Abstract
In elderly patients with acute myeloid leukemia (AML) treated intensively, no best postremission strategy has emerged yet. This clinical trial enrolled 416 patients with AML aged 65 years or older who were considered eligible for standard intensive chemotherapy, with a first randomization comparing idarubicin with daunorubicin for all treatment sequences. After induction, an ambulatory postremission strategy based on 6 consolidation cycles administered monthly in outpatients was randomly compared with an intensive strategy with a single intensive consolidation course similar to induction. Complete remission (CR) rate was 57% with 10% induction deaths, and estimated overall survival was 27% at 2 years and 12% at 4 years, without notable differences between anthracycline arms. Among the 236 patients who reached CR, 164 (69%) were randomized for the postremission comparison. In these patients, the multivariate odds ratio in favor of the ambulatory arm was 1.51 for disease-free survival (P =.05) and 1.59 for overall survival from CR (P =.04). Despite repeated courses of chemotherapy associated with a longer time under treatment, the ambulatory arm was associated with significantly shorter rehospitalization duration and lower red blood cell unit and platelet transfusion requirements than observed in the intensive arm. In conclusion, more prolonged ambulatory treatment should be preferred to intensive chemotherapy as postremission therapy in elderly patients with AML reaching CR after standard intensive remission induction.
Introduction
Advances in the treatment of younger patients with acute myeloid leukemia (AML) have been obtained with intensified postremission treatments, such as high-dose chemotherapy or allogeneic stem cell transplantation.1,2 However, approximately half of patients with newly diagnosed AML are older than 65 years and thus are not eligible for such options. In these older patients, even the benefit associated with standard intensive chemotherapy remains debated because of excessive toxicity and short response duration. Factors related to age, including a poorer performance status (PS) and comorbidities, may directly affect treatment tolerance.3–7 Factors related to disease biology, including more frequent prior myelodysplastic syndrome (MDS) and unfavorable karyotype, may lower the response rate and response duration.3–7 In a recent large retrospective study, the outcome of elderly persons with AML was very poor, with a median survival of 2 months and a 2-year survival rate of 6%; only 30% of them underwent chemotherapy treatment within 2 years after AML diagnosis.8
In patients treated with intensive chemotherapy, how to maintain complete remission (CR) remains a major issue. There is no validated postremission strategy in these patients. A frequent option is to try to apply several consolidation courses, even if a substantial proportion of patients in CR may eventually not receive the whole planned treatment as a result of acquired comorbidities.9 On the other hand, a beneficial effect of prolonged maintenance with low-dose chemotherapy has been reported in elderly patients but not in younger patients.10,11 When the present study was initiated, no study had prospectively compared an intensive to a more prolonged ambulatory consolidation. Ambulatory consolidation might be less toxic and easier to apply in most patients. Both approaches needed, however, to be prospectively compared.
The ALFA-9803 study was thus initiated in November 1999 by the Acute Leukemia French Association (ALFA). Patients aged 65 years or more with de novo or post-MDS AML and considered fit for intensive chemotherapy were prospectively enrolled with an initial randomization between daunorubicin and idarubicin for the whole therapy. A second randomization comparing a single intensive consolidation course to 6 less-intensive ambulatory courses was offered to CR patients with 2-year overall survival as primary end point.
Patients, materials, and methods
Disease eligibility
Patients aged 65 years or more with newly diagnosed, previously untreated AML with 20% or more myeloid marrow blasts, either “de novo” or evolving from a MDS, were eligible for the study in the absence of central nervous system involvement. AML was defined here as post-MDS only if MDS had been previously diagnosed on blood and bone marrow examination for a minimum period of 3 months. Patients with refractory anemia with excess of blast in transformation (RAEB-T), according to the French-American-British (FAB) classification,12–14 were thus eligible. Patients with AML evolving from a prior myeloproliferative disorder according to the WHO classification,15 as well as those with acute promyelocytic leukemia, or those with prior exposure to chemotherapeutic agents and/or radiotherapy, were not eligible.
Patient eligibility
To be eligible, patients may not have presented contraindications for intensive chemotherapy, defined as: 1) prior congestive heart failure requiring treatment and/or left ventricular systolic ejection fraction below the normal range; 2) a creatinine or bilirubin level more than twice the upper limit of normal, except if AML-related; 3) a PS score of 4; and 4) uncontrolled severe infection. All patients gave their signed informed consent before entering the study. The study was approved in June 1999 by the institutional review board (IRB) of Hôpital Pitie-Salpetriere, Paris, France, and conducted in accordance with the Declaration of Helsinki.
Study population
Between November 1999 and March 2006, a total of 429 patients from 24 participating centers in France were randomized in this ALFA-9803 study. Among these patients, 13 did not meet all inclusion criteria. The study population thus comprised 416 patients.
Cytogenetics
Cytogenetic abnormalities were grouped according to standard ISCN criteria.16 At least 15 normal mitoses were required to defined a normal karyotype. A 2-class risk classification was used for all prognostic analyses. The high-risk subset included monosomy 7, presence of abnormalities of both chromosomes 5 and 7, 3q26 abnormalities, and complex karyotype with 5 anomalies or more. All other abnormalities as well as normal karyotypes were included in the standard-risk subset.
Treatments
Patients were randomized at baseline (R1 randomization) to receive either daunorubicin (DNR) or idarubicin (IDA) as an anthracycline throughout the study with a stratification on centers and AML type (de novo AML versus post-MDS AML). Standard induction chemotherapy consisted of a 4 + 7 course with either DNR at a daily dosage of 45 mg/m2 or IDA at a daily dosage of 9 mg/m2 for 4 days (days 1-4) in combination with 200 mg/m2 cytarabine per day as a continuous IV infusion for 7 days (days 1-7). Lenograstim (Granocyte) granulocyte colony-stimulating factor (G-CSF) was administered in all patients from day 9 until myeloid recovery at a daily dosage of 263 μg/d intravenously for a maximum of 28 days. A blood and marrow aspiration smear examination was performed on day 21. A salvage course could be offered to patients with persistent leukemia. Salvage consisted of 6 1-hour IV boluses of intermediate-dose cytarabine (500 mg/m2/12 h, days 1-3) combined to mitoxantrone (MTZ) at a daily dosage of 12 mg/m2 for 2 days (days 3 and 4). Lenograstim administration was stopped before salvage onset and restarted from day 5 of the salvage course until myeloid recovery for a maximum of 28 days.
If they did not present acquired contraindication to further intensive chemotherapy (according to baseline criteria), patients reaching CR were eligible for a second R2 randomization between ambulatory and intensive postremission therapy with a stratification on centers, AML type, and R1 groups. Ambulatory consolidation consisted of 6 monthly courses with either 45 mg/m2 DNR or 9 mg/m2 IDA for 1 day (day 1) in combination with 60 mg/m2/12 h cytarabine as home subcutaneous infusions for 5 days (days 1 to 5) without G-CSF. Intensive consolidation was an exact repetition of the first induction course and administered in hospitalized patients.
Response criteria
Responses were evaluated by blood and marrow aspiration smear examination 1 week after the last infusion of lenograstim (ie on day 45 in patients without earlier myeloid recovery) and classified according to recommendations of the International Working Group.17 These recommendations define morphologic complete remission (CR), morphologic CR with incomplete blood count recovery (CRi), morphologic AML-free state (AML-FS), partial remission (PR), and resistant disease (RD). Induction death (ID) was defined as death occurring before response evaluation unless evidence of RD was provided at least 7 days after conclusion of the chemotherapy. Salvage could be offered to patients with RD or PR. Relapses were classified as AML recurrences or MDS recurrences. MDS recurrence was defined as reappearance of cytopenia with a marrow blast percentage less than 20% and no extramedullary disease for a minimum of 3 months.
Endpoints and statistical methods
Overall survival (OS) at 2 years was the primary endpoint. Event for OS was death and patients were censored at the date of last contact if alive. Events for disease-free survival (DFS) were death and first relapse (either AML or MDS recurrence). The median follow-up of patients alive was 34 months. OS and DFS were estimated by the Kaplan Meier method.18 Graphical methods were used to test the proportional hazards assumption.19 OS and DFS comparisons were performed with the log-rank test20 or stratified Cox proportional hazards models to allow the baseline hazard functions to differ in the early compared with the later follow-up.21 Cumulative incidences of relapse and death in first CR were compared by the Gray test.22 Multivariate analyses were performed using the Cox model and tested by the log likelihood ratio test. All analyses were performed on an intent-to-treat basis. Hazard ratio (HR) estimates with 95% confidence intervals (CI) are given. All calculations were performed using the STATA software, version 9.0E (Stata Corporation, College Station, TX) and the R software, version 1.5.1 (http://www.r-project.org/).
Results
Patient characteristics
Patient and AML characteristics are indicated in Table 1. Median age was 72 years (range, 65-85). Three hundred fifty-three patients had de novo AML whereas 63 patients (15%) had post-MDS AML. Cytogenetics data were available in 339 patients. Among these 339 patients, 49 were classified in the high-risk subset (14%) and 290 in the standard-risk subset. Details are given in Table 1.
Characteristics of the 416 patients
| Sex ratio, M/F, no. | 225/191 |
| Median age (range), y | 72 (65-85) |
| Performance status, no. patients | |
| 0 | 109 |
| 1 | 193 |
| 2 | 102 |
| 3 | 12 |
| Median WBC count (range), × 109/L | 5.3 (0.1-420) |
| AML morphology, no. patients | |
| de novo AML | 353 |
| RAEB-T | 26 |
| FAB-M1/2 | 173 |
| FAB-M4/5 | 112 |
| FAB-M0/6/7 | 122 |
| Unclassified | 20 |
| post-MDS AML | 63 |
| RAEB-T | 16 |
| Other | 47 |
| Karyotype, no. patients | |
| Not done | 27 |
| Failure | 50 |
| High risk | 49 |
| Monosomy 7 | 7 |
| Abnormalities of chromosome 5 and 7 | 6 |
| 3q26 abnormalities | 4 |
| Complex, 5 abnormalities or more | 32 |
| Standard risk | 290 |
| t(8;21) | 6 |
| inv(16)/t(16;16) | 6 |
| Normal | 168 |
| Isolated trisomy 8 | 17 |
| del(5q) | 13 |
| del(7q) | 9 |
| 11q23 abnormalities | 6 |
| Complex, 3 or 4 abnormalities | 8 |
| Other various abnormalities | 57 |
| Sex ratio, M/F, no. | 225/191 |
| Median age (range), y | 72 (65-85) |
| Performance status, no. patients | |
| 0 | 109 |
| 1 | 193 |
| 2 | 102 |
| 3 | 12 |
| Median WBC count (range), × 109/L | 5.3 (0.1-420) |
| AML morphology, no. patients | |
| de novo AML | 353 |
| RAEB-T | 26 |
| FAB-M1/2 | 173 |
| FAB-M4/5 | 112 |
| FAB-M0/6/7 | 122 |
| Unclassified | 20 |
| post-MDS AML | 63 |
| RAEB-T | 16 |
| Other | 47 |
| Karyotype, no. patients | |
| Not done | 27 |
| Failure | 50 |
| High risk | 49 |
| Monosomy 7 | 7 |
| Abnormalities of chromosome 5 and 7 | 6 |
| 3q26 abnormalities | 4 |
| Complex, 5 abnormalities or more | 32 |
| Standard risk | 290 |
| t(8;21) | 6 |
| inv(16)/t(16;16) | 6 |
| Normal | 168 |
| Isolated trisomy 8 | 17 |
| del(5q) | 13 |
| del(7q) | 9 |
| 11q23 abnormalities | 6 |
| Complex, 3 or 4 abnormalities | 8 |
| Other various abnormalities | 57 |
WBC indicates white blood cell count; FAB, French-American-British classification; RAEB-T, refractory anemia with excess of blast in transformation.
Response to induction therapy
Response to induction therapy is shown in Table 2. Overall, 236 patients (57%) achieved CR (223 of them after the first induction course), 17 were in CRi, 22 in AML-FS, 6 in PR, and 95 had RD. Induction death rate was 10%. Two hundred nine patients were randomized to the DNR arm and 207 patients to the IDA arm. Their characteristics were well-balanced (Table 3). Complete remission rate (54% versus 59%, P = .28) and ID rate (10% versus 9%, P = .87) were similar in each arm. The percentage of patients who reached CR after one induction course was significantly higher in the IDA arm (59% versus 48%, P = .03). Among the 120 patients with RD or PR after the first induction course (73 in the DNR and 47 in the IDA arm), 71 were considered candidates for the salvage course, and 54 actually received it (35 in the DNR and 19 in the IDA arm). Among these 54 patients, 13 eventually achieved CR (12 in the DNR and 1 only in the IDA arm).
Response to induction therapy
| . | After first induction, n . | Eligible for salvage, n . | Received salvage, n . | Overall response, n (%) . |
|---|---|---|---|---|
| CR | 223 | na* | 0 | 236 (57%) |
| CRi | 12 | na* | 0 | 17 (4%) |
| AML-FS | 21 | na* | 0 | 22 (5%) |
| PR | 4 | 4 | 1 | 6 (1%) |
| RD | 116 | 67 | 53 | 95 (23%) |
| ID | 40 | na | na | 40 (10%) |
| . | After first induction, n . | Eligible for salvage, n . | Received salvage, n . | Overall response, n (%) . |
|---|---|---|---|---|
| CR | 223 | na* | 0 | 236 (57%) |
| CRi | 12 | na* | 0 | 17 (4%) |
| AML-FS | 21 | na* | 0 | 22 (5%) |
| PR | 4 | 4 | 1 | 6 (1%) |
| RD | 116 | 67 | 53 | 95 (23%) |
| ID | 40 | na | na | 40 (10%) |
CR indicates complete remission; CRi, CR with incomplete blood count recovery; AML-FS, morphologic AML-free state; PR, partial remission; RD, resistant disease; na, not applicable.
According to the protocol, salvage was offered to PR and RD patients only.
Patient characteristics among R1 randomization arms
| . | DNR arm . | IDA arm . | P value . |
|---|---|---|---|
| No. patients | 209 | 207 | NA |
| Median age, y | 72 | 72 | .91 |
| AML type, no. patients | |||
| de novo AML | 179 | 174 | .68 |
| post-MDS AML | 30 | 33 | |
| Median WBC count, × 109/L | 5.5 | 5 | .69 |
| Karyotype, no. patients | |||
| Not done | 11 | 16 | .33 |
| Failure | 36 | 14 | .001 |
| Standard-risk | 137 | 153 | .07 |
| High-risk | 25 | 24 | .99 |
| . | DNR arm . | IDA arm . | P value . |
|---|---|---|---|
| No. patients | 209 | 207 | NA |
| Median age, y | 72 | 72 | .91 |
| AML type, no. patients | |||
| de novo AML | 179 | 174 | .68 |
| post-MDS AML | 30 | 33 | |
| Median WBC count, × 109/L | 5.5 | 5 | .69 |
| Karyotype, no. patients | |||
| Not done | 11 | 16 | .33 |
| Failure | 36 | 14 | .001 |
| Standard-risk | 137 | 153 | .07 |
| High-risk | 25 | 24 | .99 |
NA indicates not applicable.
In univariate analysis, PS score, age, and white blood cell count (WBC), were identified as predictive of ID (P = .001, .008, and .03, respectively) (Table 4). Performance status also predicted CR achievement (P = .01). ID rate was 6% in patients with a PS score of 0 or 1 compared with 17% in those with a PS score ≥ 2. It is noteworthy that WBC correlated with PS score (P = .03). Median WBC was 4.4 G/L in patients with a PS score of 0 or 1, compared with 13.9 G/L in those with a PS score ≥ 2. In multivariate analysis, only age and PS score remained predictive of ID (Table 4).
Bad prognostic factors for response to induction therapy and overall survival in the whole population of 416 patients
| . | Univariate . | Multivariate . | |
|---|---|---|---|
| P value* . | P value* . | HR (95% CI)† . | |
| For induction death | |||
| Advanced age | .008 | .02 | 1.65 (1.08-2.54) |
| High PS | .01 | .003 | 2.81 (1.42-5.56) |
| High WBC | .03 | NS | 1.04 (0.99-1.09) |
| AML type | .06 | NS | 0.34 (0.08-1.49) |
| For CR achievement | |||
| High PS | .02 | NS | 1.47 (0.90-2.40) |
| High-risk karyotype | < .001 | < .001 | 4.11 (2.11-8.03) |
| For overall survival | |||
| Advanced age | .014 | .014 | 1.23 (1.04-1.45) |
| High PS | < .001 | .05 | 1.32 (1.00-1.75) |
| High WBC | .002 | < .001 | 1.04 (1.02-1.07) |
| High-risk karyotype | < .001 | < .001 | 3.95 (2.78-5.61) |
| . | Univariate . | Multivariate . | |
|---|---|---|---|
| P value* . | P value* . | HR (95% CI)† . | |
| For induction death | |||
| Advanced age | .008 | .02 | 1.65 (1.08-2.54) |
| High PS | .01 | .003 | 2.81 (1.42-5.56) |
| High WBC | .03 | NS | 1.04 (0.99-1.09) |
| AML type | .06 | NS | 0.34 (0.08-1.49) |
| For CR achievement | |||
| High PS | .02 | NS | 1.47 (0.90-2.40) |
| High-risk karyotype | < .001 | < .001 | 4.11 (2.11-8.03) |
| For overall survival | |||
| Advanced age | .014 | .014 | 1.23 (1.04-1.45) |
| High PS | < .001 | .05 | 1.32 (1.00-1.75) |
| High WBC | .002 | < .001 | 1.04 (1.02-1.07) |
| High-risk karyotype | < .001 | < .001 | 3.95 (2.78-5.61) |
NS indicates not significant.
P values are given for age as a continuous variable, PS as a 2-class variable (0–1, ≥2), WBC as a continuous variable, AML type (de novo vs post-MDS), and karyotype as a 2-class variable (standard-risk, high-risk).
HRs are given for the high-risk as compared with the standard-risk karyotype subset, for patients with a PS ≥ 2 as compared with those with PS of 0-1, for 10-G/L WBC increment, and for 5-year age increment.
In univariate analysis, high-risk cytogenetic and high PS score were identified as predictive of no CR achievement (Table 4). CR rate was 63% in the standard-risk cytogenetic subset, 29% in the high-risk cytogenetic subset, 50% in patients in whom cytogenetic evaluation failed, and 56% in those in whom cytogenetic evaluation was not performed (P < .001). Within the standard-risk subset, no difference in CR rate was observed between patients with a normal karyotype (62.5%) and those with chromosomal abnormalities (63%). CR rate was 60% in patients with a PS score of 0 or 1 compared with 47% in those with a PS score ≥ 2 (P = .02). After adjustment, only high-risk cytogenetics remained predictive of no CR achievement (Table 4).
Overall survival
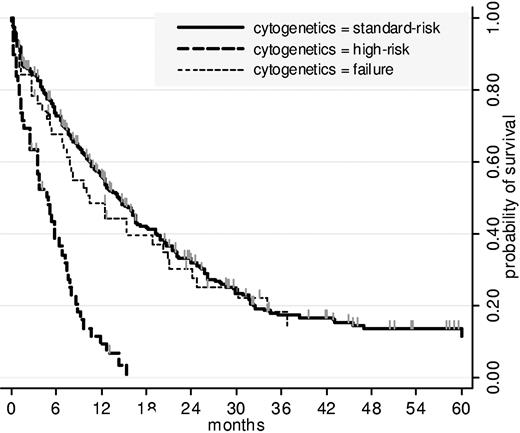
Among the 416 patients, 310 died, including 40 induction deaths. Median overall survival was 12 months. Estimated OS was 27% at 2 years (95% CI, 23-32) and 12% at 4 years (95% CI, 8-16). Overall survival estimations were similar in both DNR and IDA randomization arms (P = .37 by the log-rank test). As indicated in Table 4, 4 factors were independently predictive of shorter OS. Figure 1 shows OS among the 2 cytogenetic subgroups (standard-risk and high-risk) as well as in the subgroup of patients in whom cytogenetic evaluation failed. No clear cutoff was identified for WBC. For age, the best cutoff was 75 years. Estimated OS at 2 years was 18% (95% CI, 10 to 29) in the 84 patients aged 75 years or more versus 29% (95% CI, 24 to 35) in the remaining 332 patients (P = .008).
Overall survival, by cytogenetics. As indicated here for the 3 subgroups (high risk, standard risk, failure) cytogenetics significantly influenced OS (P < .001 by the log-rank test). No difference in survival was observed between standard-risk and failure subgroups (P = .59 by the log-rank test).
Overall survival, by cytogenetics. As indicated here for the 3 subgroups (high risk, standard risk, failure) cytogenetics significantly influenced OS (P < .001 by the log-rank test). No difference in survival was observed between standard-risk and failure subgroups (P = .59 by the log-rank test).
Outcome of CR patients and effect of postremission therapy
Among the 236 CR patients, 163 relapsed and 146 died (135 deaths after relapse and 11 deaths in first CR). Estimated OS from CR was 40% at 2 years (95% CI, 33-47) and 19% at 4 years (95% CI, 13-26).
Fifty-three CR patients were not eligible for the postremission randomization R2 because of acquired comorbidity (severe infection, 28; poor PS, 16; congestive heart failure, 5; renal failure, 2; other, 2). Nineteen eligible patients withdrew their consent, and 164 were randomized to receive either ambulatory or intensive consolidation. This represented 69% of all CR patients, but only 39% of the whole study population. Characteristics of both randomization groups (82 patients in each arm) were well-balanced (Table 5). Fifteen patients withdrew their consent just after randomization (14 in the intensive arm and 1 in the ambulatory). The overall rate of consent withdrawal in the whole population of patients eligible for R2 was thus 19%.
Patient characteristics among R2 randomization arms
| . | Ambulatory arm . | Intensive arm . | P value . |
|---|---|---|---|
| No. patients | 82 | 82 | NA |
| RI arm* | 37/45 | 42/40 | .53 |
| Median age, y | 71 | 72 | .48 |
| AML type, no. patients | |||
| de novo AML | 72 | 73 | .99 |
| post-MDS AML | 10 | 9 | .99 |
| Median WBC count, × 109/L | 5.5 | 6.1 | .81 |
| Karyotype, no. patients | |||
| Not done | 7 | 4 | .53 |
| Failure | 7 | 9 | .79 |
| Standard-risk | 63 | 66 | .70 |
| High-risk | 5 | 3 | .72 |
| . | Ambulatory arm . | Intensive arm . | P value . |
|---|---|---|---|
| No. patients | 82 | 82 | NA |
| RI arm* | 37/45 | 42/40 | .53 |
| Median age, y | 71 | 72 | .48 |
| AML type, no. patients | |||
| de novo AML | 72 | 73 | .99 |
| post-MDS AML | 10 | 9 | .99 |
| Median WBC count, × 109/L | 5.5 | 6.1 | .81 |
| Karyotype, no. patients | |||
| Not done | 7 | 4 | .53 |
| Failure | 7 | 9 | .79 |
| Standard-risk | 63 | 66 | .70 |
| High-risk | 5 | 3 | .72 |
NA indicates not applicable.
DNR/IDA.
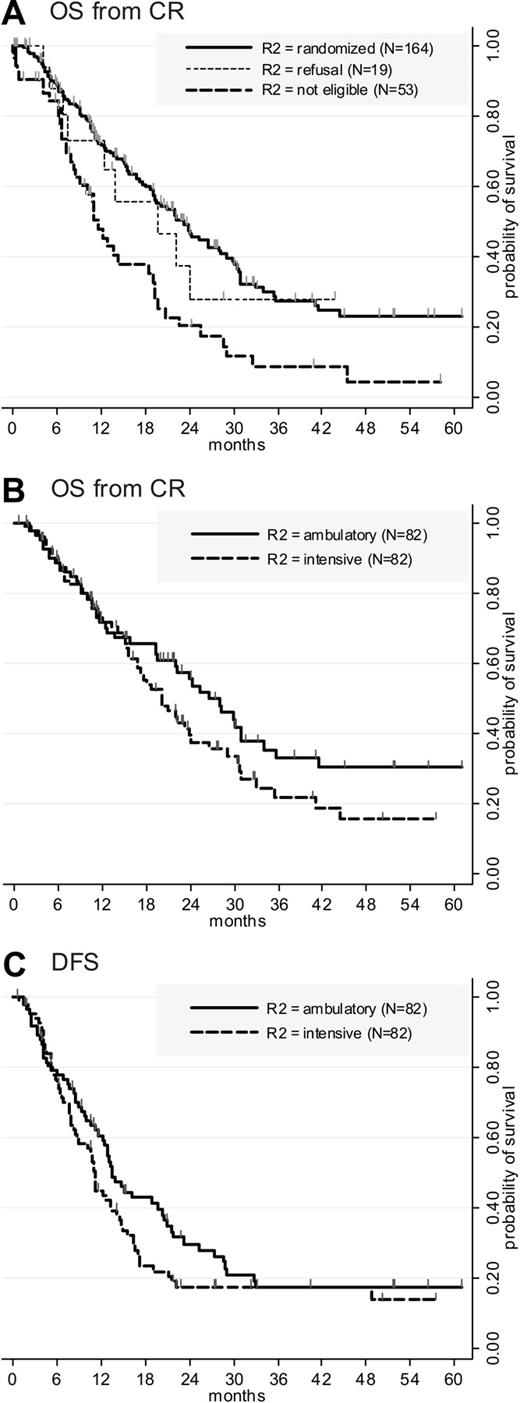
Among all CR patients, the proportion of patients aged 75 years or more was lower in those who were eventually randomized for postremission therapy than in those who were not randomized (13% versus 29%, P = .006). These 2 subsets of patients, however, were comparable regarding PS (P = .25), WBC (P = .53), AML type (P = .30), cytogenetics (P = .36), and R1 randomization arm (P = .99). As expected, OS from CR was significantly shorter in the 53 patients who were not considered for R2 randomization due to acquired comorbidity than in the 164 randomized patients (Figure 2A; P < .001 by the log-rank test). Conversely, OS from CR was equivalent in the 19 patients who refused the R2 randomization than in the 164 randomized patients (P = .71 by the log-rank test).
Outcome from CR. (A) OS from CR. Events accounting for overall survival were 97 deaths in the 164 randomized patients compared with 40 deaths in the 53 patients noneligible for R2 randomization (P < .001 by the log-rank test) and 9 deaths in the 19 patients who refused R2 randomization (P = .71 by the log-rank test). (B) OS from CR according to the postremission randomization. Events accounting for overall survival were 53 deaths in the intensive arm compared with 44 deaths in the ambulatory arm (P = .03 by stratified Cox). (C) DFS according to the postremission randomization. Events accounting for disease-free survival were 59 relapses and 4 deaths in first complete remission in the intensive arm compared with 58 relapses and no deaths in first complete remission in the ambulatory arm (P = .04 by stratified Cox). Among the 4 deaths in first complete remission, 3 occurred during the neutropenic period after intensive consolidation (2 pulmonary infections, 1 septic shock), and 1 occurred later and was probably not related to AML therapy (rapid development of a lung carcinoma).
Outcome from CR. (A) OS from CR. Events accounting for overall survival were 97 deaths in the 164 randomized patients compared with 40 deaths in the 53 patients noneligible for R2 randomization (P < .001 by the log-rank test) and 9 deaths in the 19 patients who refused R2 randomization (P = .71 by the log-rank test). (B) OS from CR according to the postremission randomization. Events accounting for overall survival were 53 deaths in the intensive arm compared with 44 deaths in the ambulatory arm (P = .03 by stratified Cox). (C) DFS according to the postremission randomization. Events accounting for disease-free survival were 59 relapses and 4 deaths in first complete remission in the intensive arm compared with 58 relapses and no deaths in first complete remission in the ambulatory arm (P = .04 by stratified Cox). Among the 4 deaths in first complete remission, 3 occurred during the neutropenic period after intensive consolidation (2 pulmonary infections, 1 septic shock), and 1 occurred later and was probably not related to AML therapy (rapid development of a lung carcinoma).
When OS from CR was analyzed on the intent-to-treat basis, a significant difference in favor of the ambulatory arm was observed (Figure 2B; P = .03 by stratified Cox). At 2 years, estimated OS from CR was 56% (95% CI, 43-66) in the ambulatory arm compared with 37% (95% CI, 26-49) in the intensive arm. After adjustment on age, AML type, and cytogenetics, ambulatory consolidation arm remained the only factor predictive for longer survival from CR in a multivariate model (Table 6)
Bad prognostic factors for DFS and OS from CR in the 164 patient randomized for postremission therapy
| . | Univariate . | Multivariate . | |
|---|---|---|---|
| P value* . | P value* . | HR (95% CI)† . | |
| For DFS | |||
| Advanced age | .01 | .04 | 1.33 (1.01-1.74) |
| AML type | .02 | .05 | 1.75 (1.01-3.02) |
| High-risk karyotype | < .001 | < .001 | 8.40 (3.27-21.6) |
| Intensive consolidation | .04 | .05 | 1.51 (1.00-2.27) |
| For OS from CR | |||
| Advanced age | .045 | NS | 1.16 (0.85-1.59) |
| AML type | .11 | NS | 0.94 (0.52-1.71) |
| High-risk karyotype | < .001 | NS | 1.81 (0.75-4.39) |
| Intensive consolidation | .03 | .04 | 1.59 (1.02-2.47) |
| . | Univariate . | Multivariate . | |
|---|---|---|---|
| P value* . | P value* . | HR (95% CI)† . | |
| For DFS | |||
| Advanced age | .01 | .04 | 1.33 (1.01-1.74) |
| AML type | .02 | .05 | 1.75 (1.01-3.02) |
| High-risk karyotype | < .001 | < .001 | 8.40 (3.27-21.6) |
| Intensive consolidation | .04 | .05 | 1.51 (1.00-2.27) |
| For OS from CR | |||
| Advanced age | .045 | NS | 1.16 (0.85-1.59) |
| AML type | .11 | NS | 0.94 (0.52-1.71) |
| High-risk karyotype | < .001 | NS | 1.81 (0.75-4.39) |
| Intensive consolidation | .03 | .04 | 1.59 (1.02-2.47) |
NS indicates not significant.
P values are given for age as a continuous variable, AML type (de novo vs post-MDS), and karyotype as a 2-class variable (standard-risk, high-risk).
HRs are given for the high-risk as compared with the standard-risk karyotype subset, for 5-year age increment, for post-MDS as compared with de novo AML, and for intensive as compared with ambulatory post-remission therapy.
Ambulatory consolidation arm was also significantly associated with longer DFS (Figure 2C; P = .04 by stratified Cox). At 2 years, estimated DFS was 28% (95% CI, 18-39) in the ambulatory arm compared with 17% (95% CI, 10-27) in the intensive arm. After adjustment on age, AML type, and cytogenetics as a 2-class variable, all these 4 factors remained associated with longer DFS (Table 6). At 2 years, cumulative incidence of death in first CR was 5% in the intensive and 0% in the ambulatory arm (P = .04, by the Gray test). Cumulative incidence of relapse was 78% in the intensive and 70% in the ambulatory arm (P = .54, by the Gray test). It is noteworthy that postrelapse survival was the same within each randomization arm (P = .91 by the log-rank test) with a median of 6 months.
When comparisons were performed on the basis of postremission treatment actually received rather than on the intent-to-treat basis, multivariate odds ratio for shorter DFS (HR = 1.62, 95% CI, 1.06-2.46; P = .025) and OS (HR = 1.64, 95% CI, 1.04-2.59; P = .03) in the intensive arm remained significant.
Details on postremission treatments actually received by the 164 randomized patients, as well as need of rehospitalization and transfusion, are given in Table 7. Despite repeated courses of chemotherapy and longer median time under therapy, ambulatory postremission arm was associated with significantly shorter rehospitalization duration and less red blood cell unit and platelet transfusions than observed after the intensive consolidation in the intensive arm. These later comparisons were performed in patients who received the whole planned postremission treatment and did not included resources associated with eventual treatment of relapse.
Details on treatments received by the 164 patients randomized for postremission therapy
| . | Ambulatory arm . | Intensive arm . | P value . |
|---|---|---|---|
| No. patients randomized | 82 | 82 | NA |
| Patients who received the planned treatment†, no. | 76 | 67 | .06 |
| Consent withdrawal after R2 | 1 | 14* | .001 |
| Treatment interruption‡ | 5 | 1 | .21 |
| Days under therapy§, median (range) | 247 (215-316) | 82 (63-160) | <.001 |
| Need of rehospitalization and transfusion§ | |||
| No. of days, median (range) | 0 (0-31) | 27 (20-84) | <.001 |
| Patients with febrile neutropenia, % | 39 | 100 | <.001 |
| Transfusion requirements | |||
| RBC units, median (range) | 2 (0-24) | 6 (0-12) | .007 |
| Platelet transfusions, median (range) | 1 (0-18) | 4 (1-15) | <.001 |
| . | Ambulatory arm . | Intensive arm . | P value . |
|---|---|---|---|
| No. patients randomized | 82 | 82 | NA |
| Patients who received the planned treatment†, no. | 76 | 67 | .06 |
| Consent withdrawal after R2 | 1 | 14* | .001 |
| Treatment interruption‡ | 5 | 1 | .21 |
| Days under therapy§, median (range) | 247 (215-316) | 82 (63-160) | <.001 |
| Need of rehospitalization and transfusion§ | |||
| No. of days, median (range) | 0 (0-31) | 27 (20-84) | <.001 |
| Patients with febrile neutropenia, % | 39 | 100 | <.001 |
| Transfusion requirements | |||
| RBC units, median (range) | 2 (0-24) | 6 (0-12) | .007 |
| Platelet transfusions, median (range) | 1 (0-18) | 4 (1-15) | <.001 |
NA indicates not applicable.
These 14 patients all received the ambulatory treatment.
For patients randomized in the ambulatory arm, it means until a total of 6 ambulatory cycles, relapse, or last contact.
Ambulatory therapy was prematurely interrupted after 3 to 5 ambulatory cycles in 4 patients while intensive therapy was not administered in 1 randomized patient, despite continuous CR.
Calculated in patients who had receive the whole planned post-remission therapy (6 ambulatory cycles or 1 intensive cycle) and had recover from all therapy-related toxicities at the lime of analysis (51 patients in the ambulatory arm vs 65 patients in the intensive arm); resources eventually associated with relapse management were not included.
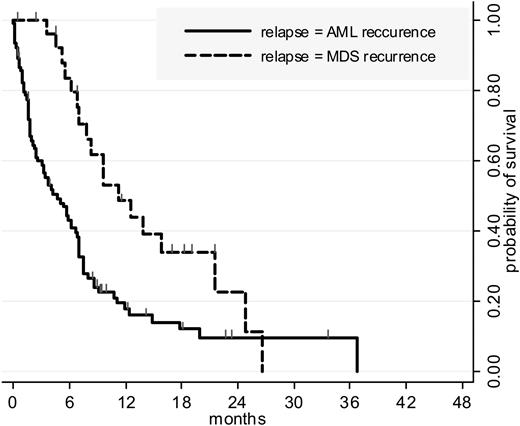
Finally, of the 117 patients who relapsed after R2 randomization, 27 (23%) had MDS rather than AML recurrence. The incidence of MDS recurrence was similar among both randomization arms and more frequent in patients treated for post-MDS than for de novo AML (44% versus 19%, P = .03). Postrelapse survival was significantly longer in patients with MDS recurrence (P = .002 by the log-rank test) (Figure 3).
Postrelapse survival, by relapse type. Postrelapse survival in patients randomized for postremission therapy. One hundred seventeen of the 164 patients randomized for postremission therapy relapsed. Twenty-seven relapses (23%) were classified as MDS recurrence (18 in the intensive versus 9 in the ambulatory arm; not significantly different). Postrelapse survival was significantly longer in these 27 patients than in other relapsing patients (P = .002 by the log-rank test). The incidence of MDS recurrence was more frequent in patients treated for post-MDS than for de novo AML (44% versus 19%, P = .03). Cumulative incidence of relapse was significantly higher in patients with post-MDS AML (59% versus 44% at 12 months, P = .005 by the Gray test) but due to a longer postrelapse survival their OS was eventually comparable with OS of patients with de novo AML.
Postrelapse survival, by relapse type. Postrelapse survival in patients randomized for postremission therapy. One hundred seventeen of the 164 patients randomized for postremission therapy relapsed. Twenty-seven relapses (23%) were classified as MDS recurrence (18 in the intensive versus 9 in the ambulatory arm; not significantly different). Postrelapse survival was significantly longer in these 27 patients than in other relapsing patients (P = .002 by the log-rank test). The incidence of MDS recurrence was more frequent in patients treated for post-MDS than for de novo AML (44% versus 19%, P = .03). Cumulative incidence of relapse was significantly higher in patients with post-MDS AML (59% versus 44% at 12 months, P = .005 by the Gray test) but due to a longer postrelapse survival their OS was eventually comparable with OS of patients with de novo AML.
Discussion
Overall results of a standard intensive chemotherapy in elderly persons with AML are considered as unsatisfactory. Some investigators have already raised the issue of the value of intensive chemotherapy itself in these patients compared with alternative options. The European Organization for Research and Treatment of Cancer (EORTC) Leukemia Group conducted a randomized study in which intensive chemotherapy resulted in a longer survival than best supportive care.23 Our French AML group conducted another randomized study in which low-dose cytarabine and intensive chemotherapy yielded similar survival duration.24 Over the past 20 years, a significant reduction in early mortality after intensive induction was nevertheless observed.25,26 This gain (< 10% induction death in the present study) may be due to a better supportive care during the neutropenic phase induced by chemotherapy but also to a better patient selection to intensive chemotherapy.
As in the ECOG study,27 the use of idarubicin during induction and consolidation phases was not associated here with higher CR rate or longer survival compared with daunorubicin. The only benefit we report is that the use of idarubicin led to a significantly higher response rate after the first induction course, as fewer patients required salvage therapy in the idarubicin compared with the daunorubicin arm. A superiority of idarubicin over daunorubicin in terms of CR rate and overall survival has been observed, however, in a systematic overview based on individual patient data of 5 studies.28
As a result of obvious patient selection associated with each trial dealing with elderly patients with AML, it is very difficult to properly compare the results of one study with another. To evaluate the degree of patient selection, the following factors are generally used: median age, performance status, proportion of patients with post-MDS AML and high-risk cytogenetics.3–7,29 The patient population of the present study does not seem to be particularly biased and, despite a higher median age, the overall outcome of patients enrolled compares favorably with that of other recent elderly AML studies. Of note, this outcome has been reached with relatively less intensive postremission treatments, even in our intensive arm as we retained a single intensive consolidation cycle in that arm. This can be a matter of debate and some investigators might consider this as suboptimal. However, in addition to the fact that repetition of intensive courses appears to be an impossible target for most patients aged more than 65 years, the few available studies that evaluated different postremission therapies failed to show any benefit associated with a more intensive treatment. In the British Medical Research Council (MRC) AML11 study, a total of 4postremission courses was compared with a single one, without significant difference in survival.9 In the Cancer and Leukemia Group B (CALBG) study, 2 relatively intensive postremission courses were compared with 4 less intensive courses based on standard-dose cytarabine, without significant difference in survival.30 The only limited randomized study which recently showed a prolonged outcome in patients receiving a more intensive postremission treatment is the HD98-B trial from the German-Austrian AML Study Group.31 However, this postremission comparison occurred after a total of 3 previous intensive cycles and, thus, significantly later than in the present study, resulting in a potential selection bias toward favorable risk factors.
We report here a longer DFS and overall survival in CR patients randomized to the less intensive and more prolonged option than in those randomized for one single intensive cycle, suggesting that a more prolonged and less toxic exposure to chemotherapy may be of benefit in this group of patients. The antileukemic efficacy of both options was at least equivalent. The ambulatory treatment was delivered as planned to most patients, with less deaths in CR, less time spent in hospital and transfusion needs, and thus probably a better cost/efficacy ratio. We consider now that this postremission therapy as our standard treatment, easily applicable to most patients in CR in this age group, to which alternative new strategies should be compared.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr F. Delabarre (CHUGAI Sanofi-Aventis, Chugai Pharma, France) for providing lenograstim and glycosylated granulocyte colony-stimulating factor (G-CSF) for the study. We are indebted to Drs S. Chevret, L. Degos, and G. Socie for indispensable advice and to S. Aprelon and Y. Costa from the Regional Clinical Research Office of the AP-HP for help in data monitoring.
This work was sponsored by Assistance Publique-Hôpitaux de Paris (AP-HP) and supported by the Programme Hospitalier de Recherche Clinique (PHRC); AP-HP ID, P970901 ID; PHRC AOM 94148. The clinicaltrials.gov study ID is NCT00363025.
Authorship
Contributions: All authors participated actively in study conception and design and acquisition of data. C.G. and P.T. contributed equally to study analysis. C.T. centrally reviewed all cytogenetic data. The statistical analysis was undertaken by H.D. The manuscript was written by C.G., P.T., and H.D and was approved by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Acute Leukemia French Association can be found in Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article.
Correspondence: Herve Dombret, Department of Hematology, Hôpital Saint-Louis, 1 Avenue Claude, Vellefaux, 75010 Paris, France. e-mail: herve.dombret@sls.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal