complications during treatment, which occur in particular during chemotherapy-induced neutropenia.1 Strategies to reduce therapy-associated toxicity have involved the use of hematopoietic growth factors such as granulocyte colony-stimulating factor (G-CSF). This hematopoietic growth factor expands circulating pools of neutrophils by stimulating proliferation and maturation of myeloid progenitor cells and in addition enhances phagocyte function ex vivo.2,3 Multiple clinical trials in pediatric patients with cancer have demonstrated that G-CSF shortens duration of neutropenia,4 but this has not necessarily translated into a demonstrable reduction of risk for infection, and data supporting a positive effect of G-CSF on overall survival are conflicting.4 The guidelines published by the American Society of Clinical Oncology (ASCO) suggest that the costly hematopoietic growth factors should be used as primary prophylaxis only when the expected incidence of febrile neutropenia is 40% or more.5 This recommendation is supported by a recent meta-analysis, which demonstrated that the use of G-CSF in this group of patients was associated with reduction in febrile neutropenia by 20%.6 On the other hand, the prophylactic administration of G-CSF in patients with AML has been a matter of controversy, since most of the myeloid leukemia cells express receptors for G-CSF, and G-CSF has been shown to induce proliferation of leukemic blasts in vitro.7,8 Whereas a number of randomized clinical trials in adult patients with AML demonstrated that administration of G-CSF had some clinical benefit without worsening overall survival,9-16 randomized studies in the pediatric population are lacking to date. In this report, we present the results of a multicenter randomized trial evaluating the impact of prophylactic G-CSF in children with de novo AML on hematologic recovery, the risk of infectious complications and, as secondary objective, on outcome.
Patients and methods
Clinical trial AML-BFM 98
This study was part of the prospective clinical trial AML-BFM 98, which has been described in detail elsewhere.17 Children up to the age of 18 years with newly diagnosed AML were eligible for the study. Patients with AML and Down syndrome were treated as protocol patients and were randomized, but received less anthracyclines and no second induction. Patients were enrolled between July 1, 1998, and June 30, 2003. The protocol was approved by the protocol review committee of the German Cancer Society, and by the ethics committee of the University of Münster. Each patient (or parent) had to sign an informed consent before inclusion into the study and separately for the randomizations. All initial smears and day-15 bone marrow aspirates were centrally reviewed at the University Children's Hospital in Münster.
In brief, the treatment plan consisted of the 8-day AIE induction (cytarabine at a dose of 100 mg/m2/day continuous infusion on days 1 and 2 followed by a 30-minute infusion every 12 hours on days 3 to 8; 12 mg/m2 idarubicin on days 3, 4, and 5 and 150 mg/m2 etoposide on days 6, 7, and 8; and intrathecal cytarabine on days 0 and 8). A second induction therapy (3 g/m2 high-dose cytarabine every 12 hours for 3 days, 10 mg/m2 mitoxantrone on days 4 and 5, and intrathecal cytarabine on day 6 [HAM]) was given to all patients, except to those with FAB M3 and to patients with Down syndrome. After induction therapy, patients were randomly assigned to receive either standard consolidation therapy (6-thioguanine, prednisolone, vincristine, cytarabine, idarubicin, and cyclophosphamide) or a 2-cycle consolidation with AI (cytarabine and idarubicin) and haM (1 g/m2 high-dose cytarabine every 12 hours for 3 days; 10 mg/m2 mitoxantrone on days 3 and 4). Consolidation therapy was followed by one intensification block (high-dose cytarabine and etoposide [HAE]), followed by cranial irradiation and maintenance therapy (thioguanine and cytarabine). Total treatment duration was 18 months. Whereas patients of the standard risk (SR) group (SR indicates FAB M1 and M2 with Auer rods and FAB M4eo with ≤ 5% blasts on day 15 in the bone marrow, and FAB M3 independent of blast count on day 15; high risk [HR] indicates all others) were not eligible for stem cell transplantation (SCT); allogeneic SCT was recommended for HR patients if they had an HLA-identical sibling.
All patients except those with more than 5% blasts in the bone marrow on day 15 and those with FAB M3 were eligible for randomization regarding G-CSF performed on day 15. The hematopoietic growth factor was administered at a dosage of 5 μg/kg/day either subcutaneously or as an intravenous infusion over at least 1 hour. Based on the data of a randomized trial evaluating the optimal schedule for administering G-CSF in chemotherapy-induced neutropenia, the initiation of the growth factor began on day 15 after the start of AIE and HAM, respectively, and continued until the absolute neutrophil count exceeded 0.5 × 109/L (500/μL) on 3 consecutive days.18
Prophylaxis and treatment of infectious complications were performed according to common standards.19,20 Empirical broad-spectrum antibiotics were started in case of fever, and systemic antifungal therapy was given for suspected or documented fungal infection or was started when fever persisted despite antibiotic therapy. All patients except for 7 who were eligible for randomization had central venous access devices.
Statistical methods and definitions
Randomization was centrally performed using the permuted block method. Patients were stratified according to risk groups. According to the results from previous AML-BFM studies, the rate of severe infections was estimated to be 30%; 135 patients per group had to be randomized to receive or not to receive G-CSF to detect a decrease of infectious complications by 15% in the G-CSF–treated group (power 80%, alpha 5%, 2-sided test).
Complete remission (CR) was defined according to the Cancer and Leukemia Group B (CALGB) criteria and had to be achieved by the end of intensive treatment.21 Early death (ED) was defined as death before or within the first 6 weeks of treatment. EFS was calculated from date of diagnosis to last follow-up or to the first event (ED, resistant leukemia, relapse, secondary malignancy, or death of any cause). Failure to achieve remission was considered as event on day 0. Survival times for patients without event were censored at the date of last follow-up. Rates were calculated according to Kaplan-Meier and compared by log-rank test. Standard errors of the estimates were obtained using the Greenwoods formula. Cumulative incidence functions of relapse and death in continuous CR (CCR) were constructed by the method of Kalbfleish and Prentice22 and the functions were compared with the Gray test.22 Cox regression analysis was used for multivariate analysis.
One cycle of chemotherapy was defined as the time from the start of chemotherapy until the day before the start of the next cycle. Neutropenia was defined as an absolute neutrophil count (ANC) of less than 0.5 × 109/L (500/μL), and thrombocytopenia was defined as a platelet count of less than 20 × 109/L (20 ×103/μL), or the need for platelet transfusion. Fever was defined as a temperature higher than 38.0°C. Infectious complications were categorized as microbiologically documented infections, which included septicemia in case of positive blood culture for bacteria or fungi, or as febrile neutropenia, which was defined as fever of unknown origin (FUO) if no source could be identified.1 Fungal infections were classified according to criteria published recently.23 Toxicity was evaluated according to the National Cancer Institute (NCI) Common Toxicity Criteria version 3.0.24 All efficacy analyses were performed according to the intent-to-treat principle. Additional analyses were done including only those patients who were treated according to the randomized arm.
Results
Patients
Out of a total of 538 patients (473 patients without Down syndrome, and 65 patients with Down syndrome), 429 were treated according to the multicenter clinical trial AML-BFM 98 were eligible for randomization; 15 patients died within the first 9 days after the start of treatment, 28 patients had the FAB M3 subtype, and 66 patients had more than 5% blasts in the bone marrow on day 15. The characteristics of randomized patients were similar to those of the study patients. A group of 161 children were randomized to the G-CSF group, whereas 156 patients were assigned to the control group that did not receive the hematopoietic growth factor (Table 1)The groups were well balanced for age, FAB subtype, initial leukocyte count, cytogenetic analysis, Down syndrome, and risk groups. Significantly more boys were randomized to receive G-CSF (Table 1). The compliance with treatment allocation was 90%; 18 patients assigned to the group without G-CSF received the hematopoietic growth factor, whereas 14 patients randomized for G-CSF did not receive it. The characteristics of patients randomized (intent-to-treat) were comparable to the characteristics of patients who were treated as assigned (data not shown). Median follow-up of patients alive was 4.1 years (range, 1.3-7.1 years). Of the randomized patients, 27 underwent SCT in first remission.
Patient characteristics by randomized treatment group
| . | No G-CSF . | G-CSF . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| All patients | 156 | 100.0 | 161 | 100.0 |
| Sex, male/female | 72/84* | 46.2/53.8 | 96/65* | 59.6/40.4 |
| Age | ||||
| Younger than 1 y | 41 | 26.3 | 56 | 34.8 |
| 1-9 y | 47 | 30.1 | 52 | 32.3 |
| 10 y or older | 68 | 43.6 | 53 | 32.9 |
| Leukocytes | ||||
| Less than 20 × 103/μL | 100 | 64.1 | 87 | 54.0 |
| Between 20 and less than 100 × 103/μL | 37 | 23.7 | 44 | 27.3 |
| 100 × 103/μL or more | 19 | 12.2 | 30 | 18.6 |
| CNS leukemia | 12 | 7.7† | 19 | 11.8† |
| FAB types | ||||
| M0 | 8 | 5.1 | 8 | 5.0 |
| M1 | 25 | 16.0 | 18 | 11.2 |
| M1 with Auer | 14 | 9.0† | 8 | 5.0† |
| M2 | 39 | 25.0 | 41 | 25.5 |
| M2 with Auer | 28 | 17.9† | 36 | 22.4† |
| M4 | 31 | 19.9 | 26 | 16.2 |
| M4eo | 14 | 9.0 | 17 | 10.6 |
| M5 | 23 | 14.7 | 30 | 18.6 |
| M6 | 7 | 4.5 | 4 | 2.5 |
| M7 | 23 | 14.7 | 34 | 21.1 |
| Other | 1 | 0.6 | 0 | 0 |
| Karyotypes and cytogenetics | ||||
| Favorable‡ | 32 | 20.5† | 32 | 19.9† |
| Other | 102 | 65.4† | 106 | 65.8† |
| Down syndrome | 18 | 11.5 | 27 | 16.8 |
| Standard risk | 63 | 40.4 | 58 | 36.0 |
| High risk | 75 | 48.1 | 76 | 47.2 |
| . | No G-CSF . | G-CSF . | ||
|---|---|---|---|---|
| No. . | % . | No. . | % . | |
| All patients | 156 | 100.0 | 161 | 100.0 |
| Sex, male/female | 72/84* | 46.2/53.8 | 96/65* | 59.6/40.4 |
| Age | ||||
| Younger than 1 y | 41 | 26.3 | 56 | 34.8 |
| 1-9 y | 47 | 30.1 | 52 | 32.3 |
| 10 y or older | 68 | 43.6 | 53 | 32.9 |
| Leukocytes | ||||
| Less than 20 × 103/μL | 100 | 64.1 | 87 | 54.0 |
| Between 20 and less than 100 × 103/μL | 37 | 23.7 | 44 | 27.3 |
| 100 × 103/μL or more | 19 | 12.2 | 30 | 18.6 |
| CNS leukemia | 12 | 7.7† | 19 | 11.8† |
| FAB types | ||||
| M0 | 8 | 5.1 | 8 | 5.0 |
| M1 | 25 | 16.0 | 18 | 11.2 |
| M1 with Auer | 14 | 9.0† | 8 | 5.0† |
| M2 | 39 | 25.0 | 41 | 25.5 |
| M2 with Auer | 28 | 17.9† | 36 | 22.4† |
| M4 | 31 | 19.9 | 26 | 16.2 |
| M4eo | 14 | 9.0 | 17 | 10.6 |
| M5 | 23 | 14.7 | 30 | 18.6 |
| M6 | 7 | 4.5 | 4 | 2.5 |
| M7 | 23 | 14.7 | 34 | 21.1 |
| Other | 1 | 0.6 | 0 | 0 |
| Karyotypes and cytogenetics | ||||
| Favorable‡ | 32 | 20.5† | 32 | 19.9† |
| Other | 102 | 65.4† | 106 | 65.8† |
| Down syndrome | 18 | 11.5 | 27 | 16.8 |
| Standard risk | 63 | 40.4 | 58 | 36.0 |
| High risk | 75 | 48.1 | 76 | 47.2 |
Significant difference between G-CSF and control group (P = .02).
Percentage of patients with data.
Definition of favorable cytogenetics: t(8;21), inv16.
Hematologic recovery
Neutrophil recovery after both AIE and HAM was significantly shorter in the G-CSF group than in patients of the control group (intent-to-treat analysis: median, 23.0 vs 18.0 days, P = .02, and 16.0 vs 11.0 days, P = .001, respectively; Table 2). This difference was seen in particular in HR patients (median, 24.0 vs 18 days, P = .03, and 15.5 vs 11.0 days, P = .008, respectively). The hematopoietic growth factor did not affect the time of platelet recovery (Table 2). Similarly, the administration of G-CSF after induction therapy had no impact on hematologic recovery after consolidation therapy and after HAE (data not shown).
G-CSF and hematologic recovery
| . | Intent-to-treat . | Treated-as-assigned . | ||||
|---|---|---|---|---|---|---|
| No G-CSF . | G-CSF . | P . | No G-CSF . | G-CSF . | P . | |
| AIE, median d | ||||||
| Total | ||||||
| Neutropenia | 23.0* | 18.0* | .01* | 22.0 | 18.0 | .11 |
| Thrombocytopenia | 6.0 | 6.0 | .97 | 4.5 | 7.0 | .46 |
| SR | ||||||
| Neutropenia | 22.0 | 18.0 | .21 | 20.5 | 18.5 | .49 |
| Thrombocytopenia | 9.0 | 7.5 | .81 | 8.0 | 8.0 | .83 |
| HR | ||||||
| Neutropenia | 24.0* | 18.0* | .03* | 22.5 | 18.0 | .12 |
| Thrombocytopenia | 6.0 | 8.0 | .28 | 5.0 | 8.5 | .13 |
| Down syndrome, median d | ||||||
| Neutropenia | 22.5 | 19.0 | .37 | 23.0 | 21.0 | .54 |
| Thrombocytopenia | 2.0 | 1.0 | .15 | 2.0 | 1.0 | .15 |
| HAM, median d | ||||||
| Total | ||||||
| Neutropenia | 16.0* | 11.0* | .001* | 16.0* | 11.0* | .002* |
| Thrombocytopenia | 4.0 | 5.0 | .79 | 3.0 | 4.0 | .69 |
| SR | ||||||
| Neutropenia | 17.0* | 11.0* | .02* | 17.5 | 12.0 | .07 |
| Thrombocytopenia | 5.0 | 5.0 | .98 | 4.5 | 5.0 | .78 |
| HR | ||||||
| Neutropenia | 15.5* | 11.0* | .008* | 15.0* | 10.0* | .007* |
| Thrombocytopenia | 2.0 | 4.5 | .79 | 2.0 | 3.0 | .97 |
| . | Intent-to-treat . | Treated-as-assigned . | ||||
|---|---|---|---|---|---|---|
| No G-CSF . | G-CSF . | P . | No G-CSF . | G-CSF . | P . | |
| AIE, median d | ||||||
| Total | ||||||
| Neutropenia | 23.0* | 18.0* | .01* | 22.0 | 18.0 | .11 |
| Thrombocytopenia | 6.0 | 6.0 | .97 | 4.5 | 7.0 | .46 |
| SR | ||||||
| Neutropenia | 22.0 | 18.0 | .21 | 20.5 | 18.5 | .49 |
| Thrombocytopenia | 9.0 | 7.5 | .81 | 8.0 | 8.0 | .83 |
| HR | ||||||
| Neutropenia | 24.0* | 18.0* | .03* | 22.5 | 18.0 | .12 |
| Thrombocytopenia | 6.0 | 8.0 | .28 | 5.0 | 8.5 | .13 |
| Down syndrome, median d | ||||||
| Neutropenia | 22.5 | 19.0 | .37 | 23.0 | 21.0 | .54 |
| Thrombocytopenia | 2.0 | 1.0 | .15 | 2.0 | 1.0 | .15 |
| HAM, median d | ||||||
| Total | ||||||
| Neutropenia | 16.0* | 11.0* | .001* | 16.0* | 11.0* | .002* |
| Thrombocytopenia | 4.0 | 5.0 | .79 | 3.0 | 4.0 | .69 |
| SR | ||||||
| Neutropenia | 17.0* | 11.0* | .02* | 17.5 | 12.0 | .07 |
| Thrombocytopenia | 5.0 | 5.0 | .98 | 4.5 | 5.0 | .78 |
| HR | ||||||
| Neutropenia | 15.5* | 11.0* | .008* | 15.0* | 10.0* | .007* |
| Thrombocytopenia | 2.0 | 4.5 | .79 | 2.0 | 3.0 | .97 |
Significant differences.
Infectious complications
Infection-associated mortality and the incidence of life-threatening sepsis during induction therapy did not differ between the G-CSF and control groups (intent-to-treat analysis: 5 vs 2 patients and 1 vs 5 patients, P = .45 and P = .12, respectively; Table 3). Microbiologically documented infections occurred in AIE in 36 patients who received G-CSF (24.3%) and in 27 patients who did not receive the hematopoietic growth factor (20.1%; P = .47), whereas in the second induction, HAM, microbiologically documented infections were seen in 19 patients of the G-CSF group (16.1%) and in 25 patients of the control group (20.8%, P = .4; Table 3). Similar results were observed in patients with septicemia: for AIE, 25 patients (16.9%) with G-CSF versus 23 patients (17.2%) without G-CSF (P > .999); and for HAM, 16 patients (13.6%) with G-CSF versus 22 patients (18.3%) without G-CSF (P = .38; Table 3). Pathogens isolated from the blood of patients receiving G-CSF included 26 Gram-positive organisms, in particular staphylococci (n = 7, including coagulase-negative staphylococci [CoNS] [n = 5]) and viridans group streptococci (VGS; 6), and 12 Gram-negative organisms, among them Escherichia coli (3), Pseudomonas aeruginosa (2), and Klebsiella spp (2). In addition, Candida spp was isolated from the blood from 3 patients who received G-CSF. In the control group, 36 Gram-positive isolates, mainly staphylococci (8, including 6 CoNS) and streptococci (18, including 16 VGS), and 9 Gram-negative pathogens, including P aeruginosa (3), E coli (1), Klebsiella spp (1), and Enterobacter spp (1), were recovered from the blood. Invasive fungal infection occurred in a total of 4 patients: one patient suffered from invasive aspergillosis of the sinus, and Candida spp was isolated from the blood of 3 patients; all these patients were assigned to the G-CSF group and also received the hematopoietic growth factor (treated-as-assigned). Patients of the G-CSF group and control group had a comparable risk of suffering from febrile neutropenia without having a source identified (FUO). Specifically, FUO occurred in AIE in 78 patients (52.7%) with G-CSF versus 72 patients (51.4%) without G-CSF (P = .91), and in HAM in 37 patients (30.8%) of the G-CSF group versus 36 patients (29.8%) of the control group (P = .89; Table 3). In addition, the number of febrile days was not significantly reduced by the administration of G-CSF (Table 3). Similarly, G-CSF and control group did not differ in the use of antifungal agents (data not shown).
G-CSF and infectious complications
| . | Intent-to-treat . | Treated-as-assigned . | ||
|---|---|---|---|---|
| No G-CSF . | G-CSF . | No G-CSF . | G-CSF . | |
| Infection-associated mortality | ||||
| AIE/HAM, no. patients | 5 | 2 | 4 | 2 |
| Life-threatening sepsis | ||||
| AIE/HAM, no. patients | 1 | 5 | 1 | 4 |
| Microbiologically documented infections, no. patients (%)* | ||||
| AIE | ||||
| Total | 27 (20.1) | 36 (24.3) | 22 (18.6) | 34 (25.2) |
| SR | 9 (17.0) | 8 (14.8) | 5 (11.4) | 8 (15.4) |
| HR | 17 (25.8) | 19 (27.5) | 16 (26.7) | 19 (29.7) |
| Down | 1 (6.7) | 9 (36.0) | 1 (7.1) | 7 (36.8) |
| HAM | ||||
| Total | 25 (20.8) | 19 (16.1) | 17 (16.3) | 17 (15.2) |
| SR | 12 (21.8) | 7 (13.5) | 6 (13.3) | 7 (13.7) |
| HR | 13 (20.0) | 12 (8.2) | 11 (18.6) | 10 (16.4) |
| Septicemia, no. patients (%) | ||||
| AIE | ||||
| Total | 23 (17.2) | 25 (16.9) | 18 (15.3) | 23 (17.0) |
| SR | 9 (17.0) | 5 (9.3) | 5 (11.4) | 5 (9.6) |
| HR | 13 (19.7) | 12 (17.4) | 12 (20.0) | 12 (18.8) |
| Down | 1 (6.7) | 8 (32.0) | 1 (7.1) | 6 (31.6) |
| HAM | ||||
| Total | 22 (18.3) | 16 (13.6) | 16 (15.4) | 15 (13.4) |
| SR | 10 (18.2) | 6 (11.5) | 5 (11.1) | 6 (11.8) |
| HR | 12 (18.5) | 10 (15.2) | 11 (18.6) | 9 (14.8) |
| Episodes of febrile neutropenia, no. patients (%)† | ||||
| AIE | ||||
| Total | 72 (51.4) | 78 (52.7) | 65 (52.4) | 69 (51.1) |
| SR | 24 (42.9) | 27 (50.0) | 21 (44.7) | 25 (48.1) |
| HR | 38 (55.9) | 39 (56.5) | 35 (56.5) | 34 (53.1) |
| Down | 10 (62.5) | 12 (48.0) | 9 (60.0) | 10 (52.6) |
| HAM | ||||
| Total | 36 (29.8) | 37 (30.8) | 32 (30.5) | 35 (31.0) |
| SR | 14 (25.0) | 15 (28.3) | 13 (28.3) | 15 (29.4) |
| HR | 22 (33.8) | 22 (32.8) | 19 (32.2) | 20 (32.3) |
| Median no. d with fever (range) | ||||
| AIE | ||||
| Total | 5 (1-35) | 5 (1-27) | 5 (1-35) | 5 (1-27) |
| SR | 4 (1-17) | 4 (1-14) | 4 (1-16) | 4 (1-14) |
| HR | 6 (1-35) | 6 (1-27) | 5.5 (1-35) | 6 (1-27) |
| Down | 8 (1-16) | 4 (1-18) | 7.5 (1-11) | 1.5 (1-13) |
| HAM | ||||
| Total | 4 (1-19) | 3 (1-21) | 3 (1-19) | 3 (1-21) |
| SR | 3 (1-19) | 3 (1-21) | 3 (1-19) | 3.5 (1-21) |
| HR | 4 (1-15) | 3 (1-16) | 3 (1-15) | 3 (1-16) |
| . | Intent-to-treat . | Treated-as-assigned . | ||
|---|---|---|---|---|
| No G-CSF . | G-CSF . | No G-CSF . | G-CSF . | |
| Infection-associated mortality | ||||
| AIE/HAM, no. patients | 5 | 2 | 4 | 2 |
| Life-threatening sepsis | ||||
| AIE/HAM, no. patients | 1 | 5 | 1 | 4 |
| Microbiologically documented infections, no. patients (%)* | ||||
| AIE | ||||
| Total | 27 (20.1) | 36 (24.3) | 22 (18.6) | 34 (25.2) |
| SR | 9 (17.0) | 8 (14.8) | 5 (11.4) | 8 (15.4) |
| HR | 17 (25.8) | 19 (27.5) | 16 (26.7) | 19 (29.7) |
| Down | 1 (6.7) | 9 (36.0) | 1 (7.1) | 7 (36.8) |
| HAM | ||||
| Total | 25 (20.8) | 19 (16.1) | 17 (16.3) | 17 (15.2) |
| SR | 12 (21.8) | 7 (13.5) | 6 (13.3) | 7 (13.7) |
| HR | 13 (20.0) | 12 (8.2) | 11 (18.6) | 10 (16.4) |
| Septicemia, no. patients (%) | ||||
| AIE | ||||
| Total | 23 (17.2) | 25 (16.9) | 18 (15.3) | 23 (17.0) |
| SR | 9 (17.0) | 5 (9.3) | 5 (11.4) | 5 (9.6) |
| HR | 13 (19.7) | 12 (17.4) | 12 (20.0) | 12 (18.8) |
| Down | 1 (6.7) | 8 (32.0) | 1 (7.1) | 6 (31.6) |
| HAM | ||||
| Total | 22 (18.3) | 16 (13.6) | 16 (15.4) | 15 (13.4) |
| SR | 10 (18.2) | 6 (11.5) | 5 (11.1) | 6 (11.8) |
| HR | 12 (18.5) | 10 (15.2) | 11 (18.6) | 9 (14.8) |
| Episodes of febrile neutropenia, no. patients (%)† | ||||
| AIE | ||||
| Total | 72 (51.4) | 78 (52.7) | 65 (52.4) | 69 (51.1) |
| SR | 24 (42.9) | 27 (50.0) | 21 (44.7) | 25 (48.1) |
| HR | 38 (55.9) | 39 (56.5) | 35 (56.5) | 34 (53.1) |
| Down | 10 (62.5) | 12 (48.0) | 9 (60.0) | 10 (52.6) |
| HAM | ||||
| Total | 36 (29.8) | 37 (30.8) | 32 (30.5) | 35 (31.0) |
| SR | 14 (25.0) | 15 (28.3) | 13 (28.3) | 15 (29.4) |
| HR | 22 (33.8) | 22 (32.8) | 19 (32.2) | 20 (32.3) |
| Median no. d with fever (range) | ||||
| AIE | ||||
| Total | 5 (1-35) | 5 (1-27) | 5 (1-35) | 5 (1-27) |
| SR | 4 (1-17) | 4 (1-14) | 4 (1-16) | 4 (1-14) |
| HR | 6 (1-35) | 6 (1-27) | 5.5 (1-35) | 6 (1-27) |
| Down | 8 (1-16) | 4 (1-18) | 7.5 (1-11) | 1.5 (1-13) |
| HAM | ||||
| Total | 4 (1-19) | 3 (1-21) | 3 (1-19) | 3 (1-21) |
| SR | 3 (1-19) | 3 (1-21) | 3 (1-19) | 3.5 (1-21) |
| HR | 4 (1-15) | 3 (1-16) | 3 (1-15) | 3 (1-16) |
Includes patients with septicemia.
Patients with microbiologically documented infections excluded.
Toxicities
The administration of G-CSF had no impact on the incidence of oral and pharyngeal mucositis grades 3 and 4 after both induction chemotherapies (intent-to-treat analysis: 38 [26.6%] and 8 [6.9%] patients with G-CSF vs 33 [23.6%] and 6 [5.2%] patients without G-CSF; P = .59 each). Similarly, no significant difference between the G-CSF and control group were seen regarding diarrhea, nausea, vomiting, and hepatic and cardiovascular abnormalities (data not shown).
Outcome
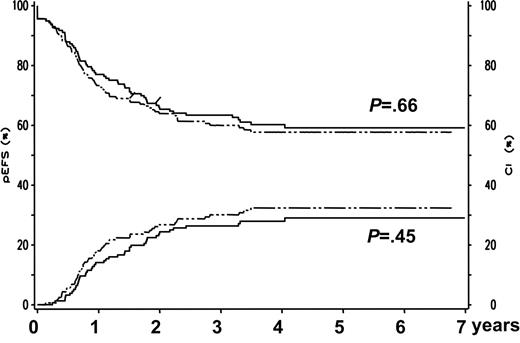
In total, 154 patients (95.7%) in the G-CSF group and 149 patients (95.5%) in the control group achieved CR (intent-to-treat analysis: P > .999; Table 4)Similarly, G-CSF had no impact on the CR rates of the different subgroups analyzed (Table 4). In addition, G-CSF did not influence the cumulative incidence of relapse at 5 years, the 5-year EFS, and the risk of death in CCR at 5 years in the total patient group and in the different subgroups analyzed (Table 4; Figure 1).Three patients in the control group and one patient who was treated with G-CSF suffered from a second malignancy. In a Cox regression analysis the following variables were included: treatment group (G-CSF, yes or no), Down syndrome present (yes or no), standard risk group (yes or no), and SCT (time-dependent covariable). There was no significant effect of G-CSF treatment on EFS (risk ratio, 1.13; 95% confidence interval [CI], 0.79-1.60; P = .50) and survival (risk ratio, 1.30; 95% CI 0.86-1.98; P = .22).
Five-year EFS and cumulative incidence of relapse for G-CSF–treated patients and controls. Five-year EFS (left y-axis, both upper curves) and cumulative incidence of relapse at 5 years (right y-axis, both lower curves) for patients treated according to AML-BFM 98 and randomized to receive prophylactic G-CSF (n = 161, dotted curves) or not to receive G-CSF (n = 156, solid curves).
Five-year EFS and cumulative incidence of relapse for G-CSF–treated patients and controls. Five-year EFS (left y-axis, both upper curves) and cumulative incidence of relapse at 5 years (right y-axis, both lower curves) for patients treated according to AML-BFM 98 and randomized to receive prophylactic G-CSF (n = 161, dotted curves) or not to receive G-CSF (n = 156, solid curves).
Treatment results with and without prophylactic G-CSF
| . | Intent-to-treat . | Treated-as-assigned . | ||||
|---|---|---|---|---|---|---|
| No G-CSF . | G-CSF . | P . | No G-CSF . | G-CSF . | P . | |
| CR rate, no. patients (%) | ||||||
| Total | 149 (95.5) | 154 (95.7) | >.999 | 132 (95.7) | 140 (95.2) | >.999 |
| SR | 58 (92.1) | 55 (94.8) | .72 | 48 (92.3) | 52 (94.5) | .71 |
| HR | 73 (97.3) | 72 (94.7) | .68 | 67 (97.1) | 67 (94.4) | .68 |
| Down | 18 (100) | 27 (100) | 17 (100) | 21 (100) | ||
| Cumulative incidence of relapse at 5 y, % ± SE | ||||||
| Total | 29 ± 4 | 32 ± 4 | .45 | 29 ± 1 | 34 ± 1 | .32 |
| SR | 19 ± 6 | 32 ± 7 | .10 | 17 ± 6 | 32 ± 8 | .08 |
| HR | 44 ± 7 | 42 ± 7 | .88 | 45 ± 7 | 45 ± 7 | .94 |
| Down | 0 | 7 ± 5 | .24 | 0 | 5 ± 5 | .37 |
| 5-y EFS, % ± SE | ||||||
| Total | 59 ± 4 | 58 ± 4 | .66 | 59 ± 4 | 56 ± 4 | .48 |
| SR | 68 ± 6 | 56 ± 7 | .18 | 69 ± 7 | 55 ± 7 | .15 |
| HR | 45 ± 6 | 48 ± 6 | .91 | 45 ± 6 | 46 ± 6 | .96 |
| Down | 89 ± 7 | 89 ± 6 | .98 | 88 ± 8 | 90 ± 6 | .85 |
| Cumulative incidence of death in CCR at 5 years, % ± SE | ||||||
| Total | 5 ± 2 | 5 ± 2 | .95 | 6 ± 2 | 5 ± 2 | .69 |
| SR | 3 ± 2 | 7 ± 4 | .36 | 4 ± 3 | 7 ± 4 | .46 |
| HR | 5 ± 3 | 4 ± 3 | .69 | 6 ± 3 | 3 ± 3 | .39 |
| Down | 11 ± 7 | ± 4 | .35 | 12 ± 8 | 5 ± 5 | .45 |
| Second malignancy, no. patients | ||||||
| Total | 3 | 1 | 2 | 1 | ||
| SR | 1 | 0 | 1 | 0 | ||
| HR | 2 | 1 | 1 | 1 | ||
| Lost for follow-up, no. patients | ||||||
| Total | 2 | 1 | 2 | 1 | ||
| SR | 2 | 0 | 2 | 0 | ||
| HR | 0 | 1 | 0 | 1 | ||
| . | Intent-to-treat . | Treated-as-assigned . | ||||
|---|---|---|---|---|---|---|
| No G-CSF . | G-CSF . | P . | No G-CSF . | G-CSF . | P . | |
| CR rate, no. patients (%) | ||||||
| Total | 149 (95.5) | 154 (95.7) | >.999 | 132 (95.7) | 140 (95.2) | >.999 |
| SR | 58 (92.1) | 55 (94.8) | .72 | 48 (92.3) | 52 (94.5) | .71 |
| HR | 73 (97.3) | 72 (94.7) | .68 | 67 (97.1) | 67 (94.4) | .68 |
| Down | 18 (100) | 27 (100) | 17 (100) | 21 (100) | ||
| Cumulative incidence of relapse at 5 y, % ± SE | ||||||
| Total | 29 ± 4 | 32 ± 4 | .45 | 29 ± 1 | 34 ± 1 | .32 |
| SR | 19 ± 6 | 32 ± 7 | .10 | 17 ± 6 | 32 ± 8 | .08 |
| HR | 44 ± 7 | 42 ± 7 | .88 | 45 ± 7 | 45 ± 7 | .94 |
| Down | 0 | 7 ± 5 | .24 | 0 | 5 ± 5 | .37 |
| 5-y EFS, % ± SE | ||||||
| Total | 59 ± 4 | 58 ± 4 | .66 | 59 ± 4 | 56 ± 4 | .48 |
| SR | 68 ± 6 | 56 ± 7 | .18 | 69 ± 7 | 55 ± 7 | .15 |
| HR | 45 ± 6 | 48 ± 6 | .91 | 45 ± 6 | 46 ± 6 | .96 |
| Down | 89 ± 7 | 89 ± 6 | .98 | 88 ± 8 | 90 ± 6 | .85 |
| Cumulative incidence of death in CCR at 5 years, % ± SE | ||||||
| Total | 5 ± 2 | 5 ± 2 | .95 | 6 ± 2 | 5 ± 2 | .69 |
| SR | 3 ± 2 | 7 ± 4 | .36 | 4 ± 3 | 7 ± 4 | .46 |
| HR | 5 ± 3 | 4 ± 3 | .69 | 6 ± 3 | 3 ± 3 | .39 |
| Down | 11 ± 7 | ± 4 | .35 | 12 ± 8 | 5 ± 5 | .45 |
| Second malignancy, no. patients | ||||||
| Total | 3 | 1 | 2 | 1 | ||
| SR | 1 | 0 | 1 | 0 | ||
| HR | 2 | 1 | 1 | 1 | ||
| Lost for follow-up, no. patients | ||||||
| Total | 2 | 1 | 2 | 1 | ||
| SR | 2 | 0 | 2 | 0 | ||
| HR | 0 | 1 | 0 | 1 | ||
Discussion
The data from our prospective, randomized clinical trial of prophylactic G-CSF in children undergoing therapy for AML indicate that G-CSF significantly shortens the time of neutropenia, which is comparable to the results of clinical trials in pediatric patients with acute lymphoblastic leukemia25-27 and of studies in adult patients with AML.9-16,28 Although infectious complications particularly occur during severe neutropenia,1,29 the effect of G-CSF on neutrophil recovery did not translate into a demonstrable reduction of risk for infection-associated mortality, microbiologically documented infection, and the incidence of FUO. The results were similar when excluding patients with Down syndrome who received less anthracyclines and no second induction. Our findings are corroborating the results of most randomized clinical trials in adults with AML,9-12,15 and also concur with the data of the recent pediatric clinical trial CCG-2891, where G-CSF was added to the intensive chemotherapy in a post facto attempt to mitigate against severe toxicity observed during induction therapy (Table 5)30 . Our results, however, are in contrast to a meta-analysis of randomized controlled trials that evaluated the effect of prophylactic hematopoietic growth factors in children undergoing intensive therapy for different types of cancer resulting in an incidence of 40% or higher of febrile neutropenia.6 The analysis demonstrated that the prophylactic administration of G-CSF or granulocyte-macrophage (GM)–CSF led to a reduction of febrile neutropenia by 20%. Since the treatment regimen in the presented clinical trial resulted in an incidence of febrile neutropenia even higher than 40%, the number of randomized patients is large enough to detect a potential benefit of prophylactic G-CSF with a high probability. It can only be speculated whether other therapy-induced alterations in host defense, such as impairment of innate immunity and T cells or the disruption of barriers, may have played a role in increasing the risk of infection. For example, in contrast to previous reports,25,32 the incidence of severe mucositis, associated with an increased risk of bacterial and fungal infections, was not affected in our study by the administration of G-CSF. Since most pediatric cancer centers participating in the AML-BFM trials hospitalize children with AML regardless of the neutrophil count from the time of diagnosis for at least the period of induction chemotherapy, we did not aim to evaluate whether G-CSF may shorten the length of hospitalization nor did we analyze potential cost savings by the prophylactic use of G-CSF.
Recent clinical trials of prophylactic G-CSF in patients with AML
| CSF, dose, schedule, and route . | Adult/pediatric (median age, y) . | No. patients . | Amelioration of depth and/or duration of neutropenia* . | Decrease in neutropenic fever . | Decrease in documented infections . | Decrease in antibiotic usage . | Decrease in hospitalizations . | Improvement in rate of complete remission . | Improvement in disease-free survival . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenograstim, 150 μg/m2/d, intravenously, start d 1 of chemotherapy or d 1 after completion of chemotherapy | Adult (68) | 722† | P < .001; 25 vs 20 days (no G-CSF vs G-CSF after chemotherapy) | — | — | — | P < .001; 29.7 vs 27.2 (no G-CSF vs G-CSF after chemotherapy) | P = .009; 48.6% vs 58.3% (no G-CSF vs G-CSF with chemotherapy) | NS | Amadori et al16 |
| Filgrastim, 5 μg/kg/d, subcutaneously or intravenously, start d 2 after completion of chemotherapy | Pediatric (7.6) | 512 | P = .003 and P < .001; 42 vs 38 d for cycle 1, 51 vs 44 d for cycle 2 | NS | NS | — | P < .001; 69 vs 63 d (overall results for 2 cycles) | NS | NS | Alonzo et al30 |
| Filgrastim, 200 μg/m2/d intravenously, start d 3 after completion of chemotherapy | Adult (49) | 270† | P < .001; 18 vs 12 d | NS | NS | NS | — | NS | NS | Usuki et al15 |
| Lenograstim, 293 μg/d, subcutaneously, start d 8 after completion of chemotherapy | Adult (66) | 226† | — | — | — | — | — | NS | NS | Goldstone et al14 |
| Filgrastim, 5 μg/kg/d, subcutaneously, start d 1 after completion of chemotherapy | Adult (46) | 194† | P < .001; 19 vs 12 d for cycle 1, 28 vs 20 d for cycle 2 | NS | P = .02 and P = .04; 15 vs 13 d for cycle 1, 22 vs 15d for cycle 2 | P < .001; 27 vs 24 d for cycle 1, 34 vs 29 d for cycle 2 | — | NS | Harrouseau et al31 | |
| Filgrastim, 400 μg/m2/d, intravenously, start d 4 after completion of chemotherapy | Adult (68) | 234† | P = .014; 24 vs 21 d | NS | NS | NS | NS | NS | NS | Godwin et al12 |
| Filgrastim, 5 μg/kg/d, subcutaneously, start d 1 after completion of chemotherapy | Adult (54) | 521† | P < .001; 25 vs 20 d | NS | NS | P < .001; 18.5 vs 15 d | P < .001; 25 vs 20 d | NS | NS | Heil et al11 |
| Lenograstim, 5 μg/kg/d, intravenously, start d 2 after completion of chemotherapy | Adult (71) | 173† | P < .001; 27 vs 21 d‡ | — | NS | — | — | P = .002; 47% vs 70% | NS | Dombret et al10 |
| Filgrastim, 200 μg/m2/d, intravenously, start d 2 before start of chemotherapy | Adult (45) | 58† | P < .001; 29 vs 24 d | — | NS | — | — | NS | NS | Ohno et al9 |
| CSF, dose, schedule, and route . | Adult/pediatric (median age, y) . | No. patients . | Amelioration of depth and/or duration of neutropenia* . | Decrease in neutropenic fever . | Decrease in documented infections . | Decrease in antibiotic usage . | Decrease in hospitalizations . | Improvement in rate of complete remission . | Improvement in disease-free survival . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenograstim, 150 μg/m2/d, intravenously, start d 1 of chemotherapy or d 1 after completion of chemotherapy | Adult (68) | 722† | P < .001; 25 vs 20 days (no G-CSF vs G-CSF after chemotherapy) | — | — | — | P < .001; 29.7 vs 27.2 (no G-CSF vs G-CSF after chemotherapy) | P = .009; 48.6% vs 58.3% (no G-CSF vs G-CSF with chemotherapy) | NS | Amadori et al16 |
| Filgrastim, 5 μg/kg/d, subcutaneously or intravenously, start d 2 after completion of chemotherapy | Pediatric (7.6) | 512 | P = .003 and P < .001; 42 vs 38 d for cycle 1, 51 vs 44 d for cycle 2 | NS | NS | — | P < .001; 69 vs 63 d (overall results for 2 cycles) | NS | NS | Alonzo et al30 |
| Filgrastim, 200 μg/m2/d intravenously, start d 3 after completion of chemotherapy | Adult (49) | 270† | P < .001; 18 vs 12 d | NS | NS | NS | — | NS | NS | Usuki et al15 |
| Lenograstim, 293 μg/d, subcutaneously, start d 8 after completion of chemotherapy | Adult (66) | 226† | — | — | — | — | — | NS | NS | Goldstone et al14 |
| Filgrastim, 5 μg/kg/d, subcutaneously, start d 1 after completion of chemotherapy | Adult (46) | 194† | P < .001; 19 vs 12 d for cycle 1, 28 vs 20 d for cycle 2 | NS | P = .02 and P = .04; 15 vs 13 d for cycle 1, 22 vs 15d for cycle 2 | P < .001; 27 vs 24 d for cycle 1, 34 vs 29 d for cycle 2 | — | NS | Harrouseau et al31 | |
| Filgrastim, 400 μg/m2/d, intravenously, start d 4 after completion of chemotherapy | Adult (68) | 234† | P = .014; 24 vs 21 d | NS | NS | NS | NS | NS | NS | Godwin et al12 |
| Filgrastim, 5 μg/kg/d, subcutaneously, start d 1 after completion of chemotherapy | Adult (54) | 521† | P < .001; 25 vs 20 d | NS | NS | P < .001; 18.5 vs 15 d | P < .001; 25 vs 20 d | NS | NS | Heil et al11 |
| Lenograstim, 5 μg/kg/d, intravenously, start d 2 after completion of chemotherapy | Adult (71) | 173† | P < .001; 27 vs 21 d‡ | — | NS | — | — | P = .002; 47% vs 70% | NS | Dombret et al10 |
| Filgrastim, 200 μg/m2/d, intravenously, start d 2 before start of chemotherapy | Adult (45) | 58† | P < .001; 29 vs 24 d | — | NS | — | — | NS | NS | Ohno et al9 |
NS indicates not significant; —, no analysis of this endpoint performed in the study.
Neutropenia is defined as 500 neutrophils/μL except otherwise specified.
Design of the study includes randomization with a control group.
Neutropenia defined as ANC below 1.0 × 109/L.
Although it has been shown that AML blast cells have G-CSF receptors and that G-CSF could increase the kinetics of leukemia cell growth,7,8 we and others9-16,28,30 did not observe a significant impact of prophylactic G-CSF on long-term outcome of patients with AML (Table 5). It has to be mentioned, however, that all but 19-16,28,30 of these studies enrolled predominantly adult patients older than 40 years. On the other hand, we recognize that our study was not sufficiently powered to address this question. Given a baseline of 60% probability of 5-year EFS, a difference of about 15% could have been detected with the actual sample size (power 80%, alpha 5%, 2-sided test). Our results indicate that the CR rate was not affected by the administration of G-CSF, an observation which was also made in most of the trials in adult AML (Table 5).9,11,12,14,15,28 In contrast, Alonzo et al30 reported that pediatric patients with hypercellular day-7 marrow who received G-CSF had a higher remission rate than children who did not receive G-CSF. This fact might be explained by the timing of G-CSF administration, which began 2 days after completion of the first cycle of chemotherapy and continued through the second cycle. Similarly, a recent trial in adult AML indicated that G-CSF administered concomitantly with standard-induction chemotherapy results in a higher CR rate than chemotherapy alone.16 In contrast to a retrospective analysis of 22 consecutive pediatric patients undergoing autologous bone marrow transplantation for treatment of primary AML, which showed that the 12 patients who received GM-CSF as part of their transplantation protocol had a significantly higher relapse rate than did patients who did not receive GM-CSF (90% vs 36%),33 we did not see a significant difference in the cumulative incidence of relapse at 5 years between patients who received G-CSF and patients in the control group. It has to be investigated whether molecular aberrations of the G-CSF receptor or a dysregulation in the downstream signal transduction might determine an increased risk of relapse in individual patients. For example, mutations in the G-CSF receptor gene resulting in truncation of the carboxy-terminal region, which transduces maturation signals and suppresses the receptor's proliferative signal, have been detected in a subset of patients with severe congenital neutropenia who developed AML.34
In conclusion, the results of our randomized study of prophylactic administration of G-CSF in children undergoing therapy for AML indicate that G-CSF reduces time of neutropenia but did not result in fewer episodes of infections or in improved outcome. Therefore, one cannot advocate the general prophylactic use of G-CSF in children with de novo AML. Further trials have to determine if there are subgroups of children with AML who could benefit from G-CSF therapy.
Authorship
Author contributions: T.L. contributed to the analysis and the interpretation of the data and drafted the manuscript; M.Z. contributed to the analysis and the interpretation and revised the manuscript; D.R. contributed to the design, the analysis, and the interpretation of the data, and revised the manuscript; M.D. contributed to the design, the analysis, and the interpretation of the data; J.S. contributed to the design, the analysis, and the interpretation of the data; and U.C. contributed to the conception, design, analysis, and the interpretation of the data, and revised the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Thomas Lehrnbecher, Pediatric Hematology and Oncology, Childrens Hospital III, Johann Wolfgang Goethe-University, Theodor-Stern-Kai 7, D-60590 Frankfurt, Germany; e-mail: thomas.lehrnbecher@kgu.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Supported by the Deutsche Krebshilfe and the Czech Ministry of Education MSM0021620813.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal