Abstract
Progress to uncover the molecular and cellular regulators that govern human hematopoietic stem cell (HSC) fate has been impeded by an inability to obtain highly purified fractions of HSCs. We report that the rhodamine 123 (Rho 123) dye effluxing fraction of the Lin−CD34+CD38− population contains SCID-repopulating cells (SRCs) capable of long-term repopulation in primary nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Purification based on Rho uptake led to a 4-fold enrichment of SRCs in the Lin−CD34+CD38− fraction, with a frequency of 1 SRC in 30 Lin−CD34+CD38−Rholo cells. The Lin−CD34+CD38−Rholo fraction also possesses long-term self-renewal capacity as measured by serial transplantation totaling more than 20 weeks. We conclude that Rho dye efflux provides an additional means of purifying human HSCs in the quest to achieve homogeneous populations of primitive cells for both experimental and therapeutic applications.
Introduction
To identify factors that govern human hematopoietic stem cell (HSC) fate decisions (self-renewal or differentiation), pure HSC populations are required. Human HSCs are enriched in the Lin−CD34+CD38− cell fraction, as assessed by the functional SCID-repopulating cell (SRC) assay.1,2 The frequency of SRCs in cord blood (CB) was estimated to be 1 in 600 Lin−CD34+CD38− cells.3 Recent improvements to the nonobese diabetic/severe combined immunodeficient (NOD/SCID) model have suggested that SRC frequency in this fraction is 1% to 2%.4 Thus, while markedly enriched for HSCs, this fraction still remains heterogeneous. In the mouse, multiple markers are used to purify HSCs. One functional parameter used to purify HSCs is uptake of Rho. Rho is a fluorescent dye that binds to active mitochondria in cells, thus reflecting the metabolic state of the cell.5-7 Rho efflux is mediated by P-glycoprotein, encoded by the multidrug resistance gene-1.8 Murine long-term stem cells are enriched based on low retention of Rho.9-11 Previous studies have attempted to separate human HSCs from progenitor cells using Rho, however, the SRC activity of purified fractions was not assessed.8,12 Here, we used the SRC assay to show that differential Rho efflux can be used to enrich human HSCs in the Lin−CD34+CD38− cell fraction.
Materials and methods
NOD/SCID mouse repopulation
All animal experimental protocols were approved by the Animal Care Committee of the University Health Network. The NOD/SCID repopulation assay was performed by intrafemoral injection as described.13,14 Sublethally irradiated NOD/SCID mice (3.5 Gy) were treated with 200 μg/mouse anti-CD122 antibody 24 hours prior to transplantation.15 Mice were killed at 3 to 10 weeks after transplantation and the injected right femur (RF) and noninjected femur, tibia, and pelvis (BM) were analyzed by flow cytometry for human engraftment. Whole bone marrow from either RF or BM was injected into 2 separate preconditioned secondary mice, without further purification or adjustment for human-cell content. The transplanted cell dose from the RF and BM of mice that received a transplant of Lin−CD34+CD38−Rholo cells was 9.0 ± 0.6 × 106 and 29 ± 2.6 × 106 cells, respectively; and from the RF and BM of mice that received a transplant of Lin−CD34+CD38−Rhohi cells was 7.5 ± 1.1 × 106 and 20.5 ± 2.6 × 106 cells, respectively.
Sample collection and purification
Samples of cord blood (CB) were obtained according to procedures approved by the institutional review board of Trillium Hospital and University Health Network. Human CB samples were depleted of lineage-positive and CD38+ cells by negative selection using the StemSep system (Stem Cell Technologies, Vancouver, BC). Rho staining was performed as described.9 Briefly, cells were incubated at 37°C in 0.1 μg/mL rhodamine 123 dye (Eastman Kodak, Rochester, NY) for 30 minutes and destained at 37°C for an additional 30 minutes. CD38 and CD34 staining was performed as described.12 Cells were sorted on a MoFlo (Dako Cytomation, Fort Collins, CO). Twenty percent cutoffs were used to sort Lin−CD34+CD38−Rholo and Lin−CD34+CD38−Rhohi cell fractions as shown in Figure 1A. Sorted cells were injected at a dose of 3500 cells per mouse unless cells were injected for limiting dilution analysis (see “Limiting dilution analysis”).
Lin−CD34+CD38−Rholo cells provide a significantly higher level of human engraftment than Lin−CD34+CD38−Rhohi cells in NOD/SCID mice. (A) Lineage and CD38-depleted CB cells were sorted into Lin−CD34+CD38−Rholo and Lin−CD34+CD38−Rhohi fractions. The gates for Rho sorting were set as the bottom and top 20% of Rho uptake within the Lin−CD34+CD38− population. Equal numbers of cells (3500 per mouse) from sorted fractions were transplanted and mice were evaluated for erythroid (CD45−CD36+GlyA+, ▨) and lymphomyeloid (CD45+, ▪) human engraftment at 3 weeks (n = 21 mice) (B) and lymphomyeloid engraftment at 7 weeks (n = 16 mice) (C) in both the injected right femur (RF) and remaining bone marrow (BM). (*) P < .05 versus Lin−CD34+CD38−Rhohi cells for CD45+ grafts in both panels B and C. Error bars represent SE.
Lin−CD34+CD38−Rholo cells provide a significantly higher level of human engraftment than Lin−CD34+CD38−Rhohi cells in NOD/SCID mice. (A) Lineage and CD38-depleted CB cells were sorted into Lin−CD34+CD38−Rholo and Lin−CD34+CD38−Rhohi fractions. The gates for Rho sorting were set as the bottom and top 20% of Rho uptake within the Lin−CD34+CD38− population. Equal numbers of cells (3500 per mouse) from sorted fractions were transplanted and mice were evaluated for erythroid (CD45−CD36+GlyA+, ▨) and lymphomyeloid (CD45+, ▪) human engraftment at 3 weeks (n = 21 mice) (B) and lymphomyeloid engraftment at 7 weeks (n = 16 mice) (C) in both the injected right femur (RF) and remaining bone marrow (BM). (*) P < .05 versus Lin−CD34+CD38−Rhohi cells for CD45+ grafts in both panels B and C. Error bars represent SE.
Lentivirus production, infection, and clonal analysis
A third-generation lentiviral vector encoding the enhanced green fluorescent protein (Egfp) gene was used for clonal tracking experiments as described.13,16 Lin−CD34+CD38−Rholo cells were infected at a concentration of 1.7 × 105 cells/mL with an MOI of 130 to 180. Genomic DNA from the RF and BM of mice injected with transduced cells was extracted and clonal analysis was performed as reported.16 Gene transfer efficiency (13.6%) was estimated in suspension cultures as described.17
Limiting dilution analysis
Preconditioned mice received an intrafemoral transplant of 200, 100, or 50 Lin−CD34+CD38− cells and 200, 100, or 33 Lin−CD34+CD38−Rholo cells along with 3000 irradiated (15 Gy) lineage-positive CB cells, and were killed after 7 to 10 weeks. Recipients were considered to be engrafted if the RF contained more than 0.4% CD45+ human cells by flow cytometry or was positive by Southern blot analysis using a human-specific probe as described.18 Data from 3 limiting dilution experiments (n = 65 mice) were pooled and analyzed using L-Calc software (Stem Cell Technologies).
Statistical analysis
Data are presented as mean ± standard error (SE). The significance of the differences between groups was determined by the Student t test (SigmaStat software; Jandel, Chicago, IL).
Results and discussion
Equivalent numbers of Lin−CD34+CD38−Rholo and Lin−CD34+CD38−Rhohi (hereafter referred to as Rholo and Rhohi, respectively) cells were transplanted intrafemorally into preconditioned NOD/SCID mice, and human engraftment was evaluated at 3 and 7 weeks after transplantation (Figure 1B and C, respectively) in the injected right femur (RF) and remaining bone marrow (BM: left femur, tibiae, and pelvis). This strategy enabled assessment of both repopulation and migration, properties characteristic of the most primitive SRCs.19 At 3 weeks (Figure 1B), Rholo cells provided significantly higher levels of lymphomyeloid (CD45+) engraftment in the RF than Rhohi cells (mean, 22.4% ± 3.4% versus 8.7% ± 3.5%), although only a trend was observed for the total human graft (CD45+ lymphomyeloid and CD45−CD36+GlyA+ erythroid cells). Similar to other grafts characterized at this early time point,13 we observed high levels of CD45−CD36+GlyA+ erythroid cells in the RF (mean, 37.4% ± 5.6% and 22.5% ± 8.5% from Rholo and Rhohi cells, respectively). Comparable results were observed for engraftment in BM. By 7 weeks after transplantation (Figure 1C), Rholo cells provided a significantly larger lymphomyeloid (CD45+) graft in both the RF and BM compared with Rhohi cells (mean, RF 74.5% ± 10.9% versus 23.8% ± 12.3%; BM 48.8% ± 8.5% versus 3.0% ± 1.4%), suggesting that the Rholo immunophenotype may enrich for HSCs.
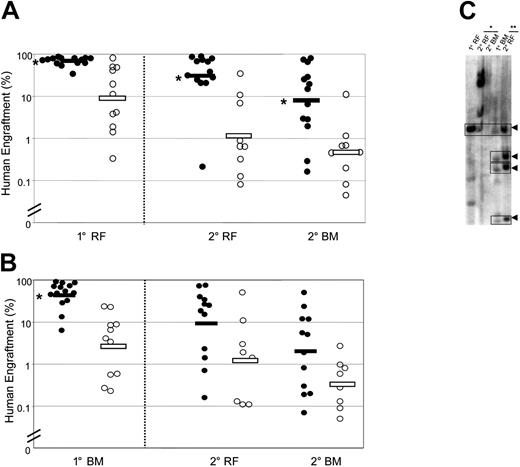
In order to evaluate the content of self-renewing HSCs in each cell population, we performed serial transplantations. Cells from the RF of primary mice that received a transplant of Rholo cells produced significantly larger human grafts in secondary mice (both in RF and BM) compared with cells from the RF of primary mice that received a transplant of Rhohi cells (geometric means in secondary mice: RF, 33.1% versus 1.3%; BM, 8.7% versus 0.5%) (Figure 2A). The same trend was seen in secondary mice that received a transplant of primary BM cells (Figure 2B). To directly examine the self-renewal of individual Rholo SRCs, we performed lentiviral-mediated clonal tracking for 3 primary mice and the corresponding 6 secondary mice. The presence of clones with the same restriction fragment size in primary mice that received a transplant of Rholo cells and their secondary recipients (Figure 2C) demonstrates the existence of self-renewing HSCs within the Rholo population. Overall, these data indicate that selection for cells with Rholo staining enables further enrichment of HSCs within the Lin−CD34+CD38− population.
Serial transplantation demonstrates superior self-renewal of Lin−CD34+CD38−Rholo SRCs compared with Lin−CD34+CD38−Rhohi SRCs. Sorted cell populations were injected intrafemorally into preconditioned primary (1°) mice. After 7 to 10 weeks, the ability of SRCs to repopulate secondary (2°) mice was assessed separately for cells from the primary injected RF (A) and remaining BM (B). Secondary recipients were killed 7 to 10 weeks after transplantation, and human cell engraftment was assessed in the injected RF and remaining BM. Primary mice were injected with Lin−CD34+CD38−Rholo cells (•) or Lin−CD34+CD38−Rhohi cells (○). Bars indicate geometric means. (*) P < .05 versus Lin−CD34+CD38−Rhohi cells. (C) Southern blot analysis of repopulating clones in representative primary mice that received a transplant of Lin−CD34+CD38−Rholo cells and corresponding secondary recipients. Lanes were loaded with DNA obtained from RF or BM of a primary recipient and from the corresponding tissues of 2 secondary recipients injected with cells from the primary RF (*) or remaining BM (**). Self-renewing clones are indicated by arrowheads.
Serial transplantation demonstrates superior self-renewal of Lin−CD34+CD38−Rholo SRCs compared with Lin−CD34+CD38−Rhohi SRCs. Sorted cell populations were injected intrafemorally into preconditioned primary (1°) mice. After 7 to 10 weeks, the ability of SRCs to repopulate secondary (2°) mice was assessed separately for cells from the primary injected RF (A) and remaining BM (B). Secondary recipients were killed 7 to 10 weeks after transplantation, and human cell engraftment was assessed in the injected RF and remaining BM. Primary mice were injected with Lin−CD34+CD38−Rholo cells (•) or Lin−CD34+CD38−Rhohi cells (○). Bars indicate geometric means. (*) P < .05 versus Lin−CD34+CD38−Rhohi cells. (C) Southern blot analysis of repopulating clones in representative primary mice that received a transplant of Lin−CD34+CD38−Rholo cells and corresponding secondary recipients. Lanes were loaded with DNA obtained from RF or BM of a primary recipient and from the corresponding tissues of 2 secondary recipients injected with cells from the primary RF (*) or remaining BM (**). Self-renewing clones are indicated by arrowheads.
Limiting dilution analysis was performed to quantify SRC enrichment. The frequency of SRCs was estimated as 1 in 30 for Rholo cell fractions (95% confidence interval: 1 SRC in 18 to 1 SRC in 51 Rholo cells) and 1 in 121 for Lin−CD34+CD38− fractions (95% confidence interval: 1 SRC in 74 to 1 SRC in 200 Lin−CD34+CD38− cells) (Table S1, available on the Blood website; see the Supplemental Table link at the top of the online article). We defined the Rholo fraction as the lower 20% of the Rho-stained Lin−CD34+CD38− population. Therefore, if all stem cells were in the Rholo fraction, the expected frequency would be 1 SRC in 24 Rholo cells. Our estimated frequency of 1 SRC in 30 Rholo cells indicates that most long-term repopulating cells are contained in this fraction. Thus, cell sorting based on Rho dye efflux provides an additional parameter for purification of human HSCs. Studies in the murine system suggest that purification of homogeneous HSC populations may not be possible as stem cells may be stochastically regulated by random intrinsic and extrinsic factors.11 However, this improved protocol is a significant step toward achieving a more homogeneous population of primitive cells for both experimental and therapeutic applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: J.L.M. and O.I.G. designed research, performed research, collected and analyzed data, and wrote the paper; K.T. designed research, performed research, collected and analyzed data, and edited the paper; M.D. performed research and collected data; J.E.D. designed research, analyzed data, and edited the paper.
J.L.M. and K.T. made equal contributions to this work.
We thank P. Scheufler, P. Savage, and the entire obstetrics unit (Trillium Hospital, Mississauga, ON, Canada) for providing CB samples; G. Mallia and S. Zere for processing the CB samples; S. Zhao (Hospital for Sick Children, Toronto, ON, Canada) for sorting; T. Tanaka (Osaka University Medical Center) for supplying the TM-β1 hybridoma cell line15 ; and Jean Wang for her critical review of the paper.
This work was supported by grants from The Stem Cell Network of National Centres of Excellence, the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society and the Terry Fox Foundation, Genome Canada through the Ontario Genomics Institute, Ontario Cancer Research Network with funds from the Province of Ontario, the Leukemia and Lymphoma Society, the Canadian Institutes for Health Research, and a Canada Research Chair.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal