Abstract
The development of inhibitory antibodies to factor VIII is a serious complication of hemophilia. FEIBA (factor VIII inhibitor-bypassing activity), an activated prothrombin complex concentrate (aPCC), and NovoSeven, recombinant factor VIIa (rFVIIa), are used as hemostatic bypassing agents in treating patients with inhibitors. The FENOC study was designed to test equivalence of the products in the treatment of ankle, knee, and elbow joint bleeding. A prospective, open-label, randomized, crossover, equivalency design was used. The parameters of interest were the percentage of patients who reported efficacy in response to FEIBA and the percentage that reported efficacy in response to NovoSeven. A difference in these percentages of no more than 15% was determined to be a clinically acceptable magnitude for equivalence of the 2 products. The primary outcome was evaluation 6 hours after treatment. Data for 96 bleeding episodes contributed by 48 participants were analyzed. The criterion for declaring the 2 products equivalent at 6 hours was not met; however, the confidence interval of the difference in percentages of efficacy reported for each product only slightly exceeded the 15% boundary (−11.4%-15.7%), P = .059. FEIBA and NovoSeven appear to exhibit a similar effect on joint bleeds, although the efficacy between products is rated differently by a substantial proportion of patients. This trial was registered at www.clinicaltrials.gov as #NCT00166309.
Introduction
The development of persistent inhibitory antibodies to factor VIII is a serious and costly complication of replacement therapy occurring in people with hemophilia.1 The ultimate goal of treatment is to permanently eradicate the inhibitor by immune tolerance induction therapy (ITI), thereby making it possible for the patient to be treated routinely with replacement therapy.2,3 ITI fails, however, in a substantial number of patients and is not generally available worldwide because of its high cost. For the nontolerized patient, surgical procedures and the treatment of bleeding episodes become unpredictable and are associated with high risk. Patients with low-responding inhibitors (ie, with peak titers ≤ 5 BU/mL) face fewer clinical problems in that hemostasis can usually be achieved by saturating the inhibitor with higher doses of the deficient factor.4,5 However, in patients with high-responding inhibitors (> 5 BU/mL), the factor will be neutralized and other treatment modalities must be used. FEIBA (factor VIII inhibitor-bypassing activity), an activated prothrombin complex concentrate (aPCC), has been used as a hemostatic bypassing agent in patients with high-responding inhibitors for decades.6 More recently, NovoSeven, recombinant factor VIIa (rFVIIa), has been added as a treatment option.7,8 The bypassing mechanisms of these agents are different and not fully understood. However, they are both able to bypass the factor VIII–dependent step in the coagulation cascade and promote hemostasis by enhancing thrombin generation.9,10 Although successfully used in a variety of challenging clinical situations with an overall efficacy of 80% to 90%,11-13 aPCC and rFVIIa are not always effective. The different mechanisms of action provide a theoretic foundation for interindividual as well as intraindividual variation in the clinical efficacy between the 2 agents. A comparative, randomized study between the 2 agents has never been performed, leaving clinicians with factors such as product availability, safety considerations, and personal experience on which to base their choice of treatment. Treatment with bypassing agents is very costly, which, in an era of cost constraint, further necessitates that the choice of therapeutic interventions be guided by evidence-based medicine. The present study, the FEIBA NovoSeven Comparative (FENOC) Study, was undertaken to address these issues.
The FENOC study evaluated 2 bypassing agents used in the treatment of joint bleeding in congenital hemophilia A complicated by inhibitors. The study was designed to test the equivalence of the 2 products, that is, the degree to which they provide the same, or nearly the same, clinical efficacy.
Patients, materials, and methods
Study population
Patients aged 2 years or older with congenital hemophilia A, an inhibitor, and the need for bypassing agents in the treatment of joint bleeding were eligible to participate. Those with other congenital or acquired bleeding disorders, symptomatic liver disease defined as an INR more than 1.5 and/or a platelet count less than 50 × 109/L (50 000 × 106/L), or a life expectancy fewer than 12 months were excluded from participation. The sample size was targeted at 60 patients, each with 2 joint bleeds (ankles, knees, or elbows). The institutional review boards of Malmö University Hospital and of collaborating institutions approved the FENOC study; informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Study design
A prospective, open-label, randomized, crossover, clinical equivalency study design was used. A difference in efficacy of no more than 15% was determined to be a clinically acceptable magnitude for equivalence. Each participant was treated with 1 dose of FEIBA (75-100 IU/kg body weight; target dose, 85 IU/kg) or 2 doses of NovoSeven (90-120 μg/kg body weight; target dose, 105 μg/kg × 2) administered intravenously in a randomized fashion, with crossover between the options for the following bleeding episode. The second dose of NovoSeven was administered 2 hours after the first dose. Neither product was supplied free of charge by the manufacturers for use in the study. FEIBA is manufactured by Baxter (Vienna, Austria); NovoSeven is manufactured by NovoNordisk (Hillerod, Denmark).
Study procedures
Prior to entry, the patient or guardian provided written consent, following which eligibility was confirmed and a physical examination was performed. Randomization in association with the first bleeding event was performed in a block of patients equally divided into 2. A randomization list specifying the order of treatment for enrolled patients was provided to each participating center.
The study participant was instructed to initiate treatment within 4 hours after the symptoms of bleeding started. Date, time, cause of bleeding, type of treatment(s), time of administration, and joint affected were recorded. The hemostatic effect of each concentrate was evaluated by the patient at 2 (before the second dose of NovoSeven), 6, 12, 24, 36, and 48 hours in terms of whether the patient thought the treatment was effective, partially effective, poorly effective, or not effective, and whether the bleeding had stopped. Additional treatments and the occurrence of rebleeding were recorded.
Statistical methods
All analyses were performed on completed pairs of evaluations according to the intention to treat. The primary outcome of the study was evaluation of the hemostatic effect at 6 hours following treatment. An effective response was defined by creating a dichotomy of the effective and partially effective response versus poorly effective and not effective. The statistical parameters of interest were the percentages of patients who reported an effective or partially effective response to FEIBA and the percentage who reported an effective or partially effective response to NovoSeven. For this equivalency study, the null hypothesis was that the difference in these percentages was more than 15%, and the alternative hypothesis was that the difference in the percentages was 15% or less. The data were analyzed using a modified version of the McNemar test proposed by Lee and Lusher.14 Rejecting the null hypothesis at the .05 level was equivalent, in this setting, to showing that the upper and lower limits of a 90% confidence interval for the difference in efficacy fell within plus or minus 15%. The same method was applied to an analysis of the patient's report of whether the bleeding had stopped.
In 2 instances, the type of product was switched during the course of the bleed from the assigned treatment to the other product. Analyses of efficacy and time at which bleeding stopped were censored at the time of the product switch. In analyses of the total infusions received and the number of infusions required to stop the bleed, the infusions of the “other” product were counted. Three patients assigned to the NovoSeven group received 1 infusion instead of the prescribed 2 infusions. In the comparison of number of infusions required to stop the bleeding, data for these 3 were combined with those of patients receiving 2 infusions. The paired signed rank test was used to compare the actual number of infusions required to stop the bleed to the number specified by the protocol (1 FEIBA and 2 NovoSeven) in the 2 treatment groups. Thus, the comparison considered 1 FEIBA infusion and 1 to 2 NovoSeven infusions “the same.” The time to bleed stop was defined as the first time point at which the participant thought that the bleeding had stopped. Generalized estimating equation (GEE)15 models were used to test for differences in the distribution of type of joint bleed by treatment, the distribution of efficacy outcomes by treatment, time from the onset of bleeding to treatment, and the effect of knee bleeds on efficacy. Logistic regression was used to investigate predictors of discordant evaluations of efficacy of the treatments. A chi-square test for equal proportions was used to test for differences in products favored among pairs with discordant outcomes. A McNemar test was used in exploratory analyses of differences between FEIBA and NovoSeven.
Results
Characteristics of the cohort
Sixty-six patients were enrolled from 27 centers in Europe and North America. Fourteen withdrew prior to treatment or were treated only once. Reasons for withdrawal included the following: no bleeding episodes in the study joints or timing of prophylactic or other infusions prevented participation (6); lack of compliance with the protocol (3); concern about use of an unfamiliar product, or change of mind about participation (2). The study was stopped by the Ministry of Health in one country because the products for treatment were not provided free of charge (1). No reason was given for the withdrawal of 2 patients. The diaries for 4 patients were not adequately completed for inclusion in the analysis. No study-related or study drug–related adverse experiences were reported. Forty-eight pairs (96 bleeding episodes) were included in the intent-to-treat analysis reported here, providing an estimated power of 54%.
At enrollment, the mean age was 27.5 years (range, 8-55 years). All participants had a factor VIII clotting activity level in plasma of less than 0.02 kIU/L, and the median inhibitor titer was 8.6 BU/mL (range, 0-1800 BU/mL). None was undergoing immune tolerance induction. The measurements of platelets and INR were very similar by treatment period, as were the causes of bleeds. Approximately 85% of the total bleeding episodes were spontaneous, and 15% were related to trauma. Twenty-seven pairs (56.3%) were bleeds in the same joint. The distribution of joints (Table 1) was different by treatment period (P < .001) with knee bleeds occurring more frequently in the NovoSeven group and elbow bleeding occurring more frequently in the FEIBA group. Three episodes of bleeding in “nonstudy” joints were treated.
Distribution of study joints by treatment
| Joint . | FEIBA, no. (%) . | NovoSeven, no. (%) . |
|---|---|---|
| Knee | 15 (31.3) | 26 (54.2) |
| Elbow | 21 (43.8) | 10 (20.8) |
| Ankle | 10 (20.8) | 9 (18.8) |
| Elbow and knee | 0 (0.0) | 1 (2.1) |
| Ankle and knee | 0 (0.0) | 1 (2.1) |
| Shoulder | 1 (2.1) | 0 (0.0) |
| Hip | 1 (2.1) | 1 (2.1) |
| Joint . | FEIBA, no. (%) . | NovoSeven, no. (%) . |
|---|---|---|
| Knee | 15 (31.3) | 26 (54.2) |
| Elbow | 21 (43.8) | 10 (20.8) |
| Ankle | 10 (20.8) | 9 (18.8) |
| Elbow and knee | 0 (0.0) | 1 (2.1) |
| Ankle and knee | 0 (0.0) | 1 (2.1) |
| Shoulder | 1 (2.1) | 0 (0.0) |
| Hip | 1 (2.1) | 1 (2.1) |
For both the FEIBA and NovoSeven categories, N = 48.
The median time from onset of bleed to treatment was 2 hours in both groups (range, 0-9.5 hours). Three of the 96 treatments were administered between 4 and 5 hours, and 2 (one in each treatment group) were administered 8 or more hours after onset of the bleed. The median initially prescribed dose of FEIBA was 84.6 IU/kg (range, 51.5-100.0 IU/kg) and of NovoSeven was 212.5 μg/kg (includes both doses) (range, 98.6-261.8 μg/kg). A total of 91.7% of FEIBA doses prescribed were within the range recommended in the protocol, with 3 patients receiving doses that were below the range. Seventy-nine percent of NovoSeven doses were within the recommended range. Seven patients received doses below the range, and 3 received doses above the range. Approximately 25% of patients reported use of analgesics in each treatment period. The distributions of type of analgesic used were quite similar.
Data regarding type of treatment and products used in the 6 months prior to enrollment were available for 46 of the 48 patients included in the analysis. Forty-three (93.5%) were on on-demand therapy, 4.4% (n = 2) were on prophylaxis, and 1 participant had been on both types of treatment. Fifty percent of the group had exposure to both FEIBA and NovoSeven, 17.4% to FEIBA only, and 23.9% to NovoSeven only. Four participants, 8.7%, had not been exposed to either product in the 6 months prior to enrollment.
Evaluation of the hemostatic effect
The rates of efficacy, confidence intervals of the differences, and P values are shown by time point and type of treatment in Table 2. Although the proportions of patients who rated both treatments effective or partially effective were fairly similar at each evaluation, the statistical criterion for declaring the 2 products equivalent was not met. At every interval examined, the ends of the confidence interval were either more than 15% or less than −15%. At 6 hours after infusion (the primary outcome), however, the confidence interval only slightly exceeded the 15% boundary (−11.4%-15.7%) with a corresponding P value of .059.
Rates of efficacy by treatment and time point
| Hours after infusion (N) . | FEIBA, % . | NovoSeven, % . | 90% confidence interval, %* . | P . |
|---|---|---|---|---|
| 2† (48) | 75.0 | 60.4 | −0.73-29.90 | .482 |
| 6 (47) | 80.9 | 78.7 | −11.42-15.67 | .059 |
| 12 (45) | 80.0 | 84.4 | −18.08-9.19 | .101 |
| 24 (42) | 95.2 | 85.7 | −1.29-20.33 | .202 |
| 36 (41) | 100.0 | 90.2 | 2.13-17.38 | .129 |
| 48 (41) | 97.6 | 85.4 | 2.05-22.34 | .325 |
| Hours after infusion (N) . | FEIBA, % . | NovoSeven, % . | 90% confidence interval, %* . | P . |
|---|---|---|---|---|
| 2† (48) | 75.0 | 60.4 | −0.73-29.90 | .482 |
| 6 (47) | 80.9 | 78.7 | −11.42-15.67 | .059 |
| 12 (45) | 80.0 | 84.4 | −18.08-9.19 | .101 |
| 24 (42) | 95.2 | 85.7 | −1.29-20.33 | .202 |
| 36 (41) | 100.0 | 90.2 | 2.13-17.38 | .129 |
| 48 (41) | 97.6 | 85.4 | 2.05-22.34 | .325 |
Efficacy is defined as effective or partially effective by patient rating. The 6-hour time point is the primary outcome.
The 90% confidence interval for the difference in the proportions of patients' rating of efficacy for each of the treatments (columns 2 and 3). Rejecting the null hypothesis at the .05 level is equivalent, in this setting, to showing that the upper and lower limits of the confidence interval for the difference in efficacy fall within plus or minus 15%.
Prior to the second dose of NovoSeven.
The study population was divided into 2 groups by location of center: North America/Western Europe and Eastern Europe/Russia, and the rates of efficacy at 2 and 6 hours were examined. While dividing the study group greatly reduced the statistical power to evaluate the equivalence of the treatments, it permitted observation of whether patient reports in 2 geographic areas that might differ in treatment practices could potentially affect outcome. Table 3 shows that there was general agreement in the evaluations of efficacy between the 2 groups.
Rates of efficacy by geographic area, hours after infusion, and treatment
| Geographic area . | FEIBA, % . | NovoSeven, % . |
|---|---|---|
| Western Europe/North America | ||
| 2 h* | 75.0 | 65.0 |
| 6 h | 73.7 | 79.0 |
| Eastern Europe/Russia | ||
| 2 h* | 75.0 | 57.1 |
| 6 h | 85.7 | 78.6 |
| Geographic area . | FEIBA, % . | NovoSeven, % . |
|---|---|---|
| Western Europe/North America | ||
| 2 h* | 75.0 | 65.0 |
| 6 h | 73.7 | 79.0 |
| Eastern Europe/Russia | ||
| 2 h* | 75.0 | 57.1 |
| 6 h | 85.7 | 78.6 |
Efficacy is defined as effective or partially effective by patient rating. For the Western Europe/North America group, N = 20; for the Eastern Europe/Russia group, N = 28.
Prior to second dose of NovoSeven.
More frequent bleeding in the treated joint was related to lower ratings of efficacy at 6 hours (P = .048), as was presence of a target joint defined as having had 8+ bleeding episodes in the year prior to enrollment (P = .026). The number of prior bleeding episodes in the treated joints did not differ by treatment period, however. The mean (median) number of prior bleeds in joints treated with FEIBA was 5.0 (4.0) and in those treated with NovoSeven was 4.9 (4.0). The effect of knee bleeds, more common in the group treated with NovoSeven, was explored. Patient rating of efficacy of treatment for knee bleeds was quite similar in both groups, and results of a GEE analysis did not show that knee bleeding was related to efficacy at any time point.
The proportion of discordant pairs (one treatment effective/the other not effective) ranged from a high of 43.8% at the 2-hour time point to a low of 9.8% at the 36-hour time point (Table 4)The maximum number of pairs for which neither treatment was effective, 5 (10.4%), was observed at the 2-hour time point. Four pairs were discordant in favor of one treatment and then switched, at a subsequent time point, to favor the other treatment. The mean number of bleeding episodes in treated joints in the year prior to enrollment was somewhat higher (P = .043), among those discordant at the 6-hour efficacy evaluation. The mean, median, and range of bleeding episodes in the discordant pair group was 7.3 (median, 5.8; range, 0.5-19.0) compared with 3.7 (median, 3.8; range, 0-16.5) among those whose evaluations of both products were concordant. A similar observation was made at 12 hours (P = .055).
Proportion of pairs discordant for rating of treatment outcome
| Hours after infusion . | Efficacy of treatment . | Bleeding stopped . | ||
|---|---|---|---|---|
| N . | % Discordant pairs . | N . | % Discordant pairs . | |
| 2* | 48 | 43.8 | 47 | 40.4 |
| 6 | 47 | 31.9 | 46 | 32.6 |
| 12 | 45 | 31.1 | 45 | 33.3 |
| 24 | 42 | 19.1 | 42 | 14.3 |
| 36 | 41 | 9.8 | 41 | 12.2 |
| 48 | 41 | 17.1 | 41 | 7.3 |
| Hours after infusion . | Efficacy of treatment . | Bleeding stopped . | ||
|---|---|---|---|---|
| N . | % Discordant pairs . | N . | % Discordant pairs . | |
| 2* | 48 | 43.8 | 47 | 40.4 |
| 6 | 47 | 31.9 | 46 | 32.6 |
| 12 | 45 | 31.1 | 45 | 33.3 |
| 24 | 42 | 19.1 | 42 | 14.3 |
| 36 | 41 | 9.8 | 41 | 12.2 |
| 48 | 41 | 17.1 | 41 | 7.3 |
Efficacy is defined as effective or partially effective by patient rating.
Prior to second dose of NovoSeven.
Among those discordant at one or more time points, 19 favored FEIBA and 10 favored NovoSeven (P = .095). Nineteen patients had no preference (were concordant at all time points) or alternated between favoring one, then the other, over the course of the follow-up period.
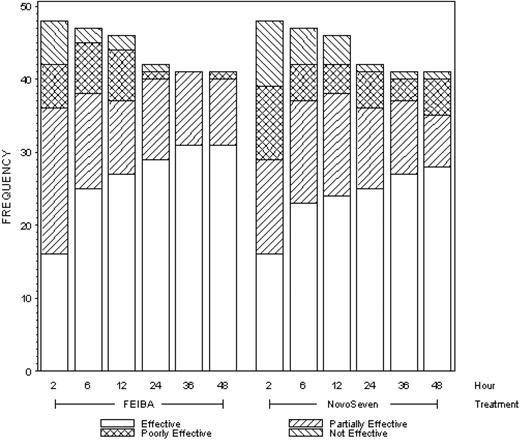
The full distribution of efficacy outcomes (effective, partially effective, poorly effective, not effective) is shown in Figure 1 for all time points. There were no statistically significant differences in the distribution of outcomes by treatment at any time point, and in exploratory analyses neither product was shown to be superior, in terms of the proportions effective or partially effective, at any time point.
Frequencies (counts) of efficacy outcomes by time point and type of treatment.
Stopping the bleeding
The proportions of patients reporting that bleeding had stopped, confidence intervals of the differences, and P values are shown by time point and treatment in Table 5. The percentages reporting stopping of bleeding were similar for both treatments over time with the ends of the confidence intervals within plus or minus 15% at 24 (P = .038) and 48 (P = .001) hours. As observed in the evaluation of efficacy, the highest proportion of discordant pairs, 40.4%, was observed at the 2-hour time point (Table 4). The rate of discordance decreased to 7.3% by the 48-hour evaluation. It was also at 2 hours that the maximum number of pairs, 16 (34.0%), reported that neither treatment had stopped the bleed. By 48 hours, this had decreased to 1 pair of bleeds (2.4%) for which neither treatment was able to stop the bleeding.
Proportion reporting that bleeding had stopped by treatment and time point
| Hours after infusion (N) . | FEIBA, % . | NovoSeven, % . | 90% confidence interval, %* . | P . |
|---|---|---|---|---|
| 2† (47) | 53.2 | 38.3 | 0.06-29.72 | .495 |
| 6 (46) | 76.1 | 65.2 | −2.73-24.47 | .309 |
| 12 (45) | 77.8 | 75.6 | −11.92-16.37 | .069 |
| 24 (42) | 90.5 | 85.7 | −4.75-14.28 | .038 |
| 36 (41) | 95.1 | 87.8 | −1.45-16.09 | .075 |
| 48 (41) | 95.1 | 92.7 | −4.48-9.36 | .001 |
| Hours after infusion (N) . | FEIBA, % . | NovoSeven, % . | 90% confidence interval, %* . | P . |
|---|---|---|---|---|
| 2† (47) | 53.2 | 38.3 | 0.06-29.72 | .495 |
| 6 (46) | 76.1 | 65.2 | −2.73-24.47 | .309 |
| 12 (45) | 77.8 | 75.6 | −11.92-16.37 | .069 |
| 24 (42) | 90.5 | 85.7 | −4.75-14.28 | .038 |
| 36 (41) | 95.1 | 87.8 | −1.45-16.09 | .075 |
| 48 (41) | 95.1 | 92.7 | −4.48-9.36 | .001 |
The 90% confidence interval for the difference in the proportions of patients' rating of whether the bleeding had stopped for each of the treatments (columns 2 and 3). Rejecting the null hypothesis at the .05 level is equivalent, in this setting, to showing that the upper and lower limits of the confidence interval fall within plus or minus 15%.
Prior to the second dose of NovoSeven.
The number of infusions administered in each treatment group and the number required to stop the bleeding were examined. When using FEIBA, patients received a mean of 1.3 infusions (median, 1; range, 1-4); when using NovoSeven, they received a mean of 2.4 infusions (median, 2; range, 1-8). Additional doses were administered in cases where the protocol treatment regimen was not sufficient. The timing of the additional doses varied. A small number (n = 2) were administered within the first 6 hours after onset of treatment; the remainder, during the balance of the 48-hour observation period. The number of infusions required to stop the bleeding in the FEIBA group was 1.2 (median, 1; range, 1-3) and in the NovoSeven group, 2.1 (median, 2; range, 1-6). In 2 of the 3 patients who received only 1 infusion of NovoSeven (instead of 2), bleeding had stopped by the 2-hour time point. Bleeding had stopped by 6 hours in the third. No rebleeding occurred in the NovoSeven patients who received only 1 dose of the product. With 1 to 2 infusions of NovoSeven considered the “same” as 1 infusion of FEIBA, there was no difference between the 2 products in the number of infusions required to stop the bleed (P = .325).
Three rebleeds occurred in 3 patients during their NovoSeven treatment period. These occurred at approximately 4, 12, and 40 hours after the onset of treatment.
From exploratory testing, neither product was shown to be superior, in terms of proportion of patients for whom the bleeding had stopped, at any time point.
Intention to treat versus per protocol
The results presented are based on the intention-to-treat analysis. Observed deviations from the protocol included administration of more frequent or less frequent doses than recommended, delays in initiation of treatment (> 4 hours after onset of bleed), treatment of nonstudy joints, and use of an antifibrinolytic agent within 6 hours of onset of treatment. There were 2 errors in randomization in which the order of administration of the products was switched. A per-protocol analysis excluding these patients was completed with results very similar to those presented. At 6 hours after onset of treatment, 81.3% and 78.1% of recipients of FEIBA and NovoSeven, respectively, reported the treatment as effective or partially effective. The confidence interval of the difference in those proportions was −15.4% to 21.6% (P = .146). The products were found to be equivalent with respect to patient rating that the bleeding had stopped at 24, 36, and 48 hours (all P < .05).
Discussion
This is the first randomized study attempting to evaluate and compare the effect of aPCC and rFVIIa. As no head-to-head comparison between the 2 products has ever been performed, clinicians have relied on anecdotal evidence and opinions based on their own experience in making choices between the agents.
While it is clear that there are differences in the products in terms of mechanism and manufacture, clinical experience suggested the selection of a study design to evaluate equivalence in clinical efficacy.11-13 Difference in efficacy of no more than 15% was deemed a clinically acceptable magnitude for equivalent or “nearly the same.” While the patient ratings of effective were generally similar between the 2 products at the time intervals evaluated, the statistical criteria for equivalence were met infrequently over the time points and outcomes (efficacy and stopping the bleeding) evaluated. To some degree, particularly for efficacy at the 6-hour time point, this was probably related to a lack of statistical power. Although the products did not meet the statistical criteria for equivalence at the majority of time points examined, this cannot be construed as evidence that one product was different or better. In exploratory analysis comparing the 2, neither product was superior to the other, either in efficacy or ability to stop bleeding at any time point examined.
The FENOC study was also initiated to provide documentation of the rates of efficacy of each product, as determined by patient report. Based on available reports in the literature, both products appear to be effective in the majority of bleeding events, but in a substantial number of bleeds the hemostatic effect is not satisfactory.11-13,16 Because the products promote thrombin generation by different mechanisms, there is reason to believe that the action of the agents may differ in individual cases.9,10
The dosing used in this study was selected because it was consistent with that recommended by the manufacturers, and supported in the literature by single-product studies.12,13,17 Both products were associated with an efficacy rate of from 80% to 90% at some time points, consistent with previous reports.11-13,17 Another important observation was the high frequency of discordant pairs. At the 6-hour evaluation, the primary outcome, almost one third of the pairs were discordant. This is a higher proportion than expected and the reason for this is unclear. At 6 and 12 hours, discordance was somewhat more common in patients with higher mean numbers of bleeds in the treated joints in the year prior to enrollment. This was not the case at 2 hours, prior to the second dose of NovoSeven, whether because the observations at 6 and 12 hours were spurious findings or that other factors obscured the importance of number of prior bleeds at the earliest time point. Another possibility to consider is antibody reactivity toward coagulation factors other than the deficient factor in hemophilic inhibitor plasma.18 Whether this is relevant for the FENOC findings remains to be studied. A random switching in and out of the responder categories (measurement “noise”) should also be considered.
Participants were instructed to initiate treatment within 4 hours after the symptoms of bleeding started because early treatment of acute joint bleeding has been associated with greater success in terms of response, and fewer doses needed.19 The early time point set for the primary outcome, 6 hours after onset of treatment, was chosen for several reasons: (1) An effective product should provide a prompt clinical effect on the bleed, (2) symptoms in the joint that might be related to inflammation secondary to the bleeding should be avoided as much as possible in the rating of efficacy, and (3) rating of the later time points in the natural course of a bleed is less informative as most, if not all, joint bleeds stop by themselves with time due to pressure built up in the joint. The high response observed at 6 hours speaks to efficacious action of the products on the clotting system. On the other hand, the declining rate of discordance with time may be secondary to the self-limiting effect of the bleed. A reasonable interpretation, though, is that different patients have a different early response to the 2 products reflected in a high rate of discordance early in the course of the bleed, and a decrease over time. From a pathophysiological standpoint, an early response would be beneficial, resulting in lower pressure in the joint, less blood and blood constituents, and therefore less iron deposition and inflammatory response over time.16,20 Thus, a product with a good hemostatic effect during the first few hours of a bleed could reduce the risk of cartilage destruction, which is particularly important in a population of patients who may bleed frequently.
There are several factors that need to be considered in the interpretation of the FENOC study results. Lack of treatment blinding and the loss of almost 20% of the cohort prior to study completion must be addressed. Although 14 patients enrolled and subsequently withdrew before receiving one or both treatments, the reasons for withdrawal were primarily related to inability to meet protocol requirements or because of center withdrawal for reasons unrelated to the treatment. Two patients withdrew because of reluctance to take an unfamiliar product. Blinding of a study, however desirable, is almost impossible with 2 very different products, therefore necessitating the open study design. On the other hand, participation by a large number of centers over a vast geographic area (ie, the United States and Western and Eastern Europe) increases the generalizability of the study, thereby strengthening the findings. Of note is that no geographic difference in evaluation of efficacy was observed when data from centers in the United States and Western Europe were compared with those from centers in Eastern Europe and Russia.
The FENOC study is the first to evaluate the use of bypassing agents in inhibitor patients in a comparative way; therefore, these data are of significance to the treating community. The main finding is that FEIBA and NovoSeven appear to exhibit a similar effect on joint bleeds, although the efficacy between products is rated differently by a substantial proportion of patients, especially during the first 12 hours after the start of symptoms. This is a very important time period, as early resolution of a bleed may be critical for prevention of cartilage destruction. The interindividual response to bypassing agents is important to clarify, from a pathophysiological and pharmacological point of view. In addition, in vitro measurements to estimate and to predict the in vivo effect may allow an even higher efficacy rate than that demonstrated in the FENOC study. It is also imperative to develop improved treatment protocols as well as algorithms for the nonresponding patient.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the FENOC Study Group appears as a data supplement to the online version of this article.
Contribution: J.A. designed research, performed research, participated in oversight of data collection and analysis, and wrote the paper; S.M.D. had oversight of data collection and analysis and wrote the paper; D.M.D. and A.G. performed research and participated in writing the paper; S.A.G. performed data analysis and participated in writing the paper; J.W. coordinated data collection and participated in the analysis; and E.B. designed research, performed research, participated in oversight of data collection and analysis, and wrote the paper.
Acknowledgments
We wish to acknowledge the contributions of M. L. Lee, University of California–Los Angeles (UCLA) School of Public Health, to protocol and analysis plan development; L. Aledort, Mount Sinai School of Medicine, New York, NY, for oversight of data and safety monitoring; E. Mattson, Malmö University Hospital, Malmö, Sweden, for study coordination; and A. Lail, Rho, Chapel Hill, NC, for statistical analysis.
This work was supported by an investigator-initiated unrestricted grant from Baxter BioScience. All collection, management, and analysis of study data were completed independently by the investigators.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal